Neuroinflammation has recently been described in amyotrophic lateral sclerosis (ALS). However, the precise role of such proinflammatory cytokines as monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1β (MIP-1β) in ALS has not yet been determined. In this study, we determined cerebrospinal fluid (CSF) MCP-1 and MIP-1β levels and assessed their association with the duration and severity of ALS.
MethodsConcentrations of MCP-1 and MIP-1β were determined in the CSF of 77 patients diagnosed with ALS and 13 controls. Cytokine levels were analysed in relation to ALS duration (< 12months vs > 12months) and severity (< 30points vs > 30points on the ALS Functional Rating Scale administered at hospital admission).
ResultsHigher CSF MIP-1β (10.68pg/mL vs 4.69pg/mL, P<.0001) and MCP-1 (234.89pg/mL vs 160.95pg/mL, P=.011) levels were found in the 77 patients with ALS compared to controls. There were no differences in levels of either cytokine in relation to disease duration or severity. However, we did observe a significant positive correlation between MIP-1β and MCP-1 in patients with ALS.
ConclusionsThe increase in MIP-1β and MCP-1 levels suggests that these cytokines may have a synergistic effect on ALS pathogenesis. However, in our cohort, no association was found with either the duration or the clinical severity of the disease.
En la esclerosis lateral amiotrófica (ELA) se ha descrito recientemente la presencia de neuroinflamación. Sin embargo, no se ha definido el rol de citoquinas proinflamatrorias como la proteína quimiotáctica de monocitos-1 (MCP-1) y la proteína inflamatoria macrofágica-1β (MIP-1β) en ELA. En este estudio evaluamos niveles de MCP-1 y MIP-1β en líquido cefalorraquídeo (LCR), analizando su participación en la duración y gravedad de la ELA.
MétodosEn 77 pacientes con ELA definida y 13 sujetos controles, se comparó el nivel de citoquinas MCP-1 y MIP-1β en LCR. Se analizaron estos niveles con relación a la duración de la ELA (< 12meses vs. >12meses) y a la gravedad de esta determinada mediante el puntaje obtenido al ingreso en la escala funcional estratificada de la ELA (<30puntos vs. >30puntos).
ResultadosEn los 77 pacientes con ELA, se encontraron aumentados los niveles de MIP-1β (4,69pg/ml vs. 10,68pg/ml, p<0,0001) y MCP-1 (160,95pg/ml vs. 234,89pg/ml, p=0,011) en comparación con sujetos controles. No se observó diferencia de los niveles de estas citoquinas con la duración o la gravedad de la enfermedad. Sin embargo, observamos una correlación positiva significativa entre MIP-1β y MCP-1 en pacientes con ELA.
ConclusionesEl aumento de MIP-1β y MCP-1 sugiere que estas citoquinas parecen tener un efecto sinérgico en la patogénesis de la ELA. Sin embargo, en nuestra cohorte no se asociaron con la duración o la gravedad de la ELA.
Amyotrophic lateral sclerosis (ALS) is a fatal disease characterised by rapid deterioration due to selective death of motor neurons in the cerebral cortex, brainstem, and spinal cord.1–3 It has traditionally been regarded as a neurodegenerative process; however, its aetiology and pathogenic mechanisms are yet to be fully understood.3,4
Recent evidence suggests that neuroinflammation is a prominent pathological feature of motor neuron lesions. This process is characterised by microglial activation, astrogliosis, and infiltration of monocytes and T-cells.5 Central nervous system (CNS) inflammation has been proposed as a contributing factor in ALS pathogenesis; this process is immune-mediated and involves proinflammatory cytokines, which play a critical role in microglial activation.5–9 Cytokines are also involved in the migration of peripheral monocytes/macrophages and other types of leukocytes into the CNS in patients with ALS.5,10–13
Monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1β (MIP-1β), 2 members of the chemokine CC subfamily, are known to play a role in other neurodegenerative diseases.14–16 Increased MCP-1 expression has been observed in mouse models of ALS and in the spinal cords of patients with ALS.12,16 To our knowledge, however, no studies have reported MIP-1β expression in patients with ALS. Furthermore, it is yet to be determined whether changes in the levels of these cytokines are protective or detrimental in the context of ALS.5
In this study, we compared cerebrospinal fluid (CSF) MCP-1 and MIP-1β levels in patients with ALS and controls, and analysed the association with disease severity and duration.
