Postural and balance disorders, functionality impairment and fatigue, are the most incapacitating problems in multiple sclerosis (MS) patients. Whole Body Vibration (WBV), through the transmission of mechanical stimuli, appears to be a useful therapeutic tool in the treatment of neurological diseases. The objective of this study is to assess the effect of the WBV on postural control, balance, functionality and fatigue in patients with MS.
Material and methodsA total of 34 patients with mild–moderate MS were randomised into a control group and an intervention group. For the intervention group, the protocol consisted of 5 consecutive days, daily series of 5 periods of 1min duration of WBV at a frequency of 6Hz. Posturographic assessment using the sensory organisation test (SOT) and motor control test (MCT), the timed get up and go test, 10m test, the Berg balance scale and Krupp's fatigue severity scale were used before and after intervention.
ResultsThe analysis showed improvements in the intervention group for conditions SOT 1, SOT 3 and latency in MCT. In the comparison between groups, only the latency or reaction time in MCT improved significantly in favour of the intervention group (from 173.78±12.46 to 161.25±13.64ms; P=.04). No side-effects were found.
ConclusionsThe results of this pilot study show that WBV can improve, in the short-term, the time of response to recover the uprightness after sudden disturbances, appearing as a possible therapeutic tool maintaining balance and posture.
Los trastornos del equilibrio, junto con las alteraciones de la funcionalidad y la fatiga, constituyen los síntomas más incapacitantes en los pacientes con esclerosis múltiple (EM). La vibroterapia de cuerpo entero o whole body vibration (WBV), a través de la transmisión de estímulos mecánicos, se presenta como una herramienta terapéutica útil en el tratamiento de las alteraciones del control postural en diversas patologías neurológicas. El objetivo del presente estudio es valorar el efecto a corto plazo de la vibroterapia sobre el control postural, la funcionalidad y la fatiga en pacientes con EM.
Material y métodosTreinta y cuatro pacientes con EM con afectación leve-moderada, distribuidos aleatoriamente en un grupo control y un grupo experimental, participaron en el estudio. El grupo experimental fue sometido a WBV durante 5 días consecutivos (series diarias de 5 periodos de 1min de duración) a una frecuencia de 6Hz. Previamente y post-intervención, fueron realizadas valoraciones con posturografía dinámica computarizada, mediante el test de organización sensorial (SOT) y el test de control motor (MCT), así como con el test timed up and go, la escala de equilibrio de Berg, la prueba los 10 metros y la escala de severidad de fatiga de Krupp.
ResultadosEl análisis comparativo de datos pre y post-intervención de los grupos mostró mejoras en el grupo experimental para las condiciones SOT 1, SOT 3 y la latencia en el MCT. Realizada la comparación entre grupos, únicamente la latencia o tiempo de reacción en el MCT mejoró significativamente a favor del grupo experimental (de 173,78±12,46 a 161,25±13,64ms; p=0,04). No se registraron efectos adversos derivados.
ConclusionesLos resultados de este estudio muestran que el protocolo utilizado de WBV mejoró a corto plazo el tiempo de respuesta para recobrar la verticalidad ante estímulos desestabilizantes, pudiéndose mostrar como una opción terapéutica en el mantenimiento del control postural y el equilibrio en pacientes con EM.
Multiple sclerosis (MS) is a major cause of disability and the most common neurological disease in young adults. It has been over 140 years since the clinical and pathological features of MS were first described and yet its aetiopathogenic knowledge remains a challenge.1 Its course is progressive, varied and unpredictable, leading to a physical and cognitive deterioration of patients. At present there is no effective treatment.2,3 It mainly affects patients aged between 20 and 50 years, with its prevalence in Spain ranging between 50 and 60 cases per 100000 inhabitants.3
The clinical manifestations of this disease appear as signs and symptoms with ample clinical variability, depending on the location of the demyelinating lesions which can occur throughout the central nervous system.4 In many cases, the organisation of movement is affected in all its aspects. Simultaneously, posture control suffers the same adaptive problems, with balance disorders along with altered functionality and fatigue being the most disabling symptoms and present in up to 78% of cases.5 The result is an anomalous gait with reduced mobility caused by the involvement of balance during walking. Patients typically present an increased support base, with greater instability during the start of motion or changes of direction. This postural instability, along with gait alterations, also represents a limitation in activities of daily living and has an impact on quality of life.6
Although one of the objectives of neurorehabilitation is the training and improvement of balance, this appears as one of the symptoms most resistant to therapeutic interventions.7 The absence of a curative treatment for the disease, along with its chronic course, have led to the exploration of alternative interventions aimed at controlling any of these disabling symptoms. Unfortunately for the medical community, there are no therapeutic programmes with enough continuity to provide long-term results.
