Embolic stroke of undetermined source (ESUS) accounts for 25% of all cerebral infarcts; only 30% are associated with paroxysmal atrial fibrillation (AF). Various biochemical, electrocardiographic, and echocardiographic findings may suggest left atrial damage and increased risk of embolism in the absence of clinically documented AF or atrial flutter. In this review, we analyse the available evidence on atrial cardiopathy or atrial disease, its involvement in ESUS, and its identification through electrocardiographic, echocardiographic, and serum markers and its possible therapeutic implications.
DevelopmentA systematic search was conducted on MEDLINE (PubMed) using the following MeSH terms: MeSH [ESUS]+[atrial cardiopathy]+[atrial fibrillation]+[interatrial block]+[treatment]. We selected what we considered to be the most useful original prospective or retrospective studies and systematic reviews. We then read the full texts of the articles and checked the references cited in each article.
We analyse epidemiological and demographic variables of patients with ESUS, as well as recent evidence related to presentation and prognosis and factors associated with recurrence and mortality. We review the contribution of atrial cardiopathy diagnosis prior to the detection of AF and the clinical, electrocardiographic, and echocardiographic variables and the biochemical markers associated with its development and its potential contribution to cerebral embolism.
ConclusionsThe systematic search of biochemical and electrocardiographic, and echocardiographic alterations can be useful to identify ESUS patients at higher risk of recurrence.
El infarto cerebral embólico de origen no determinado (ESUS por sus siglas en inglés) representa el 25% de todos los infartos cerebrales y solo el 30% se asocia con fibrilación auricular (FA) paroxística. Existen diferentes hallazgos bioquímicos, electro y ecocardiográficos que sugieren daño auricular izquierdo y aumento del riesgo de embolismo en ausencia de FA o flutter auricular clínicamente documentados. En la presente revisión analizamos la evidencia disponible sobre cardiopatía atrial o enfermedad auricular, su implicación en el ESUS y su identificación mediante marcadores electrocardiográficos, ecocardiográficos y séricos y sus posibles implicaciones terapéuticas.
DesarrolloSe realizó una búsqueda sistematizada a través de la fuente de información MEDLINE (PubMed), utilizando una estrategia diseñada con términos MeSH [ESUS]+[atrial cardiopathy]+[atrial fibrillation]+[interatrial block]+[treatment]. Se seleccionaron las publicaciones originales de estudios prospectivos, retrospectivos y de revisión consideradas como las más útiles. Se procedió a la lectura del texto completo y la bibliografía aportada en cada artículo.
Se incluyeron los factores epidemiológicos y demográficos de los pacientes ESUS, así como la evidencia reciente relacionada con su forma de presentación, pronóstico y factores asociados con recurrencia y mortalidad. Se revisó la contribución de la presencia de cardiopatía auricular previo a la documentación de FA y las variables clínicas, electro y ecocardiográficas, así como los marcadores bioquímicos asociados con su desarrollo y su contribución como fuente potencial de embolismo cerebral.
ConclusionesLa búsqueda sistemática de alteraciones bioquímicas, electro y ecocardiográficas pueden ser de utilidad para identificar pacientes ESUS con mayor riesgo de recurrencia.
Up to 25% of all strokes are of unknown cause (cryptogenic). Most cases are of emboligenic origin, although the source cannot be determined. The concept of “embolic stroke of undetermined source” (ESUS) includes non-lacunar strokes, which present clinical and radiological characteristics suggestive of stroke but lack a clear cardioembolic source (Tables 1 and 2).1 Up to 30% of ESUS are associated with paroxysmal atrial fibrillation (AF), but the cause of stroke or association with AF cannot be confirmed in the remaining cases.2,3
Diagnostic criteria for embolic stroke of undetermined source.
| 1. Non-lacunar stroke detected by CT or MRIa |
| 2. Absence of extracranial or intracranial atherosclerosis causing ≥ 50% luminal stenosis in arteries supplying the area of ischaemia |
| 3. No major-risk cardioembolic source of embolismb |
| 4. No other specific cause of stroke identified (eg, arteritis, dissection, migraine/vasospasm, drug abuse) |
CT: computed tomography; MRI: magnetic resonance imaging.
