Neuromyelitis optica spectrum disorders (NMOSD) are immune-mediated inflammatory disorders of the central nervous system involving astrocytes, B lymphocytes, anti-aquaporin 4, and such inflammatory mediators as interleukin-6. Several immunosuppressants are used in their treatment. Tocilizumab, an interleukin-6 receptor antagonist, may be a treatment option.
MethodWe performed an observational, retrospective study analysing parameters of effectiveness (annualised relapse rate, disability, and radiological progression) and safety of tocilizumab in patients with NMOSD in whom previous immunosuppressant treatment had failed. We aimed to evaluate the effectiveness and safety of tocilizumab in clinical practice in patients with NMOSD not responding to other immunosuppressants.
ResultsFive patients with NMOSD were analysed. Sixty percent of patients were women; mean age at diagnosis was 50±5.3 years and mean progression time was 4.5±3.6 years. Previously administered immunosuppressants were rituximab (in all 5), cyclophosphamide (2), and azathioprine (1). Mean time of exposure to tocilizumab was 2.3±1 years. Mean annualised relapse rate was 1.8±1.3 in the year prior to the introduction of tocilizumab and 0.2±0.4 the year after (P<.05), representing a reduction of 88.9%.
ConclusionsIn our experience, tocilizumab is safe and effective in patients with NMOSD showing no response to other immunosuppressants.
El espectro de la neuromielitis óptica (ENMO) es una enfermedad inflamatoria del sistema nervioso central con patogenia inmunomediada que implica a astrocitos, linfocitos B, anticuerpos antiacuaporina 4 y mediadores inflamatorios como la interleucina 6. En su tratamiento se utilizan distintos inmunosupresores. Tocilizumab, un antagonista del receptor de interleucina 6, podría ser una opción de tratamiento.
MétodoEstudio observacional y retrospectivo en el que se analizan parámetros de efectividad: tasa anualizada de recaídas, discapacidad y evolución radiológica, y seguridad de tocilizumab en pacientes con ENMO (Wingerchuk 2015) y fracaso de inmunosupresores previos. El objetivo es evaluar la efectividad y seguridad de tocilizumab en la práctica clínica en pacientes con diagnóstico de ENMO sin respuesta terapéutica a otros inmunosupresores.
ResultadosSe analizan 5 pacientes (60% mujeres) con ENMO. Características basales (media±DE): edad al diagnóstico 50±5,3 años; tiempo de evolución desde el diagnóstico 4,5±3,6 años; inmunosupresores previos rituximab (5), ciclofosfamida (2) y azatioprina (1); tiempo de exposición a tocilizumab 2,3±1 años; tasa anual de recaídas un año pretocilizumab vs. un año postocilizumab 1,8±1,3 vs. 0,2±0,4 (p <0,05). Reducción de la tasa anual de recaídas al año de postocilizumab: 88,9%.
ConclusionesEn nuestra experiencia, tocilizumab es seguro y efectivo en pacientes con ENMO sin respuesta a otros inmunosupresores.