In recent years there have been reports that the transmission of vibratory stimuli throughout the organism produces a series of beneficial physiological responses which depend on the characteristics of these stimuli. Vibration generated by a platform and transmitted to the body (Whole Body Vibration, WBV), activates a multitude of sensory receptors, from cutaneous to muscular. This has a particular impact on the stretching of muscle spindles through reflex activation of the alpha motor neurons which cause a tonic vibration reflex responsible for reflex muscle contraction.8,9 When combined with voluntary muscle contraction,10 this leads to an increase in synchronisation of motor units (MU), which in turn helps to improve muscle strength and functionality.11
There also appears to be an activation of higher motor centres, with an improved muscular and proprioceptive response. This could explain the improvements in balance obtained through its application.12,13
However, several authors have evaluated the acute effects on young adults of a single exposure to vibration therapy, reporting a transient improvement in some cases14,15 and no effect in others.16–18 Similar results have been obtained with prolonged exposures.19 Currently, its application is targeting neurological disorders such as cerebrovascular disease,20 cerebral paralysis21 or Parkinson's disease (PD).22,23
Generally, the performance of a traditional exercise programme in MS is limited by fatigue. The appearance of multiple devices in the market, along with the ease and comfort of their use, makes it possible to think of WBV as a therapeutic measure that could alleviate some of the symptoms and signs present in MS patients, requiring less effort and causing less fatigue,24 as well as lowering costs. However, there are still very few studies on the subject and they provide contradictory results.7,10,19,25
The objective of this study was to assess the short-term effectiveness of vibration therapy on postural control, balance, functionality and fatigue in MS patients.
Patients and methodsSubjectsSubjects were recruited from the Multiple Sclerosis Association in Móstoles (Spain) and were included if they presented mild or moderate disability, were able to stand and walk independently, with or without support, had an Expanded Disability Status Scale (EDSS)≤5 and were in a stable phase of the disease. We excluded individuals with prior experience using vibration platforms, as well as those who had suffered an MS flare in the 2 months preceding the survey, or who presented depression or some of the contraindications for the application of WBV, such as pregnancy, implants, epilepsy or tumours. In order to avoid the interaction of possible negative effects on the assessment of balance, we also considered the presence of serious adverse reactions as an exclusion criterion in those subjects who were following pharmaceutical treatment for fatigue and spasticity.
From a total of 36 patients recruited initially, 34 were included in the study.
This study was approved by the ethics committee of Universidad Rey Juan Carlos in accordance with the Helsinki Declaration.
DesignThis study was a randomised clinical trial (RCT). Patients were randomly distributed (using the QuickCalcs application from GraphPad Software) into an experimental group (n=18) or a control group (n=16). None of the subjects received physical therapy during the intervention period. All assessments were conducted by an investigator blinded to the study groups established.
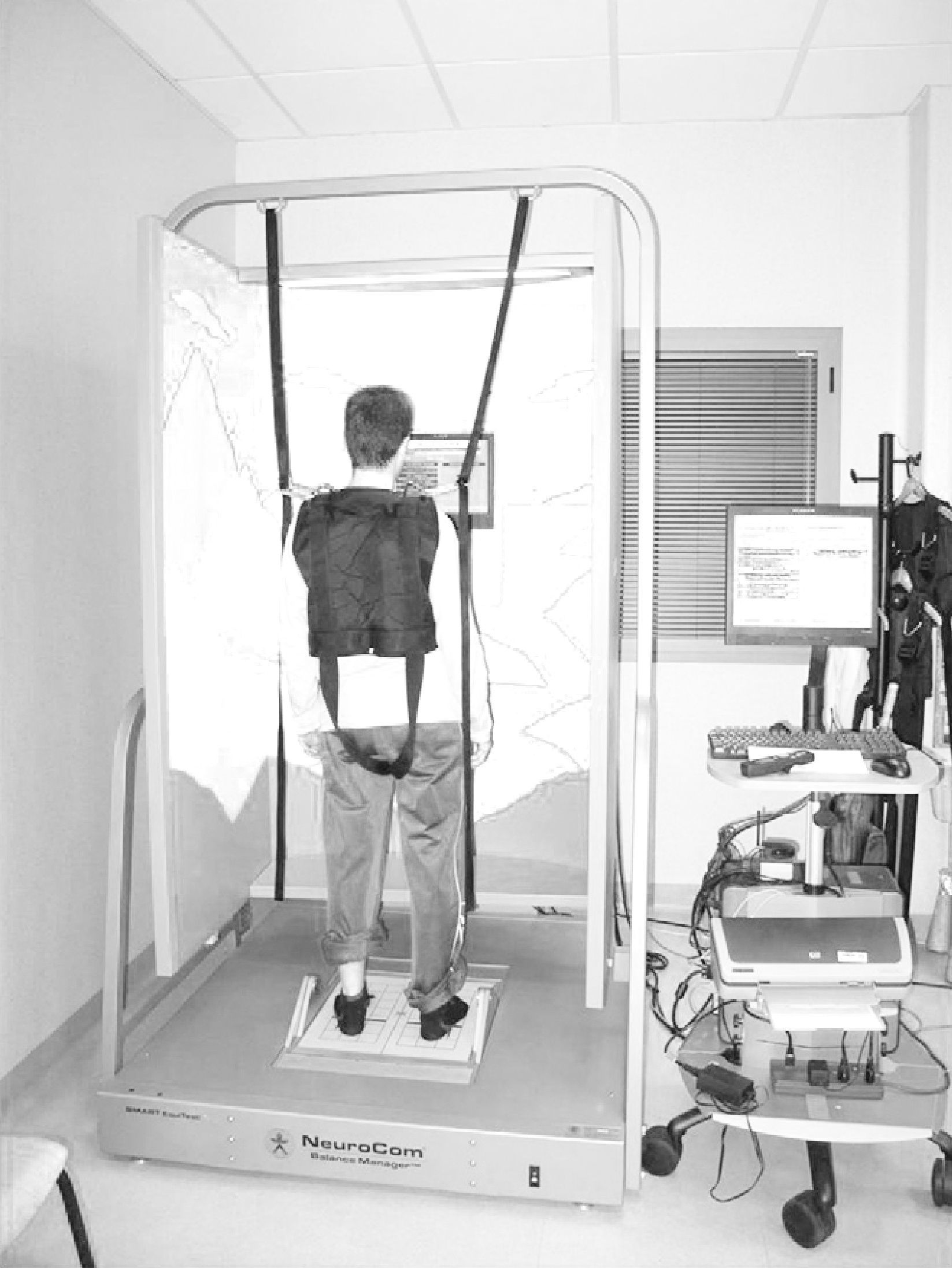
InterventionThe experimental group was subjected to WBV for 5 consecutive days, with daily vibration therapy series of 5 periods of 1min duration and 6Hz frequency at the Motion, Biomechanics, Ergonomics and Motor Control Analysis Laboratory of Universidad Rey Juan Carlos. Based on previous studies in subjects with neurological pathologies,7,10,24 we used amplitudes below 4mm (A=3mm) and inserted pauses of 1min between periods. The total duration of the intervention, taking into account the periods of vibration and breaks, was 10min. Patients had to remain in a semi-squat position and support was allowed when necessary. We used the Zeptoring® multidirectional stochastic platform (Scisen GmbH, Germany) (Fig. 1).
VariablesThe variables were evaluated prior to the start of the study and post-intervention (fifth day) by an independent investigator blinded to the allocation of patients. Although most previous studies in patients with neurological disorders evaluated results immediately after intervention, in our study this assessment was carried out 10min after the application of WBV in order to reduce the possible impact of fatigue.