Patients and methodsParticipantsWe recruited 77 patients with ALS (mean age [standard deviation]: 50.18 years [10.85]) and 13 controls (mean age: 39.15 years [11.32]) attended by the neurology departments of Hospital Zambrano Hellion (Instituto Tecnológico y de Estudios Superiores de Monterrey, Mexico) and Hospital Universitario “Dr. José Eleuterio González” (Universidad Autónoma de Nuevo León, Mexico). Controls were selected from among patients with headache attending the emergency departments of both centres; they showed no CSF or brain MRI alterations and were finally diagnosed with tension-type headache.
In the ALS group, diagnosis was established by a neurologist, according to the El Escorial criteria.17 We gathered the following clinical data at the time of inclusion: time from onset to diagnosis, time from diagnosis to inclusion in the study, and score on the revised ALS Functional Rating Scale (ALSFRS-R).18 The study was approved by the research ethics committees of the medical schools of both participating universities.
Preparation of CSF samples and analysis of chemokine levelsAfter obtaining written informed consent, we gathered CSF samples from all participants. Samples were stored at −70°C before analysis. MCP-1 and MIP-1β levels were quantified using a Bio-Plex Pro kit (Bio-Rad Laboratories, Inc., Hercules, USA), following the manufacturer's instructions: 50μL samples were incubated with 50μL of magnetic microspheres covered with specific antibodies for each of the 2 cytokines. Cytokines were discriminated with fluorescence spectroscopy using a laser flow cytometer. After washing, a detection antibody was added to each sample to form sandwich complexes; we subsequently added a streptavidin phycoerythrin conjugate. Finally, buffer solution was added to each well to stop the reaction. Samples were analysed with the Bio-Plex 200 system. The detection limit for each cytokine was 1pg/mL.
Statistical analysisNormally distributed data are expressed as means (standard deviation), and non-normally distributed data as medians (interquartile range). The non-parametric Mann–Whitney U test was used to compare CSF MCP-1 and MIP-1β levels between patients and controls. Patients were classified in 2 groups according to the time from diagnosis to inclusion in the study: less than 12 months of progression (ALS < 12months) and over 12 months of progression (ALS ≥ 12months). We also compared chemokine levels between patients scoring < 30 points on the ALSFRS-R and those scoring ≥ 30 points. The effect of disease severity and duration on MCP-1 and MIP-1β levels was analysed with the Mann–Whitney U test. Simple bivariate correlations were analysed with the Spearman rank correlation. Statistical analysis was conducted using SPSS version 20.0.
ResultsPatients with ALSForty-eight of the 77 patients included were men (62.3%). Onset was bulbar in 20 patients, spinal in 53, and mixed in 4. Median time from diagnosis to inclusion in the study was 11 months (range, 6-19), and median time from onset to diagnosis was 10 months (range, 7-18).
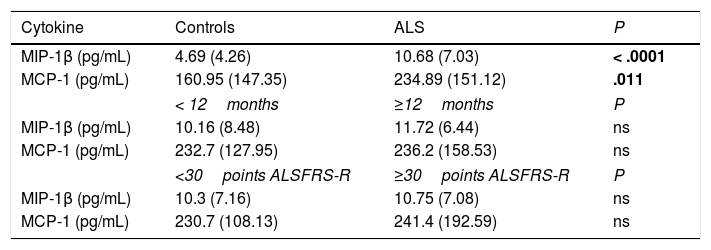
Chemokine levels in CSFThe median CSF MCP-1 level was 234.89pg/mL (151.12) in patients with ALS and 160.95pg/mL (147.35) in controls (P=.011). The median CSF MIP-1β level was 10.68pg/mL (7.03) in patients with ALS and 4.69pg/mL (4.26) in controls (P<.0001) (Table 1). No differences in MCP-1 or MIP-1β levels were observed between sexes.