Neuromyelitis optica spectrum disorders (NMOSD) are inflammatory diseases of the central nervous system; the main pathogenic mechanism is mediated by the action of aquaporin-4 antibodies (AQP4-IgG), which target aquaporin-4 channels (AQP4), the most abundant water channels in the central nervous system. The clinical course includes recurrent outbreaks (only 5%-10% are monophasic) of optic neuritis or transverse myelitis, although today the disease is considered to affect not only the optic nerves and the spinal cord, as initially described, but also the brainstem and brain hemispheres. Outbreaks are usually severe and the risk of disabling sequelae is high.1
Immunosuppressants are the main disease-modifying treatments (DMT) used; however, no randomised controlled clinical trial has provided a sufficient level of evidence to conclusively support the use of any specific drug. The low incidence of the disease, together with the availability of effective treatment options, probably further decreases the number of cases available to be included in placebo-controlled trials. In clinical practice, therapeutic management is supported by results from small series of patients receiving different immunosuppressants. Although optimal responses to such treatments as azathioprine, mycophenolate mofetil, methotrexate, and mitoxantrone have been reported, the best outcomes were obtained with rituximab (RTX), a monoclonal antibody targeting the CD20 antigen of B cells, which has obtained response rates between 50% and 93%.2–8 The meta-analysis by Damto et al.,9 which included 46 studies (n=438) of patients with NMOSD and treated with RTX, showed that RTX is an effective treatment for reducing relapses; however, due to the lack of controlled studies with results on safety, it should be used with caution as a first option. In spite of these results, between 7% and 47% of patients treated with RTX, according to different series, may present relapses, even with complete depletion of B cells.2–8
Different studies on the pathogenesis of NMOSD have reported significantly higher levels of proinflammatory interleukin 6 (IL-6) in serum and cerebrospinal fluid than in other autoimmune neurological diseases,10,11 which may mean that IL-6 has a relevant pathogenic role in these disorders. IL-6 would prolong the survival of certain B cell subclasses and the secretion of anti-AQP4 antibodies.12–14
Tocilizumab is a recombinant humanised monoclonal IgG1 antibody targeting the human IL-6 receptor. It is indicated as treatment for rheumatoid arthritis, and is dosed at 8mg/kg body weight, administered intravenously every 4 weeks. Tocilizumab has been shown to be effective in other autoimmune diseases, such as systemic lupus erythematosus.15
Some studies using tocilizumab to treat NMOSD have shown decreased intrathecal levels of IL-6 in the cerebrospinal fluid, which are correlated with decreased disease activity, both clinically and in imaging studies.16 Tocilizumab may be beneficial for treatment of some of the most frequent symptoms, such as fatigue and neuropathic pain.14
Material and methodsThe aim of the study is to assess the effectiveness and safety of treatment with tocilizumab in patients with NMOSD who have not previously responded to other DMTs.
This is an observational study including a retrospective analysis of a series of patients diagnosed with NMOSD at 3 reference hospitals for inflammatory and demyelinating diseases of the central nervous system. Inclusion criteria were: diagnosis of NMOSD according to the criteria proposed by Wingerchuck et al.17 in 2015, previous ineffectiveness of one or several DMTs, and having used tocilizumab dosed at 8mg/kg body weight every 4 weeks after suspension of the last DMT due to lack of therapeutic response.
Lack of response to a previous DMT was defined as the presence of a clinical relapse (optic neuropathy, myelitis, or brainstem syndrome) during exposure to the drug.
We retrospectively gathered demographic data and information on disease progression from diagnosis of NMOSD to the time of drafting this study; therefore, tocilizumab exposure time is different in each patient. After being diagnosed, patients were periodically assessed by a neurologist specialising in demyelinating diseases.
The primary outcome variable was the annualised relapse rate (ARR), determined by dividing the total number of relapses by a period of time in years, and calculated before and after each treatment. Secondary variables were the degree of disability, radiological progression in magnetic resonance imaging (MRI) studies, and the degree of tolerance and safety.
To quantify the degree of disability we used the Expanded Disability Status Scale (EDSS), which is frequently used in multiple sclerosis. We collected all the data on brain and spinal MRI available during clinical follow-up, analysing gadolinium-enhanced T2-weighted, FLAIR, and T1-weighted sequences. We also analysed all the laboratory and clinical results related to safety from tocilizumab treatment onset.
Differences between quantitative variables were measured with the paired samples t test.
Patients signed informed consent forms and the drug was administered after its approval by the corresponding regulatory body as an off-label treatment.