The assessments conducted included the timed up and go test (TUG) developed by Podsiadlo et al,26 who eliminated the subjectivity of the get up and go test by replacing its grading scale with a more objective temporal measurement. The TUG test has shown good interobserver (ICC=0.99) and intraobserver (ICC=0.99) reliability in subjects with MS.27 The TUG test measures the time in seconds that an individual takes to rise from a chair, walk 3m in a straight line and return to sit on the same chair. It assesses dynamic balance and functional mobility, with a value above 20s implying a high risk of falling.
We also administered the Berg balance scale (Table 1). This is a quantitative measurement of the functional status of balance, sensitive to clinical changes and considered useful in predicting falls.28–30 It consists of 14 steps that assess static and dynamic aspects of postural control and its use has been validated in MS patients.26 At present, it is considered as the “gold standard” in the clinical evaluation of balance.31
Berg balance scale.
| Test | Score |
| Test 1: transition from sitting to standing | 4. Able to stand without using hands, stabilising independently |
| The subject is asked to stand up | 3. Able to stand independently, using hands |
| 2. Able to stand using hands after several attempts | |
| 1. Needs minimum help to stand up or stabilise | |
| 0. Needs maximum or moderate help to stand up | |
| Test 2: transition from standing to sitting | 4. Sits safely with minimal use of hands |
| 3. Controls descent using hands | |
| 2. Places the back part of legs against chair to control descent | |
| 1. Sits independently but does not control descent | |
| 0. Needs help to sit down | |
| Test 3: transfers | 4. Able to transfer safely and with minimal use of hands |
| Two chairs, one with armrests and another without them, touching at an angle of 45°. The subject is asked to transfer from one chair to another and vice versa | 3. Able to transfer safely with use of hands |
| 2. Able to transfer with supervision | |
| 1. Needs help from a person | |
| 0. Needs help from 2 people | |
| Test 4: standing without support | 4. Able to stand safely for 30s |
| The subject is asked to remain standing for 30s without moving feet or holding on | 3. Able to stand for 30s with supervision |
| 2. Able to stand unsupported for 15s | |
| 1. Needs several attempts to stand unsupported for 10s | |
| 0. Unable to stand for 10s without assistance | |
| Test 5: sitting without support | 4. Able to sit safely and securely for 30s |
| The subject should remain seated without back support and with feet flat on the floor for 30s. Time should be stopped when protective reactions are observed in the thorax or upper limbs | 3. Able to sit for 30s with supervision or with the help of the upper limbs to maintain the position |
| 2. Able to sit for 15s | |
| 1. Able to sit for 10s | |
| 0. Unable to sit for 10s without support | |
| Test 6: standing, eyes closed | 4. Able to stand safely for 10s |
| The subject must stand with eyes closed for 10s. The best score out of 3 attempts is recorded. | 3. Able to stand for 10s with supervision |
| 2. Able to stand for 3s | |
| 1. Unable to keep eyes closed for 3s | |
| 0. Does not maintain the position, holds on or falls | |
| Test 7: standing with feet together | 4. Able to stand safely for 30s |
| The subject must stand with both feet as close as possible, without support, for 30s | 3. Able to stand for 30s with supervision |
| 2. Able to place feet together, but unable to stand for 30s | |
| 1. Needs help getting to the position, unable to stand for 30s | |
| 0. Unable to stand in the position | |
| Test 8: tandem standing | 4. Able to achieve the tandem position independently and maintain it for 30s |
| The subject is asked to stand with one foot in front of the other, toe-heel | 3. Able to achieve the tandem position independently, but unable to maintain it for 30s |
| 2. Able to take a step independently and hold the position for 30s | |
| 1. Needs help to take a step but can hold the position for 15s | |
| 0. Loses balance whilst taking a step or standing | |
| Test 9: standing with single leg stance | 4. Able to lift leg independently and hold it for 10s |
| The subject is asked to stand on one leg as long as possible without grabbing or holding on. The best score out of 3 attempts is recorded. | 3. Able to lift leg independently and hold it for 5–9s |
