Many publications consider that the Alzheimer's disease (AD) is exclusive to the human species, and that no other animal species suffers from the disease. However, various studies have shown that some species can present with some of the defining characteristics of the disease in humans, both in the neuropathological changes and cognitive–behavioural symptoms.
DevelopmentIn this work, the results, published (PubMed) on the senile brain changes in non-human primates of different degrees of evolution, are reviewed. The neuropathological changes associated with the accumulation of amyloid or highly phosphorylated tau protein are rare outside the primate order, but in all the sub-orders, families, genera and species of non-human primates that have been studied, some senile individuals have shown amyloid accumulation in the brain. Even in some species the presence of these deposits in senility is constant. Changes related to the accumulation of tau protein, are always of very little significance, and have been detected only in some non-human primate species, both little evolved and highly evolved. In different species of non-human primates, some types of cognitive–behavioural changes are present in some senile individuals with greater intensity when compared with both normal adult individuals and other senile individuals of the species. The importance of the determination of the longevity of the species in different habitats (natural habitats, new habitats, semi-liberty, captivity) is stressed in these studies.
ConclusionsMorphological and histochemical and cognitive–behavioural features similar to those observed in normal aged man are present in senile non-human primates. Moreover, other characteristics of the non-human primates could be indicative of a pathological “Alzheimer type” ageing.
En muchas publicaciones se considera que la Enfermedad de Alzheimer (EA) es privativa de la especie humana, y que ninguna otra especie animal padece dicha enfermedad. Sin embargo, diversos estudios han mostrado que existen en algunas especies algunas de las características definitorias de la enfermedad en el hombre, tanto en el aspecto neuropatológico como en el cognoscitivo-comportamental.
DesarrolloEn este trabajo se recogen los resultados mostrados en la bibliografía (PubMed) sobre alteraciones cerebrales en la senilidad en primates no humanos de diferente grado de evolución. Las alteraciones neuropatológicas relacionadas con la acumulación de amiloide o de proteína tau altamente fosforilada son muy raras fuera del orden primate, pero en todos los subórdenes, las familias, géneros y especies de primates que se han estudiado han mostrado que algunos individuos seniles presentan acumulaciones amiloideas en el cerebro. Incluso en algunas especies la presencia de acumulaciones amiloideas en la senilidad es constante. Las alteraciones neuropatológicas relacionadas con la acumulación de proteína tau, siempre de muy escasa relevancia, se han detectado solo en algunas especies de primates no humanos, tanto poco evolucionados como muy evolucionados. En diferentes especies de primates no humanos, algunos tipos de alteraciones cognoscitivo-comportamentales están presentes en algunos individuos seniles con una mayor intensidad tanto frente a los individuos adultos normales como a otros individuos seniles de la especie. Se analiza además la importancia de la determinación de la longevidad de las especies en los diferentes habitats (habitats naturales, nuevos habitats, semilibertad, cautiverio) para acreditar la condición de “senil” de los individuos en estudio.
ConclusionesExisten características morfohistoquímicas y cognoscitivo-comportamentales involutivas generales en los primates no humanos seniles similares a las observadas en el hombre anciano, y otras características que pudieran ser indicio de un envejecimiento patológico “tipo Alzheimer”.
Books and monographs about AD often state, offering no further explanation, that this neurodegenerative disease is specific or peculiar to the human species.1 The scant number of published studies on the possibility of AD affecting other animal species, even evolutionarily similar non-human primates, would seem to confirm this assumption. Yet while they may be difficult to interpret, there is sufficient data to suggest that a more in-depth analysis of manifestations and symptoms in potentially affected animals will be necessary before such a categorical answer can be given. First of all, we should start by providing the definition of AD. In the 10th edition of the International Classification of Diseases (ICD-10),2 the World Health Organization (WHO) defines AD as “a primary degenerative cerebral disease of unknown aetiology with characteristic neuropathological and neurochemical features”. Its clinical profile is dominated by dementia with specific characteristics (“insidious onset with slow deterioration” and “absence of clinical data suggesting other systemic or brain disease”). In fact, this definition, due to lack of better knowledge about possible aetiologies of AD, places special emphasis on the “duality” of the pathological changes occurring in the disease. These changes were listed by Alois Alzheimer more than one hundred years ago in his description of what he termed “a mere cerebral cortex disease”: an array of clinical neuropsychological symptoms indicating dementia (based on an in vivo clinical diagnosis and requiring additional studies in order to rule out other causes of dementia) and a set of neuropathological signs that are manifestations of cellular and molecular morphological and functional changes in the brain (based on post-mortem anatomical pathology diagnosis). This invented duality arises both from the different fields in which the disease is present or treated (clinical medicine, social care networks, research), and our inability to establish correlations between the involutionary changes in neuronal circuits that gradually take place in AD patients and the implications of such changes.
Concluding that a specific mammal species can suffer from AD might be as simple as demonstrating the presence of both mental/behavioural and neurodegenerative disorders. Regarding the first type of disorder, the ICD-10 defines dementia as “a syndrome due to disease of the brain…in which there is a disturbance of multiple higher cortical functions, including memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgement”. It also causes significant behavioural disorders (apathy, aggressiveness, etc.).3 Logically enough, only humans can suffer from this syndrome if the above definition is applied strictly. Not even our closest primate relatives can be said to present a significant number of these disturbances, because they also lack most of the higher functions displayed by humans. However, some specific disturbances may be present in certain individuals of other species during senile involution, such as a decrease in learning capacity. Behavioural disorders, such as apathy, may appear at the same time. Perhaps the main point to ponder is the meaning of “disturbance of multiple higher cortical functions”. Without entering into a weighty debate on a topic (mental functions) that is not our main focus, if we consider each separate species to have its own type of ‘higher cerebral functions’, individuals of other species could therefore develop and suffer species-specific AD in cases in which multiple disturbances of these functions and morphological and functional abnormalities typical of cortical neurodegeneration are both present.
The definitions of ‘higher functions’ for each different species may be very different and controversial among different fields of science in general, and in biomedicine in particular. In fact, there are differences between ‘behaviour’, ‘cognition’ and ‘consciousness’, although they are subtle and somewhat abstract. ‘Behaviour’ may be defined as the way in which living beings solve the problems that they face throughout their lives.4 They do so by creating model responses ranging from the simplest reflex actions to highly complex strategies. ‘Cognition’ may be considered as a combination of sensory perceptions and the way they are processed according to the subject's intrinsic brain structures.5 ‘Consciousness’ is the personality structure in which psychological phenomena are fully perceived and understood by a person.6 According to these general definitions, behaviour and cognition can be ascribed to all animals, according to their specific degrees of complexity. However, it would be extremely questionable to state that animal consciousness exists, even if we were to consider only the most highly evolved non-human primates.7,8
In addition to these conceptual differences between different higher functions, differences also exist between similar or equivalent functions in species of the same genus, or species of different genera. There are also differences between the functions linked to specific cortical areas in different species. In most experimental cognitive studies of AD carried out in animals, researchers determine potential disturbances in learning, memory, and attention by using a wide variety of systems in which animals must resolve a situation or perform a task.1,9 These studies often obtain contradictory or unexpected results with respect to the higher function to which we believe a task is related. For example, injuries to cholinergic nuclei in the basal forebrain that innervate the cerebral cortex do not always produce similar changes in cortical function in different species. Abnormalities on learning and attention tests were shown to be different in monkeys and rats after lesion to the nucleus basalis. This complicates the task of defining and delimiting higher cortical functions, and also makes it difficult to establish equivalences among functions pertaining to different species.1,9 Despite the above, animal models are essential in order to continue in the search for answers regarding the development of neurodegenerative diseases and their potential treatments, whether palliative or preventive.