Lacunar stroke is defined as a subcortical infarct ≤1.5 cm (≤ 2.0cm on MRI diffusion images) in largest dimension, including on MRI diffusion-weighted images, and in the distribution of the small, penetrating cerebral arteries. Visualisation by CT usually requires delayed imaging > 24-48hours after stroke onset.
Cardioembolic sources include permanent or paroxysmal atrial fibrillation, sustained atrial flutter, intracardiac thrombus, prosthetic cardiac valve, atrial myxoma or other cardiac tumours, mitral stenosis, recent (< 4 weeks) myocardial infarction, left ventricular ejection fraction < 30%, valvular vegetations, or infective endocarditis.
Diagnostic assessment of embolic stroke of undetermined source.
| Brain CT or MRI |
| 12-lead ECG |
| Transthoracic and transoesophageal echocardiography |
| Cardiac monitoring for ≥24hours |
| Imaging of both the extracranial and intracranial arteries supplying the area of brain ischaemia (catheter, MRI, or CT angiography, or cervical duplex plus transcranial Doppler ultrasonography) |
ESUS was first described in 2014. Since then, several series have reported frequencies ranging from 9% to 25% (mean, 17%) of all strokes. ESUS presents at a mean age of 65 years, is more common in men (60%), and is less frequently associated with the classic vascular risk factors. This type of stroke is less severe (median NIHSS score of 5) and is associated with a recurrence rate of 4.5% at 2–3 years of follow-up. Most patients with ESUS are treated with antiplatelets.4
This suggests that ESUS may be caused by cardioembolic mechanisms; the failure to detect AF in these patients may underscore the need to search for other predictors or change the way in which AF is detected.5 Secondary prevention strategies should also be reinforced, given that patients with ESUS are younger and present less severe episodes. This review aims to analyse the available evidence on atrial cardiopathy, its role in the pathogenesis of ESUS, its potential therapeutic implications, and its identification with electrocardiographic, echocardiographic, and serum markers.
Embolic stroke of undetermined source. Clinical and neuroimaging characteristicsThe clinical and radiological characteristics of ESUS are similar to those of cardioembolic stroke. In a series of 275 patients with ESUS, 74% presented severe neurological impairment at stroke onset, and neuroimaging studies revealed predominantly cortical ischaemic lesions or multiple cortical-subcortical ischaemic lesions in different arterial territories, with early recanalisation and haemorrhagic transformation.6 ESUS and cardioembolic stroke also present similar recurrence rates, although ESUS is associated with lower long-term mortality rates, probably due to the lower severity of this type of stroke.6–8
Age and CHADS2 and CHA2DS2-VASc scale scores seem to be the most useful predictors of ESUS. Compared to patients younger than 60 years, the risk of recurrence is 3 times higher and the mortality rate is 8 times higher in individuals older than 80 years.9,10 High-risk patients (according to CHA2DS2-VASc scores) present a three-fold increase in the risk of recurrence and a thirteen-fold increase in the risk of death, compared to low-risk patients.11
Embolic stroke of undetermined source and atrial fibrillationSystematic screening for AF has considerable therapeutic implications: in the absence of AF or a clear cardioembolic source, current treatment guidelines recommend antiplatelets, whereas patients with AF or other potential sources of cardiac embolism should receive oral anticoagulants.12–14
A clear association has been found between duration of cardiac monitoring and detection of AF.15 Detection rates are 3% for admission ECG, 5%-8% for inpatient continuous telemetry, 3%-6% for 24-48 hours Holter monitoring, 16% for mobile continuous outpatient telemetry, and 30% for implantable loop recorders (ILR).2,3,16