| 2. Able to lift leg independently and hold it for 3–4s | |
| 1. Attempts to lift leg; unable to maintain leg up for 3s | |
| 0. Unable to try or needs assistance in order not to fall | |
| Test 10: 360° turn | 4. Able to turn 360° safely within 4s or less in each direction (less than 8s in total) |
| The subject is asked to turn completely around in a full circle and then stop and turn in the opposite direction | 3. Able to turn 360° safely only in one direction in 4s. Completing the turn in the other direction requires more than 4s |
| 2. Able to turn 360° safely but slowly | |
| 1. Needs constant vigilance and verbal commands | |
| 0. Needs help while turning | |
| Test 11: rotation of thorax | 4. Looks back over each shoulder; weight changes include thorax rotation |
| The subject is asked to stand with feet still, fixed at a position | 3. Looks back over each shoulder with thorax rotation: weight changes in the opposite direction towards the shoulder level |
| Command: “Follow this object while I move it, without moving your feet” | 2. Turns the head to look at shoulder level; no thorax rotation |
| 1. Turns the head to look, without thorax rotation; needs supervision | |
| 0. Needs help to maintain balance, turns chin | |
| Test 12: pick up an object from the floor | 4. Able to pick up the object with ease and confidence |
| The subject is asked to pick up an object from the floor, placed at a distance equal to the length of the feet, and in front of the dominant foot | 3. Able to pick up the object, but needs to be monitored |
| 2. Unable to pick up the object, but reaches 2–5cm distance from the object, maintains balance independently | |
| 1. Unable to pick up the object, but tries | |
| 0. Unable to try, needs help to maintain balance | |
| Test 13: climb and descend a step | 4. Performs the task independently and safely, completes 8 steps in 20s |
| The subject is asked to go up and down on a step, alternating feet, until each foot has touched the step and the ground 4 times | 3. Performs the task independently, completes 8 steps in 20s |
| 2. Able to complete 4 steps without aid, needs to be monitored | |
| 1. Able to complete 2 steps, needs minimal assistance | |
| 0. Needs help to maintain balance, unable to try | |
| Test 14: leaning forward with outstretched arms whilst standing | 4. Able to safely lean forward more than 25cm |
| The subject is asked to lean forward as far as possible with arms stretched at 90° without falling, and without taking a step forward from a line marked on the floor. The MCP joint is used as reference for measurements. The mean score of 3 attempts is recorded. | 3. Able to safely lean forward less than 12.5cm |
| 2. Able to safely lean forward less than 5cm | |
| 1. Able to lean forward but needs to be monitored | |
| 0. Loses balance trying, requires external support | |
| Total score: /56 |
Modified from Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83 Suppl. 2:S7–11.
We also conducted the 10m walking test, which assesses the performance of gait through its speed. Patients were asked to walk with their usual cadence for a distance of 10m, allowing the use of walking aids if necessary. This test was performed twice, with the time spent being measured and the variable being recorded as the mean value.
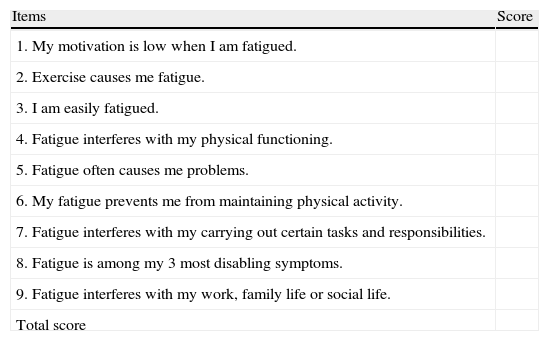
Since its description in 1989, the Krupp fatigue severity scale (Table 2) has been one of the most widely used scales for the assessment of fatigue attributed to a multifactorial source5 in MS.32 It consists of 9 items that must be assessed by the patient with a score between 0 and 7, yielding a mean value. The cut-off point of this scale is arbitrary, although most authors use 5.0 as the reference value to distinguish the presence or absence of symptoms.33,34
Krupp fatigue severity scale.
| Items | Score |
| 1. My motivation is low when I am fatigued. | |
| 2. Exercise causes me fatigue. | |
| 3. I am easily fatigued. | |
| 4. Fatigue interferes with my physical functioning. | |
| 5. Fatigue often causes me problems. | |
| 6. My fatigue prevents me from maintaining physical activity. | |
| 7. Fatigue interferes with my carrying out certain tasks and responsibilities. | |
| 8. Fatigue is among my 3 most disabling symptoms. | |
| 9. Fatigue interferes with my work, family life or social life. | |
| Total score |
Each item is scored on a scale from 1 to 7. A score of 1 is given when the patient completely disagrees with the sentence. A score of 7 is given when the patient completely agrees with the sentence. Scores between 1 and 7 can be graded.