The second group of abnormalities that characterise human AD, neuropathological abnormalities, might be described as a set of objective signs that may be analysed systematically in all species, since in theory, we can identify and measure the cellular and molecular changes occurring in brain tissue in each individual. By this logic, in order to diagnose a mammal with AD in a laboratory, we would merely have to observe whether the alterations present in human AD were present in the animal brain in the necessary quantities. These changes in human brains primarily occur in or affect neurons, and disrupt the normal functioning of neural circuits,3,10–14 although involvement of glial cells (astroglia, microglia, and oligodendroglia) is also extremely important in AD pathogenesis.15 The most important neuropathological and neurochemical features of AD are loss of neurons and neural connections, gliosis, and the production and accumulation of abnormal proteins, whether intraneuronal (forming neurofibrillary tangles) or extraneuronal (forming diffuse amyloid plaques and clumps), plus increased oxidative stress, dysfunctional cellular communication systems (neurotransmitter and non-neurotransmitter systems), and the activation of (pro) neuroinflammatory systems.3,10–19
In our research into whether this neurodegenerative profile has been observed in other species, whether spontaneously or after inducement, we found that only a few systematic studies had been completed (or published). Most focus on a limited number of morphological and histochemical changes, especially those involving amyloid. In addition, we must point out that neuropathological alterations were not linked to changes in higher cerebral functions in individuals in most of these studies, and that these changes have not been contrasted with normal ageing processes that are to be expected in each particular species. The scarcity of published articles could be due to Alzheimer's disease not being present in a specific species, but it could also be caused by the difficulties involved in completing all of the pertinent research in certain species. Consider rodents, for example. Rats and mice, the most common laboratory animals, have been studied carefully up to the end of their life spans without there being any evidence of amyloid plaques or neurofibrillary tangles appearing spontaneously. This is true even in strains or individuals showing mild deficits in memory or learning functions in addition to other significant changes such as increased gliosis or oxidative stress.20 Experimental Alzheimer models in rodents, especially rats with lesions in cholinergic nuclei in the basal forebrain, have shown that major deficits in certain cortical functions may develop, but this is not always the case and it depends on the conditions of the model subtype.1,9 However, (a) discrepancies are almost always present between observed neuropathological changes and behavioural deficits1,9 and (b) formation of intraneuronal or extraneuronal fibrillary abnormalities has never been observed,1,9 although APP synthesis may increase after the lesion is induced,21 and marked changes in neurons and pronounced gliosis may occur over the medium and long term.1,22 This apparently manifest inability to experience amyloid alterations, and/or specific resistance to the changes that must take place in order for amyloid plaques to develop in these species, may be due to both an existing block of pro-amyloid factors and the technique for detecting cellular and molecular changes in the amyloid production system. Transgenic rats created by inserting altered human genes (APP, PS1) from subjects with family Alzheimer's disease can form plaques similar to those found in humans, but plaques do not cause changes in higher cerebral functions due to defence mechanisms specific to the production of amyloid from murine APP.20,23–26 On the other hand, the use of new techniques consisting of certain amphetamine treatments in rats with no signs of neuropathological alterations, but with intense levels of oxidative stress, has been shown to induce greater immunoreactivity to amyloid antibodies (APP, 6E11, AG8) and an increase in beta-amyloid protein, as detected by infrared and Raman spectroscopy.27,28 The possibility of inducing apoptosis and accumulation of amyloid substances in the rat hippocampus, in conjunction with cognitive deficits, was published for the first time in 2005.29 The study used animals that were either senile or had a vitamin E deficiency under conditions of pronounced oxidative stress. In some, but not all, subspecies of rabbits (order lagomorpha), it is possible to find neurodegeneration showing very similar brain lesions to those in the brains of humans with Alzheimer's.30,31 In other cases, some dogs (canines),32,33 cats (felines),34 bears (plantigrades),35 and a few other mammals such as wolverines (Gulo gulo, a mustelid)36 have shown extraneuronal amyloid alterations and/or fibrillary intraneuronal alterations (especially in the wolverine). However, these alterations have not been shown to coincide with higher cerebral function deficits. Nor do we find evidence suggesting potential differences between normal results in geriatric subjects and those that share pathological characteristics with AD in humans.
If we are to attempt to clarify whether or not AD exists in non-human animal species, it seems that identifying the existence of an AD-like illness in our nearest relatives – non-human primates – is the logical place to begin. This study engages in an in-depth analysis of the international literature referring to potential characteristics of AD in non-human primates, especially those neuropathological aspects most closely related to post-mortem diagnosis.
Literature analysis and original researchWe performed an analysis of the Medline database (http://www.ncbi.nlm.nih.gov/pubmed) for the period between August 1950 and April 2011. For purposes of this search, we used generic keywords (in English) such as ageing, Alzheimer, tangles, tauopathy, amyloid, amyloid clusters, plaques, dementia, and names of concrete animal species. Performing searches based on the terms primate, non-human primate, ape, etc. was not possible since search engines for this database do not recognise these keywords (as confirmed by Medline representatives in the U.S.). Searches by the scientific names of different species delivered only a few hits in the literature. Of the more than 23000 articles recovered, we then selected about 700 whose title or summary mentioned Alzheimer-type changes or senile disorders in primates. Further reading of these articles showed that only 227 listed studies of this topic. Most of the authors/research teams had written more than one article about the same study or research project.
Our original research into a colony of stump-tailed macaques (Macaca arctoides), mainly described in the second section of this article, was carried out by following brain study protocols used in Alzheimer patients. We completed a programmed cognitive–behavioural analysis of these animals during their geriatric period.
