Cardioembolic stroke accounts for 20%-30% of all ischaemic strokes.1 With the introduction of the term “embolic stroke of undetermined source” (ESUS), and given the considerable number of non-lacunar cryptogenic strokes, the hypothesis of an unknown embolic source has led to extensive study. This includes prolonged cardiac monitoring, which increases sensitivity for identifying paroxysmal atrial fibrillation (AF).2 Although the ability of these paroxysmal episodes to cause ischaemic events was previously unknown, studies into the pathophysiology of cardioembolic stroke have shown that paroxysmal AF, which usually lasts 5-6minutes, increases the risk of stroke.3,4
Given their short duration and the fact that they are often asymptomatic and may not co-occur with neurological symptoms, these episodes are classified under stroke of undetermined cause.3,5 The heterogeneity of this group, combined with the limited use of prolonged cardiac monitoring (whether due to patient intolerance or to the unavailability of monitoring equipment and differences between devices), has led researchers to investigate parameters correlated with greater incidence of AF,6 with a view to increasing the utility of the technique. These parameters include electrocardiographic, biochemical, and echocardiographic variables, which have been found to be associated not only with AF but also with ischaemic stroke and recurrent stroke.1,7
We present the case of a patient with acute ischaemic stroke and morphological and functional signs of left atrial cardiopathy in an echocardiography study; these findings were correlated with paroxysmal AF.
The patient was a functionally independent 77-year-old woman who came to the emergency department due to right hemiparesis and mixed aphasia. She scored 1 on the NIHSS due to language impairment, which resolved completely 3hours after symptom onset. A baseline head CT scan revealed no signs of haemorrhage or acute ischaemia; the patient scored 7 on the ABCD2 scale, and was admitted to the stroke unit for cardiac monitoring. She remained asymptomatic for 18hours, after which she presented an episode of isolated mixed aphasia (NIHSS: 4). No changes were observed in CT images. Intravenous fibrinolysis achieved progressive recovery.