Krupp et al32
Computerised dynamic posturography (CDP) is a quantitative method for assessing and treating balance disorders.35,36 It is based on the use of a dynamometric platform that measures pressure centre displacement through sensors that register the different pressure stimuli exerted by the body in static and dynamic situations. It has been considered by several authors as the “gold standard” for the study of postural control.37 The CDP device employed in this study was Smart Equitest® version 8.2 (NeuroCom International Inc., Clackamas, OR, USA) (Fig. 2). Posturography analysis was conducted using the sensory organisation test (SOT) and the motor control test (MCT). The SOT offered by Smart Equitest® made it possible to assess balance and postural control through the use of external stimuli on the visual and proprioceptive system. These were used in a combined and variable manner to calculate the degree of functional impairment and compensation of the different systems involved in balance control. In other words, it measured the contribution of these sensory afferents to the maintenance of balance.38 Subjects had to maintain their centre of gravity (cog) stable in 3 consecutive series of 20s duration for each of the 6 conditions in the test.39 In the first 3 conditions, the platform remained fixed. Condition 1 (SOT1) was conducted with open eyes, condition 2 (SOT2) with closed eyes and condition 3 (SOT3) with a mobile visual environment referenced to postural oscillations. Conditions 4 (SOT4), 5 (SOT5) and 6 (SOT6) repeated the visual conditions of the first 3 tests and added platform movement referenced to the anteroposterior oscillation of the subject, with the ankle–foot angle remaining constant, thus annulling proprioceptive sensory input. The test was scored with a value of 0 when patients needed help or took a step to maintain balance.For each test the system calculated the angle of oscillation of the cog and compared it to the stability limits established as normal, resulting in partial scores for each condition and an overall balance score (COMP) (%). Values close to 100% indicated minimum balancing and those close to 0% a fall. In addition, the system enabled quantification of the relationship between horizontal and vertical forces exerted by the user to maintain balance in each test, thus determining the “type of postural strategy” used. A score close to 100 indicated the use of ankle strategy (mainly vertical forces recorded), while a score close to 0 indicated a preferential use of a hip strategy (mainly horizontal forces recorded).40–42
The MCT assessed the coordination of the reflex motor response.39 During this test, the platform shifted back and forth at different speeds and amplitudes, allowing for detection of latency or reaction time (LAT).
So as to limit the influence of external variables, all assessments and interventions were performed at the same time of day and at the same room temperature.
Statistical analysisThe normality of each of the variables was assessed using the Kolmogorov–Smirnov test (Lilliefors correction) and the Shapiro–Wilk test, as well as the corresponding QQ graph.
We evaluated the similarity between groups before starting the study using the Student t test for independent samples of quantitative variables with normal distribution and using the Mann–Whitney U-test for quantitative variables without normal distribution.
For quantitative variables with normal distribution, we conducted a Student t-test for paired data, making within-group comparisons (pre/post), and a Student t-test for independent samples, making between-group comparisons (mean difference).
For quantitative variables without normal distribution, we applied the Wilcoxon test for paired data for within-group comparisons (pre/post) and the Mann–Whitney U-test for independent samples in the between-group comparative analysis (mean difference).
We used SPSS version 17 for the analysis of data. The significance level considered was P<.05.
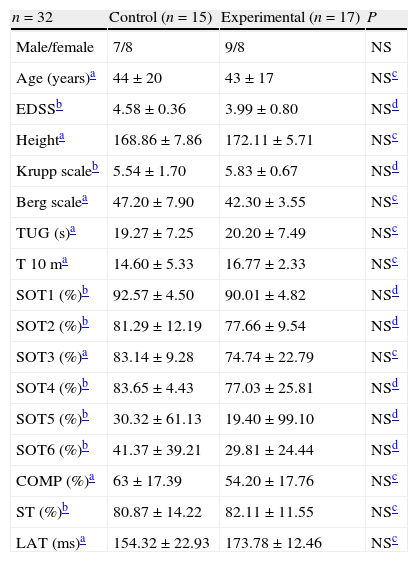
ResultsOut of 36 subjects selected initially, 34 were included in the study and 32 completed it. One subject in the experimental group (N=17) left the study due to the onset of an MS flare and another subject in the control group (N=15) was unable to complete the assessment due to fatigue. The mean±standard deviation age of the sample, which consisted of 18 males and 14 females, was 43±6 years, with a mean score of 4.1 on the EDSS. In the control group, 7 patients had EDSS scores considered as mild and 8 as moderate. In the experimental group, 10 and 7 patients had mild and moderate scores, respectively.
We did not find differences between groups for any of the variables studied at baseline (Table 3).