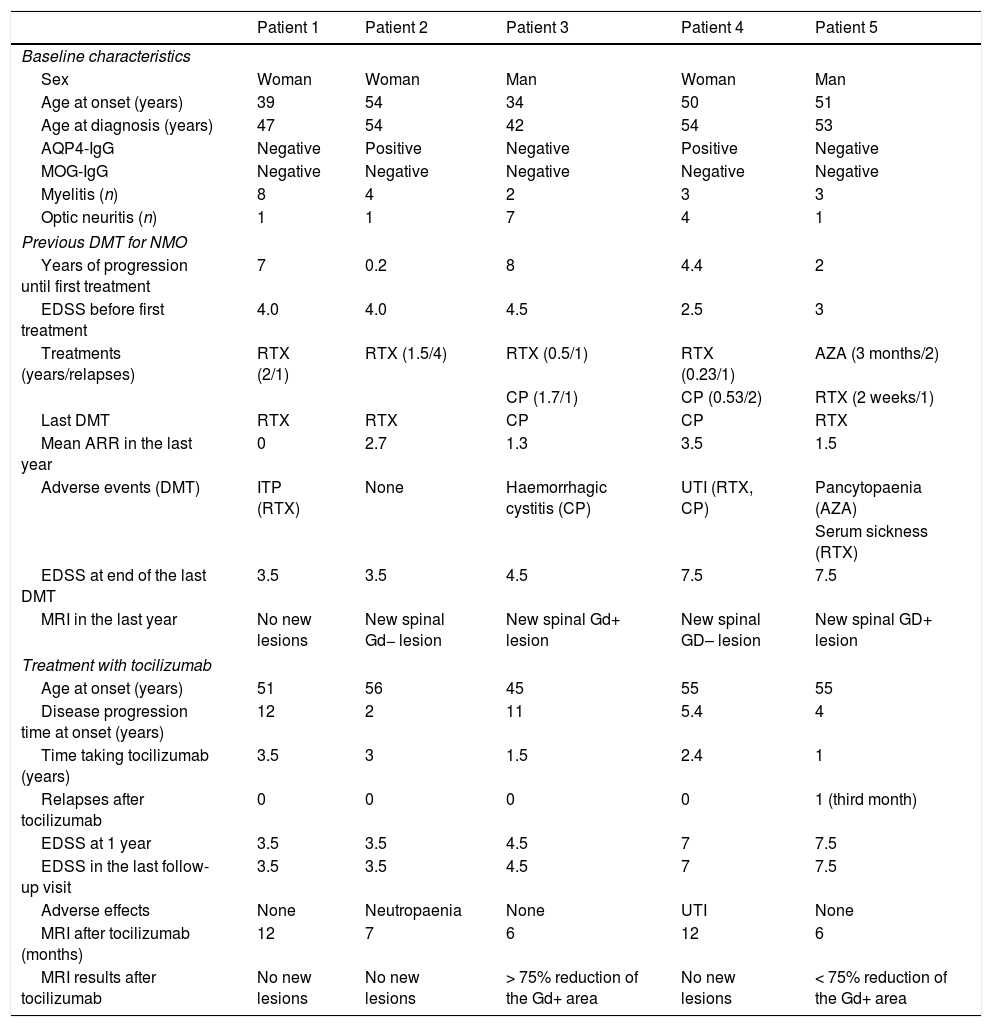
ResultsDemographic and baseline clinical characteristicsOf all the patients with NMOSD analysed, we identified 5 who met the inclusion criteria. Three patients (60%) were women. Mean age (SD) at diagnosis was 50 (5.3) years, and all patients had presented at least one episode of optic neuritis and one episode of longitudinally extensive transverse myelitis. No patient presented comorbid autoimmune diseases. Two patients presented AQP4-IgG in serum and none presented MOG-IgG. The transfected cell technique was used to quantify AQP4-IgG antibodies.
As is required for other conditions treated with tocilizumab, latent tuberculosis and active infections were ruled out before treatment onset. No specific washout period was established (the minimum time elapsed was 6 months), but titres of total leukocytes, lymphocytes, neutrophils, and platelets had to be within the normal range before treatment administration.
All patients had received RTX as a DMT at some point during progression (dosed at 375mg/m2 weekly for 4 weeks and a minimum of 1g after one year). Two had also been treated with cyclophosphamide and one with azathioprine. One patient had been previously diagnosed with multiple sclerosis and received sequential treatment with interferon beta and natalizumab before diagnosis of NMOSD.
Despite the DMTs administered, patients presented a mean ARR (SD) of 1.8 (1.3) in the year prior to tocilizumab treatment onset and a mean EDSS score of 5.3 (2). At baseline (before starting on tocilizumab), 4 of the 5 patients (80%) presented considerable disease activity in terms of number and severity of relapses (Table 1).