Our patient presented hypertension, dyslipidaemia, and type 2 diabetes mellitus; treatment adherence was good and blood analysis results were within normal ranges. In 2012, she underwent implantation of a DDDR pacemaker due to complete atrioventricular block associated with sinus dysfunction. An echocardiography performed in 2016 revealed mild left ventricular hypertrophy and mitral valve regurgitation, with no alterations in atrial morphology or other signs of structural heart disease. In view of the 2 episodes of sudden-onset focal neurological signs (with one episode characterised by isolated aphasia), we requested the pacemaker records from the cardiology department.
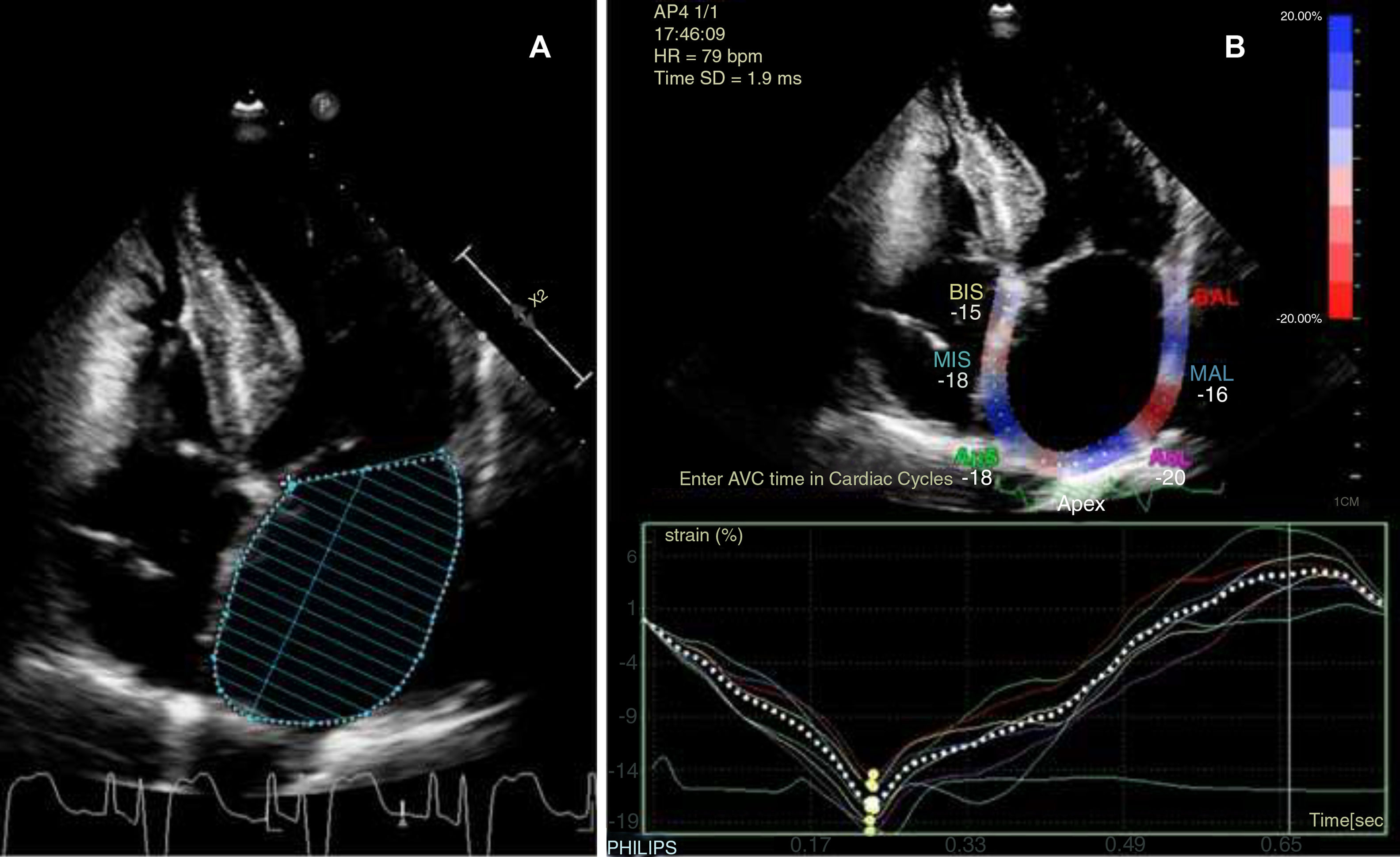
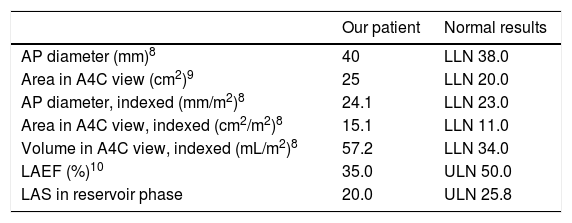
The patient continued under observation while we awaited the pacemaker records. Twenty-four-hour Holter monitoring revealed sinus rhythm and no arrhythmia; the patient was therefore transferred to the neurology ward. A Doppler ultrasound of the supra-aortic trunks revealed atheromatous plaques in the carotid territory bilaterally, without haemodynamic impairment; transcranial Doppler ultrasound did not detect stenosis. Table 1 shows the findings from transthoracic echocardiography, adjusted for body surface area (1.657m2). Atrial diameter and area were compatible with mild left atrial enlargement, whereas volume and such functional activity parameters as left atrial ejection fraction (LAEF) and left atrial strain (LAS) indicated more severe dysfunction of the cardiac chamber (Fig. 1). The pacemaker data revealed an episode of paroxysmal AF lasting 17hours, which occurred 3 months previously, and another episode occurring 4 days after the cerebrovascular event; the patient presented sinus rhythm during neurological symptoms.
Transthoracic echocardiography findings: left atrium morphological and functional parameters, adjusted for body surface area and sex.
| Our patient | Normal results | |
|---|---|---|
| AP diameter (mm)8 | 40 | LLN 38.0 |
| Area in A4C view (cm2)9 | 25 | LLN 20.0 |
| AP diameter, indexed (mm/m2)8 | 24.1 | LLN 23.0 |
| Area in A4C view, indexed (cm2/m2)8 | 15.1 | LLN 11.0 |
| Volume in A4C view, indexed (mL/m2)8 | 57.2 | LLN 34.0 |
| LAEF (%)10 | 35.0 | ULN 50.0 |
| LAS in reservoir phase | 20.0 | ULN 25.8 |
A4C: apical 4-chamber; AP: anteroposterior; LAEF: left atrial ejection fraction; LAS: left atrial strain; LLN: lower limit of normal; ULN: upper limit of normal.
The patient was asymptomatic at discharge and was diagnosed with ischaemic stroke of cardioembolic origin secondary to paroxysmal AF. She started treatment with oral anticoagulants; her functional status at 3 months was similar to that before hospital admission (mRS: 1).