Baseline characteristics of the sample.
| n=32 | Control (n=15) | Experimental (n=17) | P |
| Male/female | 7/8 | 9/8 | NS |
| Age (years)a | 44±20 | 43±17 | NSc |
| EDSSb | 4.58±0.36 | 3.99±0.80 | NSd |
| Heighta | 168.86±7.86 | 172.11±5.71 | NSc |
| Krupp scaleb | 5.54±1.70 | 5.83±0.67 | NSd |
| Berg scalea | 47.20±7.90 | 42.30±3.55 | NSc |
| TUG (s)a | 19.27±7.25 | 20.20±7.49 | NSc |
| T 10 ma | 14.60±5.33 | 16.77±2.33 | NSc |
| SOT1 (%)b | 92.57±4.50 | 90.01±4.82 | NSd |
| SOT2 (%)b | 81.29±12.19 | 77.66±9.54 | NSd |
| SOT3 (%)a | 83.14±9.28 | 74.74±22.79 | NSc |
| SOT4 (%)b | 83.65±4.43 | 77.03±25.81 | NSd |
| SOT5 (%)b | 30.32±61.13 | 19.40±99.10 | NSd |
| SOT6 (%)b | 41.37±39.21 | 29.81±24.44 | NSd |
| COMP (%)a | 63±17.39 | 54.20±17.76 | NSc |
| ST (%)b | 80.87±14.22 | 82.11±11.55 | NSc |
| LAT (ms)a | 154.32±22.93 | 173.78±12.46 | NSc |
Values expressed as mean±standard deviation.
COMP: global balance; EDSS: Expanded Disability Status Scale; LAT: MCT latency; NS: not significant; SOT1: condition 1 SOT; SOT2: condition 2 SOT; SOT3: condition 3 SOT; SOT4: condition 4 SOT; SOT5: condition 5 SOT; SOT6: condition 6 SOT; ST: postural strategy; T 10 m: 10m test; TUG: timed up and go test.
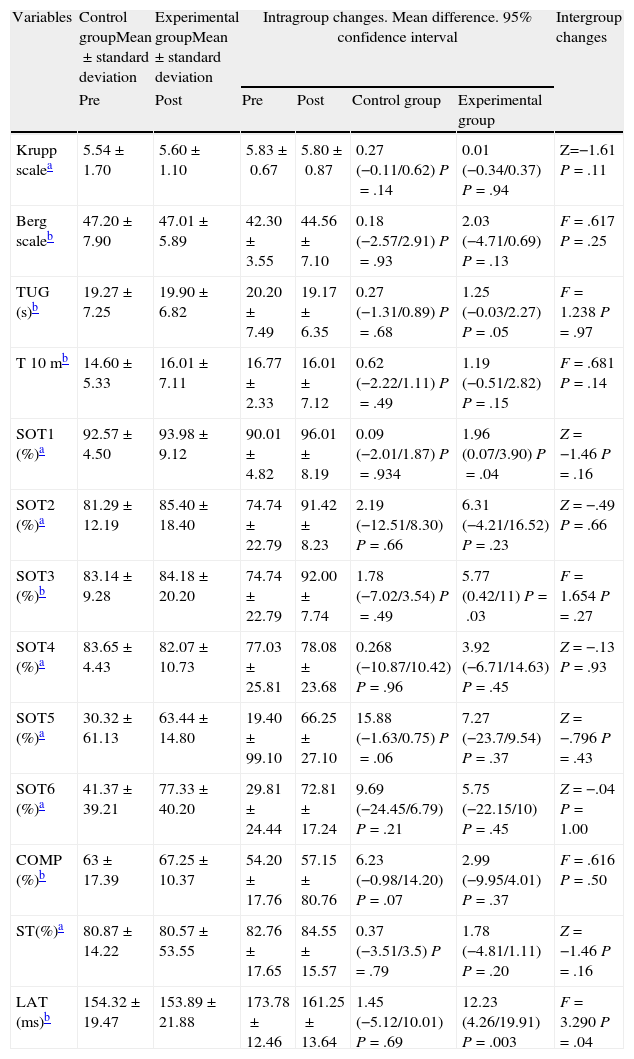
The results of comparisons of the variables studied are summarised in Table 4. In relation to within-group changes, significant improvements were found in favour of the experimental group in the variables SOT1 (P=.04), SOT 3 (P=.03) and LAT (P=.003) and a trend towards statistical significance in TUG (P=.05).
Results of comparisons of the variables studied between the control group and the intervention group.