In the CRYSTAL-AF study, AF was detected in 8.9% of patients with ILRs, compared to 1.4% of patients undergoing standard ECG studies (HR: 6.4; 95% CI, 1.9-21.7; P<.001). The detection rate increased to 12.4% in the group of patients with ILRs when monitoring was continued for 12 months (HR: 7.3; 95% CI, 2.6-20.8; P<.001).2 The EMBRACE study randomised 572 patients with cryptogenic stroke or transient ischaemic attack (TIA) in the previous 6 months, and no known history of AF, to undergo either 30-day non-invasive cardiac monitoring (intervention group) or conventional 24-hour Holter monitoring (control group). AF lasting ≥30seconds was detected in 16.1% of patients in the intervention group and 3.2% of controls (P<.001). The number needed to screen was 8 for detecting a new case of AF. Furthermore, AF lasting ≥ 2.5minutes was detected in 9.9% of patients in the intervention group and 2.5% of controls (P<.001).3
Atrial cardiopathyThe lack of evidence on a temporal association between AF and ESUS, despite multiple cardiac monitoring studies,17 has led researchers to reconsider the nature of this relationship and suggests that left atrial cardiopathy may be the cause of stroke, independently of AF.18,19 Atrial cardiopathy increases the risk of AF and stroke in patients with no known history of AF and showing sinus rhythm on different studies (ECG, Holter and events monitors, etc). Markers of left atrial dysfunction can be classified into 3 groups: electrocardiographic markers (P-wave terminal force in lead V1, episodes of subclinical atrial tachyarrhythmia, atrial extrasystoles, interatrial block),19–24 echocardiographic markers (left atrial size, volume, and/or function),25–27 and biochemical markers (cardiac troponin, NT-proBNP, von Willebrand factor).28–31
Atrial cardiopathy may cause thromboembolism before AF occurs; therefore, the latter should not be considered a necessary condition for stroke, but rather a common manifestation of an underlying atrial cardiopathy. In the light of the above, studying atrial cardiopathy may provide useful data about the pathophysiological mechanisms of ESUS and help improve secondary prevention.18,32,34
Electrocardiographic markers of atrial cardiopathyP-wave terminal force in lead V1P-wave terminal force in lead V1 (PTFV1) measures electric conduction through the atria. It was described by Morris et al.21 as an electrocardiographic sign of left atrial involvement. Prolonged PTFV1 reflects left atrial changes that alter electric conduction due to left ventricular fibrosis, hypertrophy, or increased left ventricular filling pressure.35 Prolonged PTFV1 has been proposed as a predictor of AF.22 Prolonged PTFV1 reveals underlying atrial cardiopathy, which may predispose to embolic events, even in the absence of AF.19,23,33,36
Episodes of subclinical atrial tachyarrhythmiaA subanalysis of the ASSERT trial evaluated the significance of episodes of subclinical atrial tachyarrhythmia (SATa) detected by ILRs; these were defined as episodes of atrial rate > 190 beats per minute for more than 6minutes. SATa was associated with increased risk of clinical AF (HR: 5.56; 95% CI, 3.78-8.17; P<.001) and of stroke or systemic embolism (HR: 2.49; 95% CI, 1.28-4.85; P=.007) during the first 3 months of monitoring, compared to patients without SATa. Furthermore, in the time-dependent analysis, which compared all episodes of SATa lasting > 6minutes against patients without SATa, SATa was associated with increased risk of stroke or systemic embolism (HR: 1.76; 95% CI, 0.99-3.11; P=.05).20
Using data from the TRENDS study,37 Ziegler et al.38 analysed the incidence of AF in 1368 patients with ILRs and no history of AF, stroke, or TIA. The researchers found an incidence of 30% (atrial tachycardia/AF > 5minutes on any day) during a mean follow-up period (SD) of 1.1 years (0.7), regardless of stroke risk factors as measured by the CHADS2. An analysis of the association between stroke risk factors and atrial tachycardia/AF > 6hours found a significant increase in the incidence of AF (12%, 15%, and 18% in patients with intermediate, high, and very high risk of stroke, respectively; P=.04). Daoud et al.39 analysed a subgroup of patients from the TRENDS study,37 and concluded that the temporal association between stroke or systemic embolism in patients with ILRs may involve cardioembolic mechanisms other than AF.
In patients with ESUS, SATa is associated with increased risk of thromboembolism. However, the association between SATa and risk of stroke recurrence is yet to be determined, and no precise indications for anticoagulation treatment have been established for these patients.