We were presented with the case of a patient with no signs of significant intra- or extracranial atherosclerosis, displaying sinus rhythm during 24-hour Holter monitoring. In view of the strong association between isolated aphasia and cardioembolic arrhythmia,12 we performed a comprehensive transthoracic echocardiography study to evaluate parameters that may support prolonged cardiac monitoring, in case the pacemaker records showed no relevant data, considering the potential for episodes of AF not to co-occur with stroke onset.5
The echocardiographic parameters most frequently used in our hospital to characterise the left atrium are area and anteroposterior diameter; whenever possible, these are adjusted for body surface area to avoid sex-related variability. However, these parameters tend to underestimate the true size of the atrium (as compared to volumetry) since linear measurements do not reflect differences in deformation on different planes.8 Left atrial volume is more accurate but has the limitation that it is difficult to measure. Technical advances have made it easier to calculate, making this the morphological parameter of choice in clinical practice.8,9 Another potentially useful structural parameter is the analysis of left atrial appendage morphology with transoesophageal echocardiography, due to the potential association between certain morphological patterns and increased risk of thromboembolism.13
Left atrial function parameters are less frequently used, although they are also reported to be correlated with increased risk of AF and ischaemic stroke regardless of the morphology of this chamber.10,11 Even in patients with normal left atria, this association represents a shift from the traditional classification of atrial damage based exclusively on chamber enlargement. Two useful left atrial function parameters are LAEF and LAS. The latter measures myocardial deformation using speckle tracking echocardiography; this technique analyses atrial contractility, which has been found to be associated with structural degeneration.11
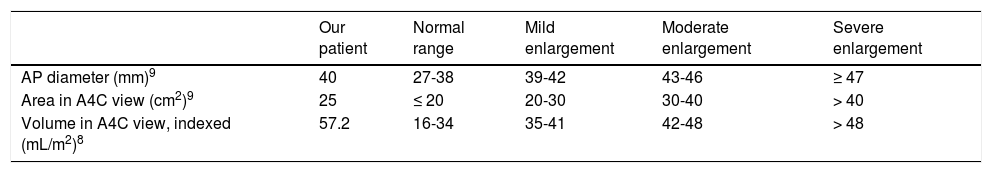
Based on the data shown in Table 1 and the considerations discussed with regard to different echocardiographic parameters, the transthoracic echocardiography study typically performed at our hospital (with the left atrium assessed by measuring anteroposterior diameter and area) would only have shown mild left atrial enlargement; however, volumetric data indicated severe atrial enlargement (Table 2). This finding, which would have gone unnoticed had we not gathered volumetric data, points to an association between left atrial enlargement and both higher incidence of AF and recurrent stroke.14 An LAEF below 50% and an LAS below 25.8% in the reservoir phase also point to underlying paroxysmal AF.10,11
Left atrial morphology data obtained with transthoracic echocardiography and classification by severity.
| Our patient | Normal range | Mild enlargement | Moderate enlargement | Severe enlargement | |
|---|---|---|---|---|---|
| AP diameter (mm)9 | 40 | 27-38 | 39-42 | 43-46 | ≥ 47 |
| Area in A4C view (cm2)9 | 25 | ≤ 20 | 20-30 | 30-40 | > 40 |
| Volume in A4C view, indexed (mL/m2)8 | 57.2 | 16-34 | 35-41 | 42-48 | > 48 |
A4C: apical 4-chamber; AP: anteroposterior.
The aetiological study of stroke includes echocardiographic parameters and other biochemical and electrocardiographic markers (proBNP levels, P terminal force in the V1 lead, etc) to identify probable emboligenic arrhythmia; the inclusion of these parameters is based on the traditional idea of AF as a cause of cardiac remodelling, venous stasis, and subsequent thromboembolism. However, research into the association between these parameters and AF incidence suggests that many of them correlate directly with the incidence and recurrence of ischaemic stroke, regardless of whether the patient has AF.1,7 Consequently, the pathophysiology of cardioembolic stroke is thought to be more complex: the idea of atrial cardiopathy as a dysfunction capable in itself of increasing the risk of embolism is becoming more widespread; AF is still considered the cause, aggravating factor, or consequence, depending on the case, but is no longer regarded as a necessary condition for cardioembolic stroke.1,7,15
However, regardless of these new hypotheses about the pathophysiology of thrombus formation, AF continues to be a major source of cardioembolism; detection of AF is closely related to the type and duration of cardiac monitoring to be performed.15 The case presented constitutes a good example of the usefulness of certain echocardiographic parameters due to their association with higher incidence of underlying AF. Furthermore, in our patient these parameters were the only factors associated with emboligenic arrhythmia; this is particularly interesting considering the increasing importance of focused cardiac ultrasound in neurology training.
In conclusion, different tools may be used to determine whether ischaemic stroke of undetermined aetiology is due to emboligenic factors. When the most frequent emboligenic causes are ruled out, left atrial volumetry or assessment of left atrial function (LAEF and LAS) may help us to determine whether patients are eligible for prolonged cardiac monitoring to screen for AF with a view to choosing the most suitable treatment.
Please cite this article as: Lambea Gil Á, Tejada Meza H, López Perales CR, Artal Roy J, Marta Moreno J. Parámetros ecocardiográficos de cardiopatía auricular y detección de fibrilación auricular en el ictus criptogénico. Neurología. 2020;35:284–287.