| Variables | Control groupMean±standard deviation | Experimental groupMean±standard deviation | Intragroup changes. Mean difference. 95% confidence interval | Intergroup changes | |||
| Pre | Post | Pre | Post | Control group | Experimental group | ||
| Krupp scalea | 5.54±1.70 | 5.60±1.10 | 5.83±0.67 | 5.80±0.87 | 0.27 (−0.11/0.62) P=.14 | 0.01 (−0.34/0.37) P=.94 | Z=−1.61 P=.11 |
| Berg scaleb | 47.20±7.90 | 47.01±5.89 | 42.30±3.55 | 44.56±7.10 | 0.18 (−2.57/2.91) P=.93 | 2.03 (−4.71/0.69) P=.13 | F=.617 P=.25 |
| TUG (s)b | 19.27±7.25 | 19.90±6.82 | 20.20±7.49 | 19.17±6.35 | 0.27 (−1.31/0.89) P=.68 | 1.25 (−0.03/2.27) P=.05 | F=1.238 P=.97 |
| T 10 mb | 14.60±5.33 | 16.01±7.11 | 16.77±2.33 | 16.01±7.12 | 0.62 (−2.22/1.11) P=.49 | 1.19 (−0.51/2.82) P=.15 | F=.681 P=.14 |
| SOT1 (%)a | 92.57±4.50 | 93.98±9.12 | 90.01±4.82 | 96.01±8.19 | 0.09 (−2.01/1.87) P=.934 | 1.96 (0.07/3.90) P=.04 | Z=−1.46 P=.16 |
| SOT2 (%)a | 81.29±12.19 | 85.40±18.40 | 74.74±22.79 | 91.42±8.23 | 2.19 (−12.51/8.30) P=.66 | 6.31 (−4.21/16.52) P=.23 | Z=−.49 P=.66 |
| SOT3 (%)b | 83.14±9.28 | 84.18±20.20 | 74.74±22.79 | 92.00±7.74 | 1.78 (−7.02/3.54) P=.49 | 5.77 (0.42/11) P=.03 | F=1.654 P=.27 |
| SOT4 (%)a | 83.65±4.43 | 82.07±10.73 | 77.03±25.81 | 78.08±23.68 | 0.268 (−10.87/10.42) P=.96 | 3.92 (−6.71/14.63) P=.45 | Z=−.13 P=.93 |
| SOT5 (%)a | 30.32±61.13 | 63.44±14.80 | 19.40±99.10 | 66.25±27.10 | 15.88 (−1.63/0.75) P=.06 | 7.27 (−23.7/9.54) P=.37 | Z=−.796 P=.43 |
| SOT6 (%)a | 41.37±39.21 | 77.33±40.20 | 29.81±24.44 | 72.81±17.24 | 9.69 (−24.45/6.79) P=.21 | 5.75 (−22.15/10) P=.45 | Z=−.04 P=1.00 |
| COMP (%)b | 63±17.39 | 67.25±10.37 | 54.20±17.76 | 57.15±80.76 | 6.23 (−0.98/14.20) P=.07 | 2.99 (−9.95/4.01) P=.37 | F=.616 P=.50 |
| ST(%)a | 80.87±14.22 | 80.57±53.55 | 82.76±17.65 | 84.55±15.57 | 0.37 (−3.51/3.5) P=.79 | 1.78 (−4.81/1.11) P=.20 | Z=−1.46 P=.16 |
| LAT (ms)b | 154.32±19.47 | 153.89±21.88 | 173.78±12.46 | 161.25±13.64 | 1.45 (−5.12/10.01) P=.69 | 12.23 (4.26/19.91) P=.003 | F=3.290 P=.04 |
COMP: global balance; EDSS: Expanded Disability Status Scale; LAT: MCT latency; SOT1: condition 1 SOT; SOT2: condition 2 SOT; SOT3: condition 3 SOT; SOT4: condition 4 SOT; SOT5: condition 5 SOT; SOT6: condition 6 SOT; ST: postural strategy; T 10 m: 10-m test; TUG: timed up and go test.
The comparison between both groups in relation to MCT showed that the reaction time elapsed from the beginning of balance alteration until the individual reflexively initiated recovery (latency) was significantly lower in the group who underwent WBV (from 173.78±12.46 to 161.25±13.64ms; P=.04).
We found no statistically significant differences in pre- and post-intervention assessments between both groups for SOT, Berg balance scale, Krupp fatigue severity scale, TUG test and 10m walk test. There were no reports of side effects resulting from the intervention using WBV in either group.
DiscussionThere are few studies published to date that assess the effect of WBV on MS.10,19,24,27 The majority use subjective methods with limited intra- and interobserver reliability. Furthermore, the scientific literature lacks studies that reflect the effect of vibration therapy on fatigue in MS.
The present study is the first one conducted in Spain that sought to evaluate the short-term effects of WBV on postural control and balance, functionality, and fatigue in MS patients. Furthermore, in order to overcome the methodological limitations of previously published studies, objective assessments have been used as a “gold standard” in conjunction with scales and tests with good intra- and interobserver reliability for MS. Specifically, for the objective assessment of postural control and balance, we employed variables that not only measured the ability to integrate sensory information (necessary for the maintenance of balance) through the SOT, but also the coordination of reflex responses triggered by MCT alterations.
An impaired static balance has been described in MS patients, especially in response to sudden and unexpected disturbances,43 as well as a longer response time that could increase the risk of falls.44 The MCT provides information on how an individual responds to such alterations. Our results have shown that 5 daily sessions of WBV can offer a benefit in this regard, significantly reducing the latency or response time needed to regain verticality after an unexpected disruption (from 175.78±18.45ms to 163.56±17.14ms; P=.04), which could lead to a reduced risk of falls.
However, the mechanism by which WBV offers benefits in various neurological diseases is not known. The hypotheses include an improvement in both motor control, through an activation of higher centres, and in proprioceptive system activation, not only of muscle and tendon receptors but also of joint mechanoreceptors.45
As expected in patients with MS, and more so in those with a moderate degree of disability (EDSS=4.4), clinical trials performed pre- and post-intervention revealed a significant effect on mobility. Values in both groups were well above what was considered normal in the TUG test (between 3.3 and 6.3 in females in their fourth decade of life and between 7.1 and 9 in males in their sixth decade of life) and above 14 points, the score considered as a cut-off point for a significantly increased risk of falls.24 The mean baseline scores recorded in the Berg scale were around the cut-off point considered (46) for an increased risk of multiple falls: 47.20 and 42.30 for the control and experimental groups, respectively. This shows the common difficulties faced by MS patients to maintain balance, which translates into a higher frequency of falls. These data corroborate those obtained by authors such as Fjeldstad et al,46 indicating greater postural instability in MS, even in cases with minimum degrees of disability.