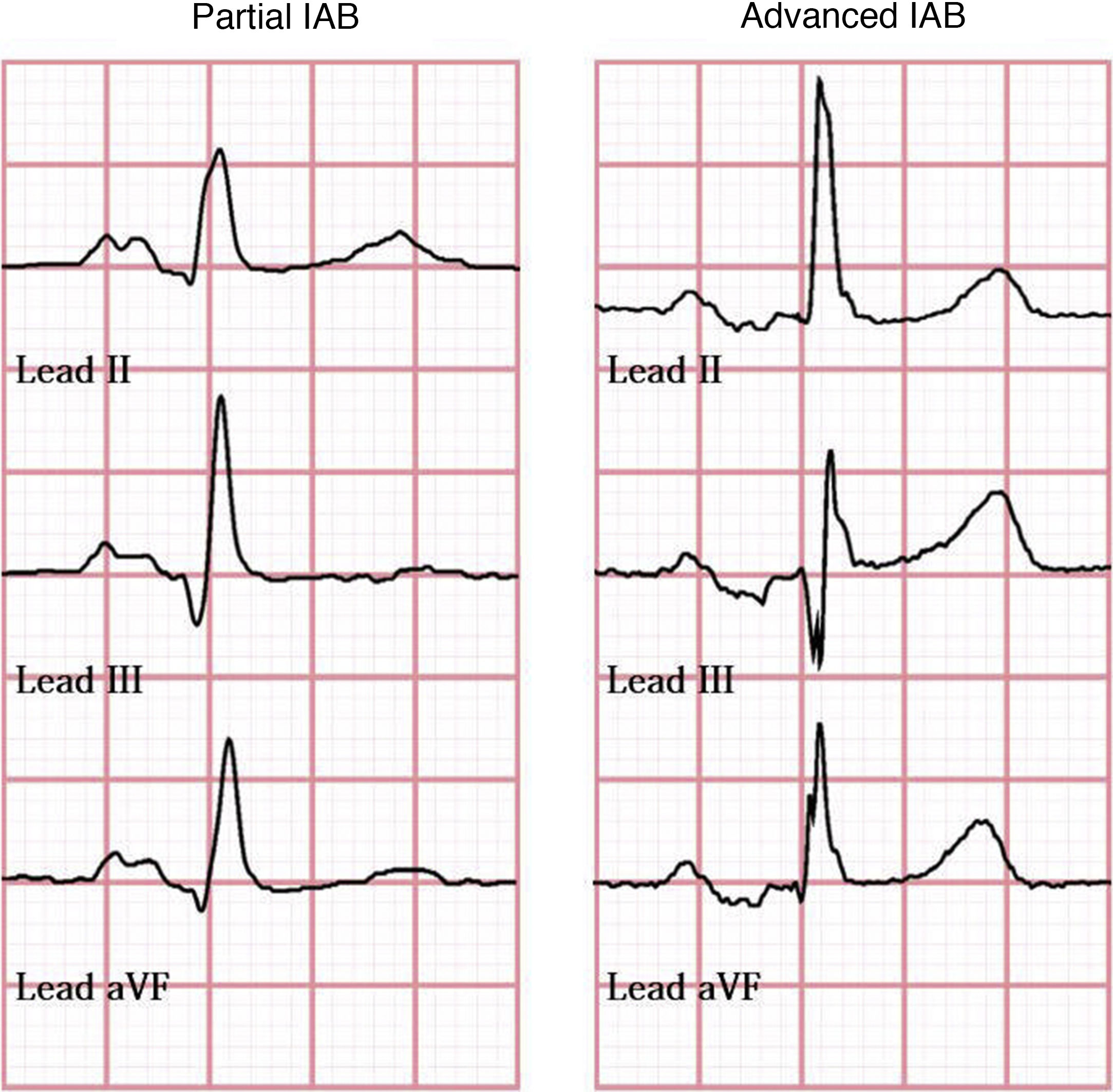
Bayés syndromeBayés syndrome is characterised by the copresence of supraventricular arrhythmia, particularly AF, and interatrial block (IAB). Bayés et al.24 classified IAB as partial, when the P-wave has a duration of ≥ 120ms and presents a negative mode, and advanced, when the P-wave has a duration of ≥ 120ms and presents a biphasic morphology in leads II, III, and aVF, resulting from caudocranial activation of the left atrium (Fig. 1). Agarwal et al.40 followed up 308 patients with AF and 308 patients with sinus rhythm for a mean (SD) of 16 months (23), and finding advanced IAB in 52% and 18%, respectively.