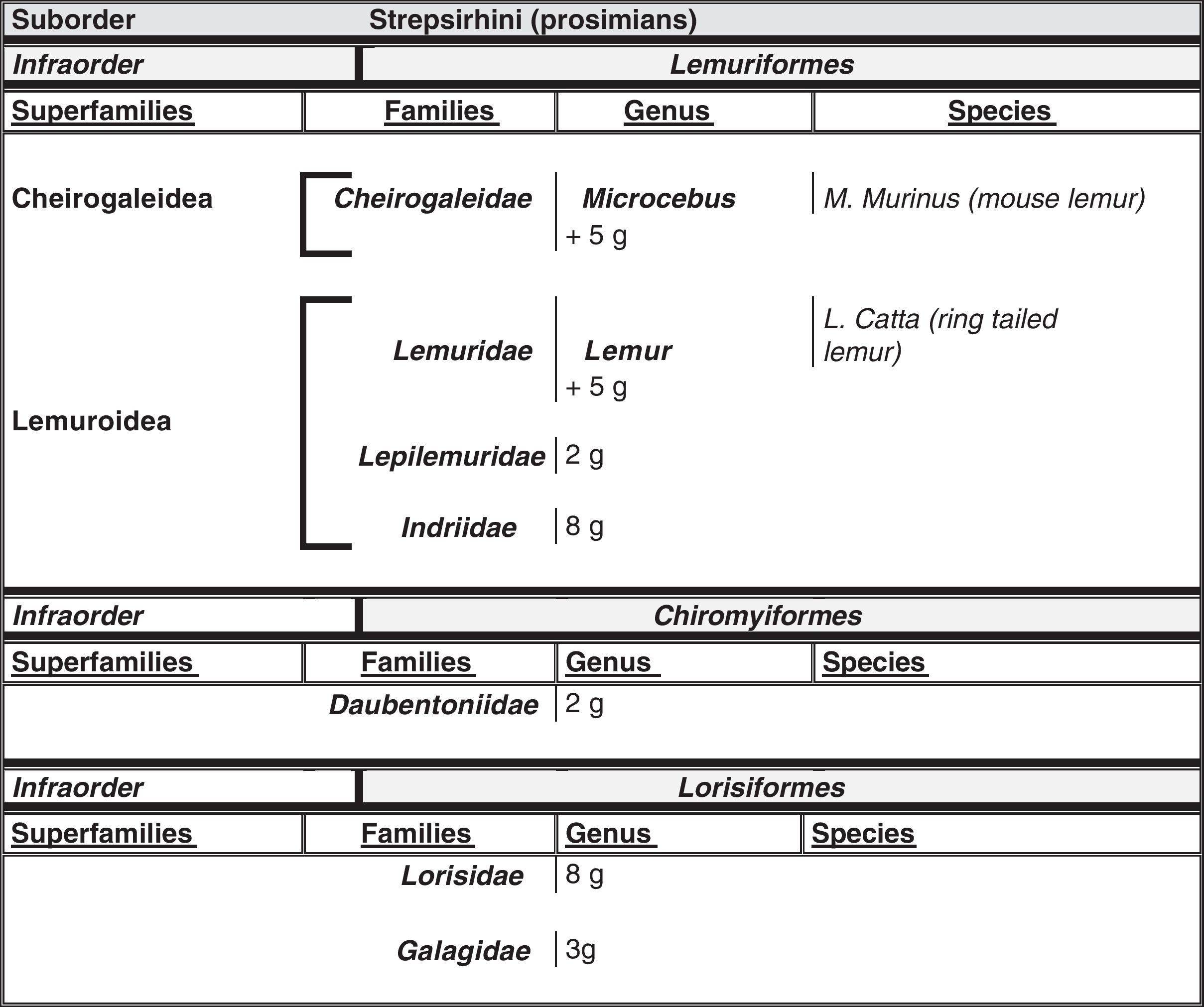
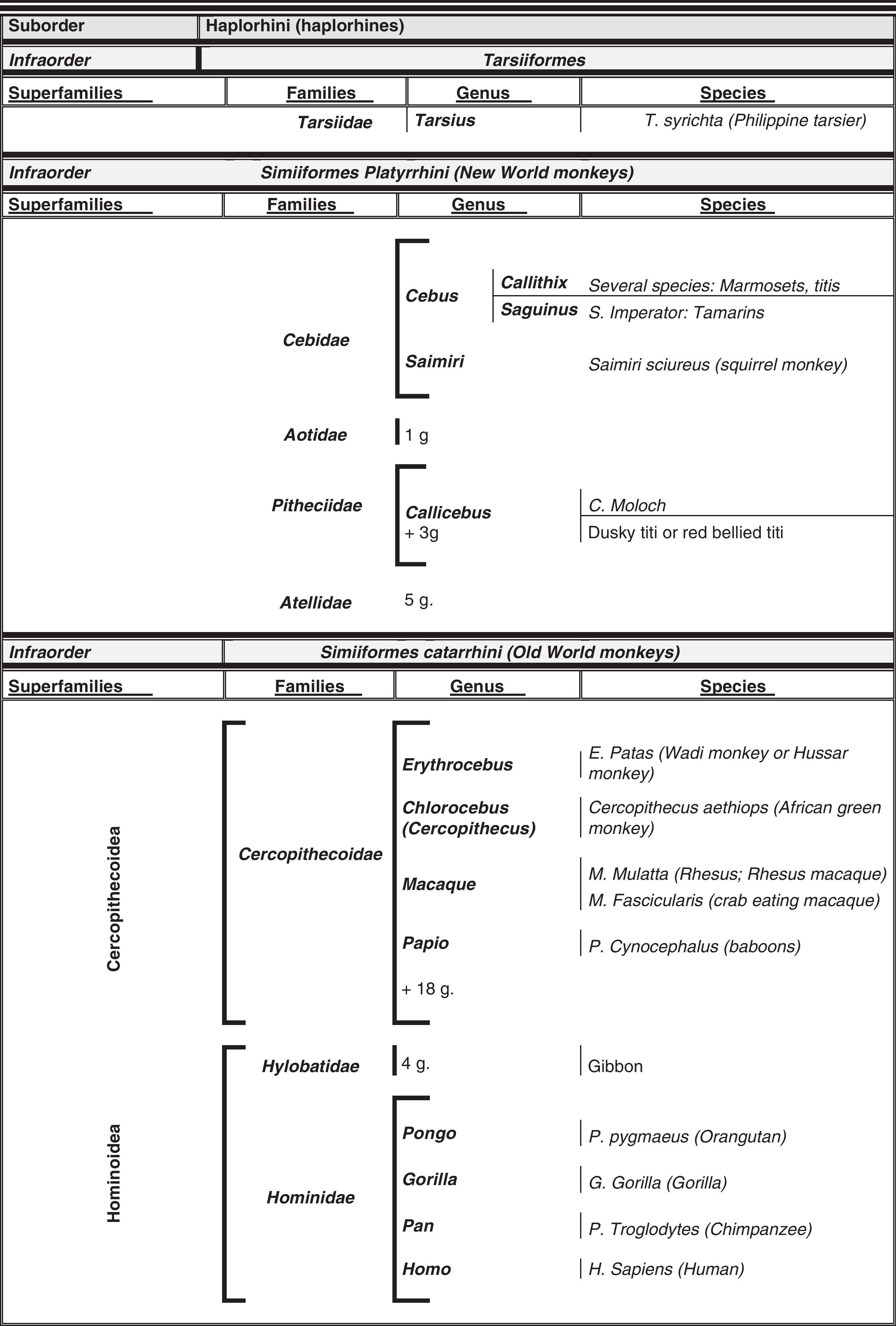
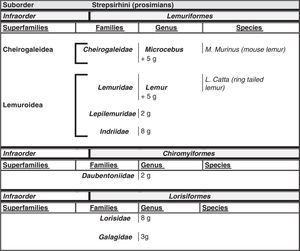
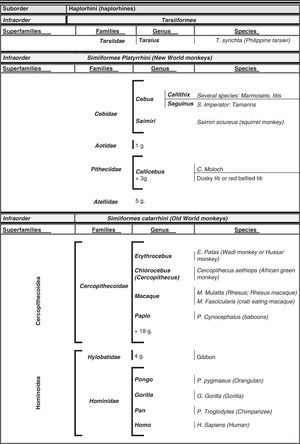
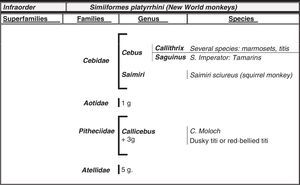
Humans within the primate orderHumans belong to the species Homo sapiens sapiens within the order of the primates. Our modern species evolved from a common anthropoid ancestor (the origin of the superfamily Hominoidea, which gave rise to hominoids and great apes). Prosimians appeared in the Cretaceous, 60 million years ago, and H. sapiens appeared 200000 years ago (Neanderthal subspecies). H. sapiens sapiens dates back to 20000 BCE (Early Modern or Cro-Magnon humans); modern humans arose in approximately 5000 BCE. Table 1 shows a cladogram with the situation of primates (some 200 species) at the top of the mammalian evolutionary scale; Figs. 1 and 2 list the families and genera of the primate order, including the Homo genus, in order of evolution. We can observe two well-differentiated suborders: prosimians (Strepsirrhini, less evolved) (Fig. 1) and simians (Haplorhini, more evolved) (Fig. 2). Within the latter group, we distinguish between Platyrrhini, or New World monkeys, and Catarrhini, Old World monkeys and apes. Humans are included in Catarrhini with the great apes (family Hominidae, genera Pongo, Gorilla, Pan and Homo). There are thought to be 22 genera of prosimians (of which only 2 have been subjects of studies relating to AD), in addition to 49 species of simians including the 8 genera of hominoids. Within the latter, more evolved infraorder, the great apes are not the most commonly studied subjects of ageing and Alzheimer research after humans. More research is done on Cercopithecidae, their evolutionary precursors. Fig. 3 shows several species of non-human primates with differing degrees of evolution. In these species, we observe a progressive increase in size, changes in appearance, and the tendency towards bipedalism as we move from prosimians to humans.
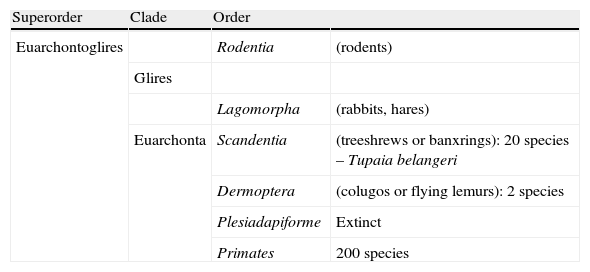