There are several factors that can affect balance in MS. Cerebellar ataxia is the most common. Other factors include loss of proprioception, spasticity, muscle weakness in the thorax and limbs, as well as physical deconditioning secondary to inactivity. In this regard, it has been demonstrated that the application of WBV decreased the MU recruitment threshold, which probably resulted in faster activation of rapid conduction muscle fibres.25 This activation, along with an increase in their synchronisation,10 would explain the improved gain in muscle strength observed with WBV compared to conventional training, with less effort and causing less fatigue. A priori, this could support WBV as a useful method for improving muscle strength, and hence balance, in MS.24 However, this aspect has not been assessed in our study. Nevertheless, lack of improvement in the perception of fatigue in the experimental group should not necessarily mean that this increase in muscle strength has not taken place, as it is known that the origin of fatigue in MS is multifactorial. Fatigue with exercise in MS has been related with alterations in the pyramidal pathway.47 Although our research group used the Krupp scale for the assessment of fatigue severity, there are other instruments that have been validated for Spanish and that have also shown good reliability and validity in patients with MS, such as the Fatigue Impact Scale for Daily Use.48
Several RCTs of moderate to high methodological quality have reported significant improvements in muscle strength and TUG scores in older adults with and without medical conditions after 6–8 weeks of WBV.49,50 Schuhfried et al7 obtained similar improvements in TUG scores with a single application of WBV (5 series of 1min duration) in 12 subjects with MS at an early stage of the disease. This work was the first study to examine the influence of WBV in MS. The authors evaluated postural control, balance and mobility through the SOT, TUG and Functional Reach Test, concluding that WBV may have beneficial effects in MS.
Jackson et al19 were the first to measure the flexor–extensor muscle strength of the knee after a single application of WBV for 30s in patients with MS. They found no significant improvement, suggesting the need for further studies before considering WBV as a viable treatment option.
The long-term effect of WBV in MS was assessed by Schyns et al10 They compared the impact on 16 subjects of 4 weeks of WBV plus physical exercise versus only the same physical exercise programme on muscle tone, spasticity, muscle strength, functionality and quality of life. They concluded that although WBV offered little benefit compared to physical exercise alone, its use could be beneficial compared with no intervention in patients with MS. The authors estimated that the duration of treatment could have been insufficient to induce neuromuscular changes that would have led to an improvement in muscle strength and functionality, and that the increasing training intensity employed could have represented an excessive strain in these patients.
Broekmans et al25 investigated the impact of 20 weeks of static and dynamic exercise on a vibration platform on muscle strength and functionality compared with no intervention in MS patients with mild to moderate impairment. Surprisingly, they found that their intervention did not improve any of the parameters explored, despite having used a similar exercise programme to that used by other authors on healthy, young and elderly subjects who did obtain significant increases in muscle strength. The authors attributed this observation to the fact that, perhaps, the training volume represented an overload in this population, although the sample described the workload as “not strenuous”, and also to the fact that since the group was from a working community, their previous level of fitness was probably better than expected, thus not leaving much room for significant improvement.
In a recent experimental study that included 3 MS patients with mild, moderate and severe disability, respectively, Wunderer et al24 evaluated the effect of 6 weeks of WBV at a rate of 2 sessions per day on muscle strength of the lower limbs and functional mobility. They obtained significant improvements in strength in all 3 patients and in mobility in 2. In addition, all improvements were maintained 4 weeks after the intervention and were more pronounced with higher volume and intensity of work, a finding corroborated by a recent, systematic review of healthy subjects.51 Other previous research has also shown the lasting effect of vibration therapy, with the positive effects obtained from the use of WBV in subjects with cerebrovascular stroke and PE being maintained 4–6 weeks after its completion.22 In the light of their results, Wunderer et al suggested that WBV may be less effective in the subjects most affected by the disease or else that both the intensity and duration of vibration should be higher in cases of moderate to severe impairment. These aspects could explain the results of our work.
Our study presents methodological limitations, among which we highlight the small sample size, the lack of a placebo group and also the lack of long-term monitoring. However, from the preliminary results observed in this study, we can conclude that WBV appears to be a useful therapeutic tool for balance control compared with no intervention in patients with mild to moderate MS. However, the protocol employed did not influence the parameters related to functionality and fatigue, possibly due to the length of the proposed treatment protocol.
Further research is essential in order to determine the best vibration therapy treatment programme, as well as the most appropriate frequency, intensity and amplitude parameters, their impact on quality of life parameters and the long-term effect in patients with MS, at different stages of the disease.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Alguacil Diego IM, et al. Efectos de la vibroterapia sobre el control postural, la funcionalidad y la fatiga en pacientes con esclerosis múltiple. Ensayo clínico aleatorizado. Neurología. 2012;27:143–53.