Patients’ baseline clinical characteristics and response to tocilizumab.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Sex | Woman | Woman | Man | Woman | Man |
| Age at onset (years) | 39 | 54 | 34 | 50 | 51 |
| Age at diagnosis (years) | 47 | 54 | 42 | 54 | 53 |
| AQP4-IgG | Negative | Positive | Negative | Positive | Negative |
| MOG-IgG | Negative | Negative | Negative | Negative | Negative |
| Myelitis (n) | 8 | 4 | 2 | 3 | 3 |
| Optic neuritis (n) | 1 | 1 | 7 | 4 | 1 |
| Previous DMT for NMO | |||||
| Years of progression until first treatment | 7 | 0.2 | 8 | 4.4 | 2 |
| EDSS before first treatment | 4.0 | 4.0 | 4.5 | 2.5 | 3 |
| Treatments (years/relapses) | RTX (2/1) | RTX (1.5/4) | RTX (0.5/1) | RTX (0.23/1) | AZA (3 months/2) |
| CP (1.7/1) | CP (0.53/2) | RTX (2 weeks/1) | |||
| Last DMT | RTX | RTX | CP | CP | RTX |
| Mean ARR in the last year | 0 | 2.7 | 1.3 | 3.5 | 1.5 |
| Adverse events (DMT) | ITP (RTX) | None | Haemorrhagic cystitis (CP) | UTI (RTX, CP) | Pancytopaenia (AZA) |
| Serum sickness (RTX) | |||||
| EDSS at end of the last DMT | 3.5 | 3.5 | 4.5 | 7.5 | 7.5 |
| MRI in the last year | No new lesions | New spinal Gd− lesion | New spinal Gd+ lesion | New spinal GD– lesion | New spinal GD+ lesion |
| Treatment with tocilizumab | |||||
| Age at onset (years) | 51 | 56 | 45 | 55 | 55 |
| Disease progression time at onset (years) | 12 | 2 | 11 | 5.4 | 4 |
| Time taking tocilizumab (years) | 3.5 | 3 | 1.5 | 2.4 | 1 |
| Relapses after tocilizumab | 0 | 0 | 0 | 0 | 1 (third month) |
| EDSS at 1 year | 3.5 | 3.5 | 4.5 | 7 | 7.5 |
| EDSS in the last follow-up visit | 3.5 | 3.5 | 4.5 | 7 | 7.5 |
| Adverse effects | None | Neutropaenia | None | UTI | None |
| MRI after tocilizumab (months) | 12 | 7 | 6 | 12 | 6 |
| MRI results after tocilizumab | No new lesions | No new lesions | > 75% reduction of the Gd+ area | No new lesions | < 75% reduction of the Gd+ area |
AQP4-IgG: aquaporin-4 antibodies; ARR: annualised relapse rate; AZA: azathriopine; CP: cyclophosphamide; DMT: disease-modifying treatment; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing lesion; Gd−: lesion without gadolinium enhancement; ITP: immune thrombocytopaenia; MRI: magnetic resonance imaging; RTX: rituximab; UTI: urinary tract infection.
Mean duration (SD) of treatment with tocilizumab was 2.3 (1) years. After tocilizumab was started, a drastic reduction was observed in the number of relapses; in fact, 4 (80%) patients did not experience relapses during follow-up. The only relapse observed, at 3 months after starting tocilizumab (patient 5, Table 1) consisted of cervical myelitis with sensory involvement of the lower limbs, which completely resolved after a megadose of steroids. Although follow-up periods for treatment with tocilizumab were variable, all patients completed a minimum of one year (Table 1). During the first year of treatment, ARRs decreased by 88.9% with regard to the previous year (1.8 [1.3] vs 0.2 [0.4]; P<.05), and no significant variations were observed in the degree of disability as measured with the EDSS (5.3 [2] vs 5.2 [1.9]; P>.05).