Primates at the top of the evolutionary scale and their kinship with proto-primates (Scandentia and Dermoptera) and rodents.
| Superorder | Clade | Order | |
| Euarchontoglires | Rodentia | (rodents) | |
| Glires | |||
| Lagomorpha | (rabbits, hares) | ||
| Euarchonta | Scandentia | (treeshrews or banxrings): 20 species – Tupaia belangeri | |
| Dermoptera | (colugos or flying lemurs): 2 species | ||
| Plesiadapiforme | Extinct | ||
| Primates | 200 species |
Images of non-human primates: Microcebus murinus (mouse lemur), Lemur catta (ring-tailed lemur), Callithrix jacchus (common marmoset), Saguinus imperator (tamarin), Saimiri sciureus (squirrel monkey), Pan troglodytes (chimpanzee) and Gorila gorila (gorilla). Photographs provided by Faunia-Madrid (Lemuriforms and Platyrrhines) and Zoo-Aquarium-Madrid (chimpanzee and gorilla).
The species H. sapiens was defined by Linnaeus in 1758,37 and its main traits were described by that author and others in later years. One of the listed distinguishing traits is consistently described as being inherent to the species: the presence of a ‘complex brain’ with ‘higher functions’.38,39 It is true that the cerebral cortex functions in humans described as ‘higher functions’ differ significantly from those of other mammals, including non-human primates. Throughout the evolutionary process, there have been different stages in which natural selection favoured those species with better functional cerebral capacity in a process known as encephalisation.40,41 Particularly in primates, if we examine gaps between less-evolved species and the next more-evolved relation, we observe development of multiple regions in the neocortex, prefrontal cortex, and centres/areas of association (Figs. 4 and 5), plus an increase in synaptic connections between neurons. These processes have both perfected higher cerebral functions and created new ones within the cognitive and behavioural spheres.42–46 Progress and morphological/functional fine-tuning in areas related to learning, different