In a sample of 32 patients with advanced IAB, Bayés et al.41 also observed a significant decrease in the incidence of supraventricular arrhythmia in patients receiving antiarrhythmic treatment for 18 months (93.7%, vs 25% in controls). An association has been observed between advanced IAB and recurrence of AF a year after pharmacological cardioversion (OR: 18.4, regardless of the antiarrhythmic drug used).42 Furthermore, presence of advanced IAB prior to pulmonary vein isolation is associated with increased risk of recurrence of AF after the procedure.43
Advanced IAB is a predictor of high risk for new-onset AF after successful cavotricuspid isthmus ablation in patients with typical atrial flutter,44 and an independent predictor of new-onset AF in patients with severe congestive heart failure following cardiac resynchronisation therapy.45 Advanced IAB may occur in different clinical scenarios, including atrial dilation and old age (it is considered a degenerative process, with a prevalence of 5.4% among patients < 20 years, vs 60% in patients > 50 years),43,44,46,47 diabetes, obstructive sleep apnoea syndrome, and metabolic syndrome.48
Presence of advanced IAB supports the hypothesis of left atrial dysfunction as an independent risk factor for stroke49; early diagnosis of Bayés syndrome is therefore essential, since these patients will probably need preventive antiarrhythmic and antithrombotic therapy.47
Echocardiographic markersMorphology of the left atrial appendageThe left atrial appendage (LAA) is an embryonic remnant of the primordial left atrium that acts as a reservoir during conditions of fluid overload.25 It is the main source of cardiac thrombi in patients with AF, due to the blood stasis caused by its morphology.50 Manning et al.51 found that up to 98% of all atrial thrombi formed during AF originated in the LAA. In a multicentre retrospective study of 359 patients with AF undergoing brain and cardiac MRI studies, Anselmino et al.50 classified LAA morphology into 4 types: chicken wing, cauliflower, cactus, and wind sock. Silent cerebral ischaemic lesions were detected in 295 patients (84.8%), with a median of 23 lesions. The population under study was stratified by quartiles according to the number of lesions: ≤ 6, 7-23, 24-43, and ≥ 44. An association was found between the number of silent cerebral ischaemic lesions and LAA morphology. Cauliflower morphology has been associated with greater numbers of lesions.26,50 Similarly, Di Biase et al.25 found that chicken-wing LAAs are associated with a 79% reduction in the risk of stroke or TIA (OR: 0.21, 95% CI, 0.05-0.91; P=.036).
Previous studies suggest that non-chicken-wing LAA may increase the risk of embolic events in patients with ESUS, constituting an echocardiographic marker of risk of recurrence.25,26
Left atrial sizeGreater left atrial size is associated with embolic events, regardless of presence of AF. In a study of 64 patients with AF undergoing transthoracic and transoesophageal echocardiography studies, Radwan27 found that indexed left atrial anteroposterior diameter (cut-off: 3cm/m2; OR: 7.5, 95% CI, 1.24-45.2; P=.02) and indexed left atrial ellipsoid volume (cut-off: 42cm3/m2; OR: 6.5; 95% CI, 1.32-32.07; P=.02) were the most reliable predictors of thromboembolic risk. Furthermore, greater percentages of left atrial fibrosis have also been associated with greater risk of thromboembolism and stroke.52
Serum biomarkersBiomarkers of cardioembolism focus on the systemic and atrial basis of the disease. Each biomarker has shown an association with risk of embolic stroke regardless of the presence of AF. We summarise the available evidence on the currently available biomarkers of atrial cardiopathy.