We analysed follow-up MRI scans performed the year after starting tocilizumab (mean time after treatment onset: 8.6 [3.1] months) and compared the results with the last available MRI from the year prior. No changes were observed between brain MRI studies. No patient presented new lesions on spinal cord T2-weighted sequences. The 2 patients with gadolinium-enhancing (Gd+) active spinal cord lesions presented reductions greater than 75% in the Gd+ area (Table 1).
The most relevant adverse events were one case of asymptomatic mild neutropaenia of 6 months’ duration (with subsequent spontaneous resolution) and one case of chronic urinary tract infection (already observed with previous DMT) in another patient.
AQP4-IgG and IL-6 levels were not quantified during follow-up.
DiscussionConsidering that NMOSD relapses are frequently disabling and present a high risk of sequelae, starting immunosuppressive DMT is essential for preventing recurrence. Currently, due to the lack of randomised clinical trials on the treatment of NMOSD, the available evidence is insufficient to recommend a specific immunosuppressive treatment; therefore, clinical experience takes on special relevance.
The patient series reported to date have shown the effectiveness of tocilizumab in reducing the rate of relapses, without relevant adverse effects; however, these studies generally present isolated cases and small series of patients showing resistance to other treatments.14,16,18–20
One of the largest series, including 7 patients,14 showed that treatment with tocilizumab in NMOSD reduces the ARR and improves EDSS score (level of evidence IV). Although the majority of patients receiving tocilizumab presented aggressive disease refractory to treatment with anti-CD20 antibodies, we suggest that the drug should be considered at earlier stages of NMOSD to improve patients’ quality of life. The series reported by Ringelstein et al.,21 including 8 patients, showed the effectiveness and safety of tocilizumab, especially in patients presenting inadequate response to RTX. Both series14,21 showed improvements in radiological activity (Gd+), in addition to decreases in EDSS scores and antibody titres (AQP4-IgG) during treatment. Both studies reported a decrease in relapse frequency and intensity (mild-moderate), with patients presenting resolution without sequelae. Neither reported severe adverse events.
Our results are similar to those reported in other series in terms of the decrease in ARRs, the absence of progression of disability, the absence of new MRI lesions, and the radiological improvement in patients with Gd+ lesions.
Several studies have suggested that the administration of treatments indicated for multiple sclerosis (such as natalizumab) in patients with NMOSD does not provide any benefit for any clinical or radiological variable, and may even lead to poorer results in these parameters; therefore, their use is not recommended.20,22,23 In our series, one patient had been treated sequentially with interferon beta and natalizumab before diagnosis of NMOSD, and during that period he presented numerous episodes of optic neuropathy, resulting in severely impaired residual visual acuity (Table 1, patient 3).
Patients treated with tocilizumab for rheumatoid arthritis have been reported to present a higher risk of infection, mainly urinary tract infections and reactivation of latent tuberculosis.24 However, a low percentage of severe infections is reported in these patients at 5 years of treatment, which shows that tocilizumab has a good long-term safety profile.25 The patients included in our series presented good tolerance to tocilizumab, and presented few adverse effects of any kind. We can mention one case of transient neutropaenia that did not lead to any secondary opportunistic infection.
Our results, like those of previous studies, are characterised by good response to tocilizumab in aggressive, refractory NMOSD, which raises the question of the drug's potential effectiveness as a first-line DMT. At the time of writing, we are aware of an ongoing phase 3 clinical trial with the molecule SA237, an anti–IL-6 receptor monoclonal antibody.
The main limitations of our study are its observational, retrospective design and the possible influence of regression toward the mean in the improvement in ARRs, although the good radiological findings lead us to suspect a limited influence.
ConclusionsIn our experience, tocilizumab (8mg/kg body weight, administered intravenously every 4 weeks) seems to be a safe and effective treatment in patients with active NMOSD and not responding to other immunosuppressants, mainly RTX. Further controlled studies with larger patient samples and longer follow-up periods are needed to assess the effectiveness of tocilizumab in NMOSD.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Carreón Guarnizo E, Hernández Clares R, Castillo Triviño T, Meca Lallana V, Arocas Casañ V, Iniesta Martínez F, et al. Experiencia con tocilizumab en pacientes con espectro de la neuromielitis óptica. Neurología. 2022;37:178–183.