types of memory, and reasoning did not occur gradually throughout the evolutionary process. During the period of time marking the gap between modern living great apes and humans (bridged by an unknown number of extinct ancestor species), humans developed their exclusive distinguishing traits: the language area, the capacity for reasoning and logic, the more complex parts of the learning and memorisation processes, etc.40–50
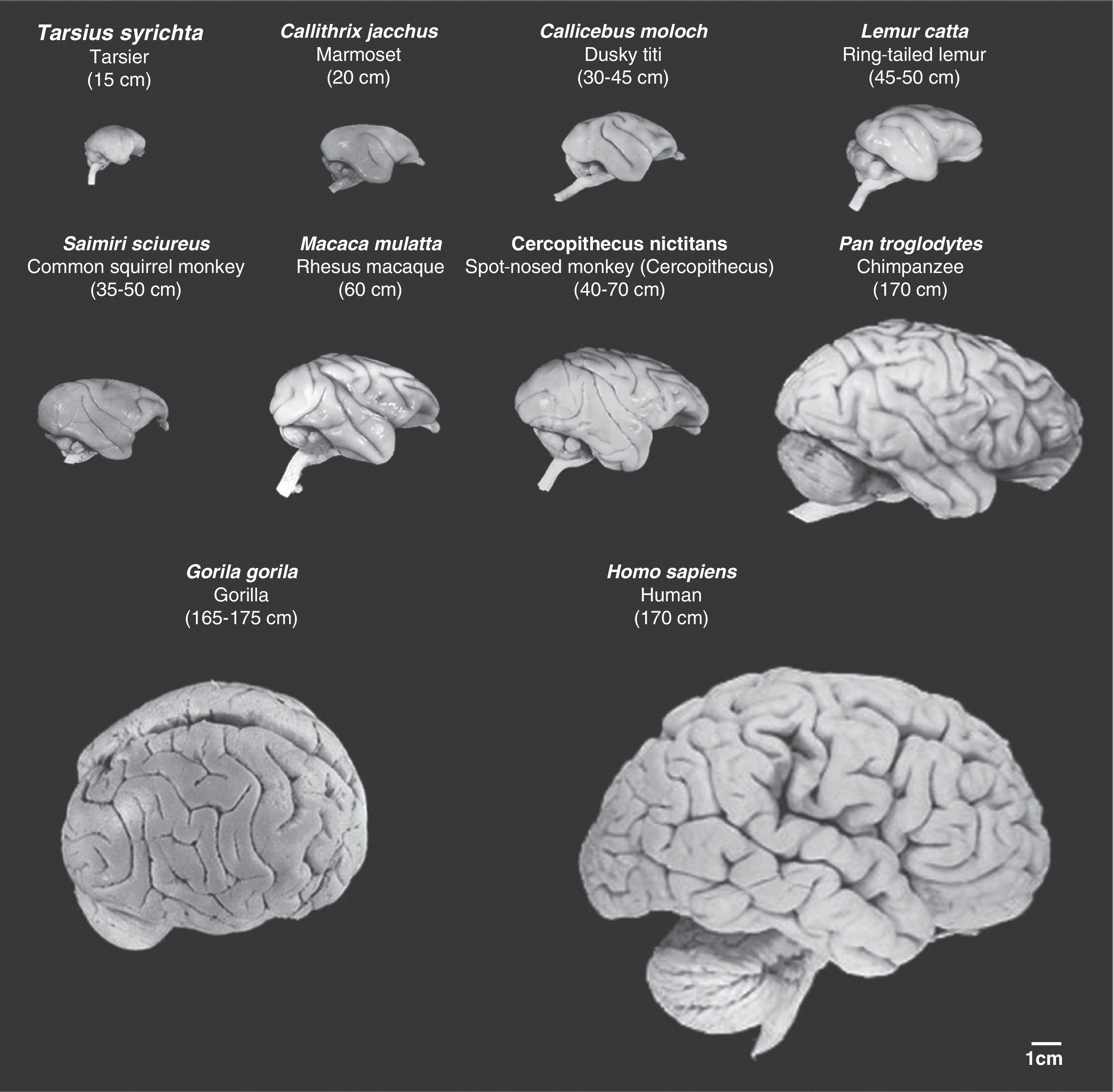
Images of non-human primate brains compared to human brains. Images are shown to the same scale. We can see that as cerebral mass increases, so do the number of cerebral folds and the volume of the prefrontal and frontal cortex. Mean body length (excluding the tail for species with tails) of the adult primate is shown in brackets. Images were taken from the University of Wisconsin and Michigan State Comparative Mammalian Brain Collections [consulted 26 April 2011]. Available at: http://brainmuseum.org/index.html.
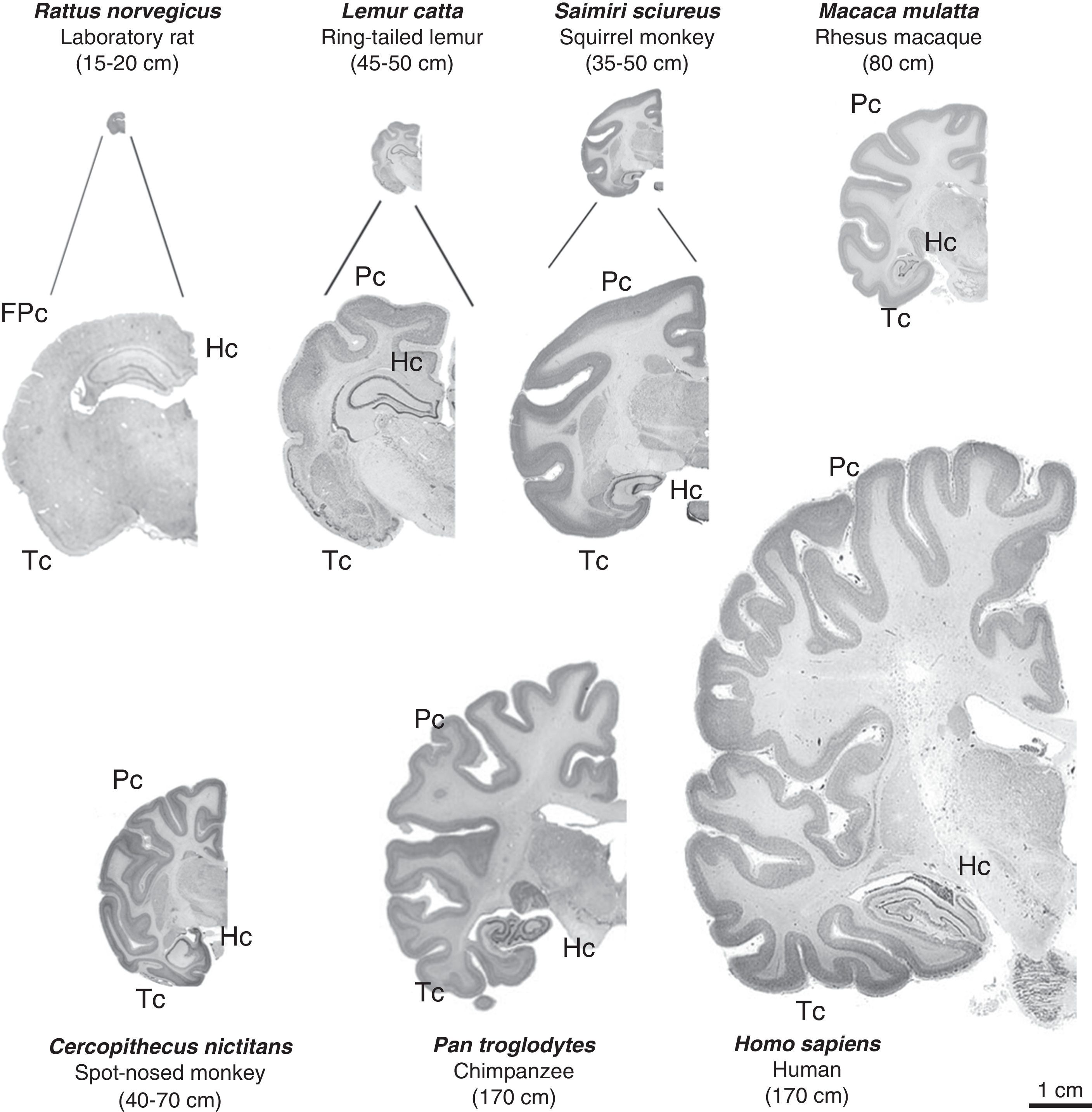
Images of coronal brain sections from non-human primates compared with rat and human brains. Images are shown to the same scale (close-ups of the first 3 are provided for better viewing). We can see that in addition to the increase in brain mass, the number of brain folds also increases progressively; brain folds are a characteristic of non-human primates, while lissencephaly is typical of rodents (FPc=frontoparietal cortex). We also see increases in parietal (Pc) and temporal (Tc) cortex volumes. The hippocampus (Hc) is located in a dorsal position in prosimians (Lemur catta), which is similar to its location in rodents (rat), while it is ventral in simians including humans. Mean body length (excluding the tail for species with tails) of the adult primate is shown in brackets. Images of non-human primates were taken from the University of Wisconsin and Michigan State Comparative Mammalian Brain Collections [consulted 26 April 2011]. Available at: http://brainmuseum.org/index.html.