Cardiac troponinCardiac troponin levels are elevated in patients with clinical and subclinical myocardial lesions and structural heart disease.53 Between 5% and 34% of patients with stroke present elevated cardiac troponin levels but no typical symptoms or electrocardiographic evidence of acute coronary ischaemia.54,55 The TRELAS study found that 24% of patients with stroke and elevated cardiac troponin levels presented coronary lesions that were responsible for either myocardial ischaemia or cardioembolism, suggesting different causes of cardiac troponin elevation in stroke.56 An association has also been observed between elevated cardiac troponin levels and cardioembolic stroke and ESUS.28,29
N-terminal pro-brain natriuretic peptideN-terminal pro-brain natriuretic peptide (NT-proBNP) is another serum biomarker of heart disease.57 Elevated NT-proBNP levels are associated with greater likelihood of detecting AF during the follow-up of patients with cryptogenic30 and cardioembolic stroke.58–60 In a study by Li et al.,61 elevated cardiac troponin and NT-proBNP levels resulted in a three-fold increase in the risk of cardioembolic stroke; the association was stronger for NT-proBNP.
Von Willebrand factorVon Willebrand factor (vWF) plays a major role in thrombus formation. A correlation has been observed between elevated plasma vWF levels and risk of atherosclerosis or thrombosis in patients with coronary heart disease; vWF level is therefore considered a marker of endothelial dysfunction.62 Some studies have demonstrated an association between high vWF levels and increased risk of stroke, particularly cardioembolic and cryptogenic stroke,63–65 which suggests that the prothrombotic effects of vWF are independent of other markers of atherosclerosis/inflammation or endothelial damage.31 However, evidence is scarce and the pathogenic mechanism seems to differ from that of atrial cardiopathy.
New treatment approachesThe optimal treatment of patients with ESUS has not been established; at present, most of them are treated with antiplatelet drugs. The benefits of anticoagulants in patients with no evidence of AF are unclear, although several controlled clinical trials have been conducted. Whereas empirical anticoagulation seems a reasonable approach in patients with recurrent ESUS, secondary prevention in the remaining cases includes blood pressure control, statins, lifestyle changes, and antiplatelet treatment.20,66
Several clinical trials have analysed whether direct oral anticoagulants reduce the risk of recurrence; 2 have concluded and another one is currently underway. The NAVIGATE ESUS trial67 compared 15mg rivaroxaban against 100mg aspirin, whereas the RE-SPECT ESUS trial68 compared 2 doses of dabigatran (110 and 150mg twice daily) against aspirin. Both treatment approaches failed to reduce recurrence rates, and rivaroxaban was associated with increased risk of haemorrhage.67 However, in the NAVIGATE ESUS trial, both treatment groups showed a recurrence rate of 5% per year, which confirms the need for better patient selection and better treatment options. Some of the electrocardiographic, echocardiographic, and biochemical markers addressed in this review may be useful in identifying patients with ESUS and high risk of recurrence. The ARCADIA trial,69 which is currently underway, compares 5mg apixaban against 81mg aspirin/day in patients aged ≥ 45 years with ESUS and evidence of atrial cardiopathy. The trial aims to demonstrate the efficacy and safety of apixaban for the secondary prevention of stroke at 4 years of follow-up; results are expected in 2022. Until further evidence is published, anticoagulation should be used with caution in patients with ESUS presenting high risk of AF and recurrence.
ConclusionsThe available evidence suggests that the left atrium plays a major role in stroke pathogenesis and prevention. Rather than focusing on AF only, we should consider atrial cardiopathy in general as a cause of stroke. In fact, AF is very likely to result from a more complex underlying heart disease.
Systematically searching for risk factors, especially P-wave morphology and duration on ECG, atrial extrasystoles, LAA morphology on echocardiogram, and such biomarkers as NT-proBNP, may be valuable in detecting patients with ESUS and high risk of recurrence.
Please cite this article as: Arauz A, Arteaga C, Zapata-Gómez C, Ramos-Ventura C, Méndez B, Otiniano-Sifuentes R, et al. Infarto cerebral embólico de origen no determinado: más allá de la fibrilación auricular. Neurología. 2022;37:362–370.