In the search for animal models that would be useful in research into human senility and diseases associated with ageing, primates have logically been selected due to being phylogenetically close to our own species. In theory, they possess two significant advantages: similarities in some cognitive processes (for example, those related to non-spatial visual ability), the ability to perform certain tasks, and structural similarity in many regions of the brain. However, only a very few primate species have been studied, as we see in Figs. 1 and 2. There are a number of reasons to explain this: raising subjects in captivity is very problematic, and so is studying wild colonies, which contain very small populations and are difficult to reach and monitor. In addition, there are a number of legal restrictions on experiments with primates (laws in the United States, European Parliament resolutions, the Great Ape Project, etc.). In addition, analysis of the topics covered by published works shows that there have been very few studies comparing cognitive/behavioural deficits with cellular and molecular changes, whether within a single species or among individuals of different species with different evolutionary levels (this is also true of human studies). This type of comparative study is completely necessary to our understanding not only of physiological ageing, but also of the pathological ageing which characterises neurodegenerative diseases. In humans, physiological changes in senile involution (considered to be those occurring in brains with a low presence of classic neuropathological characteristics) cause cognitive and behavioural alterations of little medical relevance. Neurodegeneration in patients with AD, however, leads to dementia. Rather than being a mere decrease in certain cognitive functions, dementia refers to the cumulative severe changes or abnormalities in all of the different higher cognitive functions. Different degrees of both neuropathological changes and changes in social behaviour or learning/memory have been described in some geriatric non-human primates.51 However, only a few non-human primates have been subjects of both behavioural studies during life and anatomical pathology studies after death,52 (studies currently being elaborated by the authors of the monograph; see section “Cognitive and behavioural changes in senile non-human primates”). These are the most valuable cases which shed light on the problem of whether or not non-human primates can suffer from AD. Some of these studies have found that, generically speaking, there is a pronounced difference between the social behaviour, performance of learned tasks, and assimilation of new tasks among geriatric primates with no amyloid abnormalities and little neuronal loss and among other subjects of the same species with amyloid abnormalities and more marked neuronal loss.52 However, these results cannot be interpreted as a confirmation of the presence of a universal twofold model of senile involution valid for all primates (comprising physiological senile involution and pathological-AD senile involution). This is because some studies have found, on the one hand, significant differences between elderly humans and geriatric non-human primates53–60; meanwhile, other more recent studies61 have shown that non-human primates may present neuropathological changes without any major behavioural problems. As we will see in the second part of the monograph, all the above evidence demonstrates that correlations between behavioural changes and neuropathological lesions are different, and that distinct clinical and pathological stages may be present. This suggests that there may be specific ageing models within the primate order, which includes humans.
Life expectancy in these animal species and the transition from maturity to senilityOne of the questions that must be resolved before undertaking studies on potential animal models is the age at which primates of different species are considered senile or geriatric. Results obtained through studying small colonies or lone animals cannot be applied to the general population. Life spans vary considerably between species. They depend not only on the species and subspecies, but are also heavily influenced by intrinsic factors (family and individual) and extrinsic factors (especially lifestyle). Lifestyle is a fundamentally important factor for establishing the life span of a certain species. In particular, we should highlight the fact that each species can be said to have two values for life span: one in captivity (or controlled semi-captivity) and the other in the wild. The former life span value is always longer (except where the animal completely fails to adapt to captivity), due to medical care and proper nutrition being provided. These two theoretical values change considerably (by more than 30% in some cases) as a result of a number of factors, including the extent of medical care and control over nutrition for animals in captivity or semi-captivity, and habitat characteristics for animals in the wild. Prevention of malnutrition arising from dental or digestive problems (through soft diets enriched with dietary supplements) and infectious disease prevention are especially important for geriatric animals in captivity. Sanitary conditions, presence of predators and availability of food and water are especially important for wild animals in different habitats. If calculating life span is problematic, calculating the cut-off point between maturity and senility is more so.
Many of the studies covering this topic made use of macaques (Rhesus, Cynomolgus and Arctoides), which is why many authors consider them as the model for non-human primates. The rhesus macaque (Macaca mulatta) is native to Asia and its natural range is from Afghanistan to China and Thailand, from sea level to elevations of 3000m. In captivity or semi-captivity, however, and due to introduction to new habitats, they can be found around the world. Their life expectancy and transition from maturity to senility are topics for debate, but generally speaking, their life spans in captivity or semi-captivity range from 35 to 40 years. Individuals aged more than 20 are thought to be geriatric and those in their third decade are considered extremely elderly.53,54,58,59 In comparison, the oldest non-human primate to have been studied was a 59-year-old chimpanzee raised in a laboratory.53 Differences in longevity between certain individuals of the genus Macaca raised in research centres, wildlife preserves, or protected areas (for examples, the Caribbean keys and small islands) are quite pronounced.60,61 In the United States, animals used in experiments or other specific activities must be taken to preserves where they are cared for the rest of their lives, and cases of extreme longevity have been reported among them. The oldest chimpanzee in the world lives in CHEETA Primate Sanctuary (Creative Habitats and Enrichment for Endangered and Threatened Apes) in Palm Springs, California. This chimpanzee was once thought to be one of those originally participating in Tarzan films produced between 1930 and 1940. Although an investigation put that myth to rest, it concluded that the chimpanzee was more than 50 years old,62 which is comparable to the better-documented case described above.53 Paradoxically, while the rhesus macaque is the most commonly used primate in senility studies, none of the published articles offers a clear definition of physiological or pathological senility based on well-established parametric characteristics or values, whether in morphological or behavioural terms. There are no baseline data permitting us to characterise individuals in a study independently from their age. On the other hand, the number of animals in published studies is always quite low and does not accurately represent populations in the wild.
While data having to do with senility in macaques are varied, other species seem to present more homogeneous life spans, although environmental conditions always exert an important influence on longevity and the senile involution process. A good example can be found in vervet monkey colonies (Caribbean vervet, Chlorocebus or Cercopithecus aethiops) living in Saint Kitts (western Caribbean). These colonies contain some 30000 individuals, and have been studied and controlled by the Behavioural Science Foundation (BSF) since 1968.61,63,64 The first monkeys on St Kitts arrived from Africa by slave ships in the 1630s and rapidly became acclimated to all the Caribbean islands in general. Whether wild on the islands or in captivity, these omnivorous animals live between 20 and 30 years, 5–10 years longer than the life spans of their West African ancestors, since they are not exposed to the viral pathogens that decimate their populations in Africa.62 This primate species may be of great interest in the study of neurodegenerative diseases if their ageing process, which is always accompanied by amyloid deposits, can be documented correctly.64
Compared to the longevity of macaques, which makes longitudinal studies of their illnesses difficult, other species have much shorter life spans. One example is the mouse lemur (Microcebus murinus), a tiny prosimian weighing 100g, which can be raised in small colonies in semi-captivity.65 Nevertheless, the ageing process of these animals is highly dependent on environmental factors. Their longevity in the wild is 3–4 years, while life spans in captivity or semi-captivity may exceed 10 or even 13 years.51,65 The changes in these primates’ ageing process, along with the specific characteristics of the location of the neuropathological changes in their brains, described in a further section, make this model a controversial one.
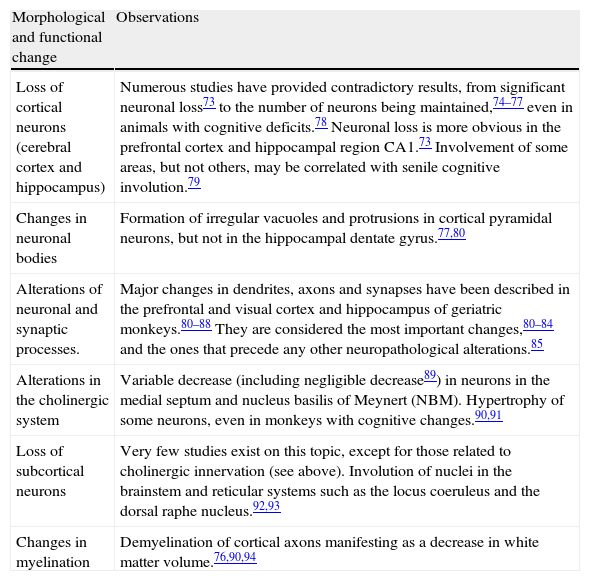
Morphofunctional physiological brain involution in non-human primatesAlterations described in elderly humans1,66–72 are generally said to be observed in non-human primates as well. The most commonly studied aspects within the field we might term ‘physiological senility’ (the morphological and functional involution of the bran with mild, sporadic losses of certain cognitive or behavioural capacities) are summarised in Table 2. They mostly involve neuronal dystrophy (changes in the soma, dendrites, and axons, with decreases in synaptic contacts) and gliosis.73–94
Age-related morphological (physiological) changes in non-human primates.
| Morphological and functional change | Observations |
| Loss of cortical neurons (cerebral cortex and hippocampus) | Numerous studies have provided contradictory results, from significant neuronal loss73 to the number of neurons being maintained,74–77 even in animals with cognitive deficits.78 Neuronal loss is more obvious in the prefrontal cortex and hippocampal region CA1.73 Involvement of some areas, but not others, may be correlated with senile cognitive involution.79 |
| Changes in neuronal bodies | Formation of irregular vacuoles and protrusions in cortical pyramidal neurons, but not in the hippocampal dentate gyrus.77,80 |
| Alterations of neuronal and synaptic processes. | Major changes in dendrites, axons and synapses have been described in the prefrontal and visual cortex and hippocampus of geriatric monkeys.80–88 They are considered the most important changes,80–84 and the ones that precede any other neuropathological alterations.85 |
| Alterations in the cholinergic system | Variable decrease (including negligible decrease89) in neurons in the medial septum and nucleus basilis of Meynert (NBM). Hypertrophy of some neurons, even in monkeys with cognitive changes.90,91 |
| Loss of subcortical neurons | Very few studies exist on this topic, except for those related to cholinergic innervation (see above). Involution of nuclei in the brainstem and reticular systems such as the locus coeruleus and the dorsal raphe nucleus.92,93 |
| Changes in myelination | Demyelination of cortical axons manifesting as a decrease in white matter volume.76,90,94 |
Most studies dedicated to researching the possible presence of AD-related neurodegenerative processes focus on the search for neuropathological changes that resemble amyloid plaques or neurofibrillary tangles. Only a few have also included parallel studies of other morphological or biochemical parameters such as the ones we are examining in a colony of stump-tailed macaques (section “Cognitive and behavioural changes in senile non-human primates”). Table 3 lists the general neuropathological traits of non-human primates that have been studied and which provide the most informative results regarding whether or not AD is present in these animals.
Age-related morphological changes suggesting pathology (such as Alzheimer's disease) in non-human primates.
| Morphological and functional change | Observations |
| Amyloid in plaques or in diffuse non-vascular deposits | Amyloid deposits of varying densities and different appearances and locations, depending on the species, have been described in all non-human primate species.51,56,59,62,64,85,95–98 However, APP and the amyloid peptide may be similar to those found in mammals in lower evolutionary orders in which deposits do not form.55 Abeta40 is predominant53 and associated with ApoE497,99 in deposits. In prosimians, it may be coexpressed with tau protein.100 The relationship with vascular deposits is quite variable.101,102 The amyloid load increases with age, mainly in the frontal and parietal lobes. Low deposit density in the occipital lobe, prefrontal cortex and parahippocampal gyrus. There is no relationship between the number of plaques and cognitive impairment95,101; amyloidosis can be induced experimentally.103,104 |
| Perivascular amyloid deposits | Marked deposits over blood vessels in numerous prosimians and less-evolved simians,52,101,102 and scant deposits in more-evolved geriatric simians.96–99 |
| Neurofibrillary tangles (intraneuronal deposits positive for phosphorylated tau protein) | This phenomenon is rarely observed in non-human primates except for prosimians. Tau protein-linked neuropathy is well-demonstrated in Microcebus murinus.52,57 Neurofibrillary tangles are rarely seen in simians, and some researchers even doubted their existence, but they were recently discovered in Chlorocebus aethiops64 and Pan Troglodytes.105 |
| Phospho-tau-positive dystrophic neurites | Present in animals with more marked involutive changes and amyloid disorder.100,106,107 |
Plaque-like beta-amyloid deposits with forms and immunoreactivities that are either similar to or different from those described in humans, and other diffuse non-vascular beta-amyloid plaques, are found in geriatric specimens in nearly all non-human primate species, and amyloid load increases alongside age. Nevertheless, only a few macaque and chimpanzee brains show degenerative changes in neuronal soma and neuritic dystrophy, especially in cerebral areas, in addition to amyloid plaques.51,95–100 Furthermore, decreases in the function of neurotransmitter systems specifically related to AD, especially the cholinergic system, have been recorded in some chimpanzees.95 Perivascular amyloid deposits may or may not be associated with other neuropathological signs, depending on the species and age of the individual. They are more numerous in prosimians and some types of less-evolved New World monkeys.57 The age at which amyloid neuropathy appears, the type of plaque, the location, and the association with other neuropathological signs all vary considerably from one species to another, and between individuals belonging to the same species, since a number of authors have provided widely divergent descriptions. These amyloid plaques have not been observed in proto-primates within the order Scandentia, such as Tupaia belangeri (the tree shrew, native to Southeast Asia).55 Plaque shapes vary considerably and depend on the species, and their presentation pattern does not correspond well with the degree of evolution of the species. Neuritic plaques, mainly in the parenchyma of the cerebral cortex, have been observed in the rhesus macaque; perivascular deposits are common in squirrel monkeys and diffuse plaques are generally observed in chimpanzees.96–99 In all cases, including that of Tupaia, researchers found that the Abeta peptide sequence was identical to that in humans, revealing as much as 98% similarity and 97% identity with the human protein. In general, non-human primates accumulate higher proportions of Abeta40 than humans do.53 Two studies have shown that non-human primates have a single isoform of ApoE associated with amyloid deposits. This isoform is analogous to human ApoE4.98,99
In contrast to what occurs with amyloid deposits, the presence of intraneuronal neurofibrillary tangles is not generally observed in non-human primates. We should mention the interesting exception of two species characterised by their high concentrations of phosphorylated and non-phosphorylated tau protein. Species in which tauopathy appears include one of the least-evolved primates (M. murinus) and one of the most evolved (Chlorocebus aethiops).51,57,64 In general, dystrophic dendrites that are immunopositive for phospho-tau protein are found in animals with greater involutive changes and the most amyloid disease. This is closely related to amyloid deposits in most of the brain regions that have been studied.100 As we will mention in a further section, recent studies using more sensitive techniques have found changes related to tau protein in Old World monkeys, but these changes do not have the same features as we observe in humans.
Metabolism of the proteins that may cause beta-amyloid or neurofibrillary deposits has only been partially studied in monkeys, but it appears to follow the steps described in humans.100,108–112
Cognitive and behavioural changes in senile non-human primatesNormal ageing in humans is accompanied by deterioration of a wide variety of mental functions corresponding to different areas. Deterioration may not occur uniformly across all areas in a single individual. If we focus on some of the higher cerebral functions, such as memory, learning, cognitive flexibility (capacity for problem-solving using different strategies), attention, and regulation of motor abilities, it would seem that the same phenomena could occur in some non-human primates based on published studies. In fact, in cases of primate senile cerebral deterioration, we may even observe improvements in certain functions (motor regulation skills, learning ability, searching for alternative problem-solving methods, etc.) if specific training is carried out, as occurs in humans.52,54,113
As we see in elderly humans, senile non-human primates require more time in order to learn new skills and develop new abilities. This has been demonstrated by behavioural testing and studies of cognitive damage such as delayed response tasks in which the animal subject has to memorise a location in space or recognise objects,52,54,113 or in cognitive flexibility tests that evaluate the ability to find alternative solutions to a single problem.114 Both short-term and long-term memories have been shown to be affected. Numerous studies of geriatric monkeys, especially macaques, have demonstrated that these animals experience increasing difficulty remembering different types of information.52,58,114–118 Many of these results are quite similar to those gathered when humans complete the same tasks, for example, being asked to recall different locations within a house when the lighting is changed.119 Results from these studies also reinforce the idea that memory improves with practice and with specific training.117,120 Other cases have revealed clear differences. While a marked loss of attention span can be observed in elderly humans, this loss of function in monkeys, recognised by a few monographs, does not seem to have the same characteristics. Likewise, in studies of rapidity of response to a stimulus, few differences have been identified between geriatric and young monkeys.52,54 Based on all of these studies of memory loss and improving memory through training, we reach an important conclusion: there are very significant variations in the results of tests carried out with subjects of the same species and age range due to the endogenous factors present in each individual. This phenomenon also occurs in humans, and it means that cognitive–behavioural deterioration does not take place in a homogeneous way. Such changes are difficult to study and measure, whether from a clinical or research perspective.119–124
While all the mentioned decreases in brain function are well-documented, we found no descriptions of cognitive–behavioural conditions equivalent to human dementia among non-human primates. Dementia is not only a decrease in cognitive function, but also the sum of the severe alterations or abnormalities in all of the higher cognitive functions. Very marked changes in social behaviour or in learning or memory capacity that distinguish some individuals from others of their species and age range have only been observed in a few primates, especially macaques.51–54 Groups of non-human primates subjected to behavioural study in life and anatomical pathology study after death are extremely scarce. However, these studies are the most helpful in the quest to determine whether AD exists in non-human primates. This handful of studies show, in general, major disorders in the areas of social behaviour, completion of learned tasks, and assimilation of new tasks among geriatric animals with significant amyloid alterations and marked neuronal loss.52,54 Many of these animals also present significant decreases in cholinergic neurons in the nucleus basalis of Meynert.90,91
FundingThis study was primarily funded through sources from laboratories which collaborated from 2010 to 2011 through a National Plan grant (CTQ 2009-09538).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Toledano A, et al. ¿Existe la enfermedad de Alzheimer en todos los primates? Afección de Alzheimer en primates no humanos y sus implicaciones fisiopatológicas (I). Neurología. 2012;27:354–69.








![Images of non-human primate brains compared to human brains. Images are shown to the same scale. We can see that as cerebral mass increases, so do the number of cerebral folds and the volume of the prefrontal and frontal cortex. Mean body length (excluding the tail for species with tails) of the adult primate is shown in brackets. Images were taken from the University of Wisconsin and Michigan State Comparative Mammalian Brain Collections [consulted 26 April 2011]. Available at: http://brainmuseum.org/index.html. Images of non-human primate brains compared to human brains. Images are shown to the same scale. We can see that as cerebral mass increases, so do the number of cerebral folds and the volume of the prefrontal and frontal cortex. Mean body length (excluding the tail for species with tails) of the adult primate is shown in brackets. Images were taken from the University of Wisconsin and Michigan State Comparative Mammalian Brain Collections [consulted 26 April 2011]. Available at: http://brainmuseum.org/index.html.](https://static.elsevier.es/multimedia/21735808/0000002700000006/v1_201305151336/S2173580812001071/v1_201305151336/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Images of coronal brain sections from non-human primates compared with rat and human brains. Images are shown to the same scale (close-ups of the first 3 are provided for better viewing). We can see that in addition to the increase in brain mass, the number of brain folds also increases progressively; brain folds are a characteristic of non-human primates, while lissencephaly is typical of rodents (FPc=frontoparietal cortex). We also see increases in parietal (Pc) and temporal (Tc) cortex volumes. The hippocampus (Hc) is located in a dorsal position in prosimians (Lemur catta), which is similar to its location in rodents (rat), while it is ventral in simians including humans. Mean body length (excluding the tail for species with tails) of the adult primate is shown in brackets. Images of non-human primates were taken from the University of Wisconsin and Michigan State Comparative Mammalian Brain Collections [consulted 26 April 2011]. Available at: http://brainmuseum.org/index.html. Images of coronal brain sections from non-human primates compared with rat and human brains. Images are shown to the same scale (close-ups of the first 3 are provided for better viewing). We can see that in addition to the increase in brain mass, the number of brain folds also increases progressively; brain folds are a characteristic of non-human primates, while lissencephaly is typical of rodents (FPc=frontoparietal cortex). We also see increases in parietal (Pc) and temporal (Tc) cortex volumes. The hippocampus (Hc) is located in a dorsal position in prosimians (Lemur catta), which is similar to its location in rodents (rat), while it is ventral in simians including humans. Mean body length (excluding the tail for species with tails) of the adult primate is shown in brackets. Images of non-human primates were taken from the University of Wisconsin and Michigan State Comparative Mammalian Brain Collections [consulted 26 April 2011]. Available at: http://brainmuseum.org/index.html.](https://static.elsevier.es/multimedia/21735808/0000002700000006/v1_201305151336/S2173580812001071/v1_201305151336/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