CSF cytokine levels in our sample; comparison between patients and controls, and between patient subgroups established according to disease duration and severity.
| Cytokine | Controls | ALS | P |
|---|---|---|---|
| MIP-1β (pg/mL) | 4.69 (4.26) | 10.68 (7.03) | < .0001 |
| MCP-1 (pg/mL) | 160.95 (147.35) | 234.89 (151.12) | .011 |
| < 12months | ≥12months | P | |
| MIP-1β (pg/mL) | 10.16 (8.48) | 11.72 (6.44) | ns |
| MCP-1 (pg/mL) | 232.7 (127.95) | 236.2 (158.53) | ns |
| <30points ALSFRS-R | ≥30points ALSFRS-R | P | |
| MIP-1β (pg/mL) | 10.3 (7.16) | 10.75 (7.08) | ns |
| MCP-1 (pg/mL) | 230.7 (108.13) | 241.4 (192.59) | ns |
Values are expressed as medians (interquartile range).
Statistically significant differences are shown in bold.
The median MIP-1β level was 11.72pg/mL (6.44) in patients with disease progression ≥ 12months and 10.16pg/mL (8.48) in those with disease progression < 12 months (P=.4). In the same patient subgroups, median MCP-1 levels were 236.2pg/mL (158.53) and 232.7pg/mL (127.95), respectively (P=.7).
Regarding clinical status, patients scoring ≥ 30 points on the ALSFRS-R showed a median MIP-1β level of 10.75pg/mL (7.08) and a median MCP-1 level of 241.4pg/mL (192.59). Those scoring < 30 points on the scale showed median MIP-1β and MCP-1 levels of 10.3pg/mL (7.16) and 230.7pg/mL (108.13), respectively. Patients with better clinical status showed higher levels of both chemokines, although differences were not statistically significant (P = .4 and P = .5 for MCP and MIP).
CorrelationsWe found a significant positive correlation between MIP-1β and MCP-1 levels in our sample as a whole (rho=0.365; P=.001), as well as in the group of patients with ALS (rho=0.309; P=.006). No correlations were observed between MIP-1β or MCP-1 levels and age or ALSFRS-R scores (data not presented).
DiscussionALS has traditionally been considered a neurodegenerative disease.1,3 However, neuroinflammatory changes, with microglial activation, have been observed in the motor cortex and spinal cord of patients with the disease.19 The process underlying cell activation and the signalling molecules that mediate cell-cell interactions in ALS are yet to be determined.7 CC chemokines (MCP-1 and MIP-1β) have been found to mediate chronic inflammation in other neurodegenerative diseases.15,20,21 Similarly, recent studies describe infiltration of peripheral monocytes/macrophages into the CNS in patients with ALS.10,11 These infiltrating monocytes express high levels of MCP-1 and other chemotactic cytokines.12 Increased MCP-1 levels have previously been described in patients with ALS16,22,23; however, the mechanisms underlying this alteration are unknown.16
We found significant increases in MCP-1 and MIP-1β levels in patients with a definite diagnosis of ALS. CSF levels of both cytokines were also significantly correlated. To our knowledge, this is the first study to suggest the involvement of MIP-1β in the pathogenesis of ALS.
Microglial cells are thought to modulate immune system alterations in ALS; the role of peripheral monocytes in the pathogenesis of the disease is more controversial.24 We found increased CSF MCP-1 and MIP-1β levels in our sample, which suggests that dendritic cells and peripheral macrophages may be involved in the pathophysiology of ALS.16 The lack of data about a correlation with serum levels of these cytokines prevents us from determining whether their origin is central or peripheral (due to blood-brain barrier disruption). Both types of immune cells may alter T-cell function and modulate innate and adaptive immunity. However, it is yet to be determined whether their role is pathogenic or protective.
In our sample, patients with longer disease duration showed slightly higher MIP-1β levels and lower MCP-1 levels than those with shorter disease duration, although these differences were not significant. Our results do not show an association between MIP-1β and MCP-1 levels and disease severity. However, it is yet to be determined at which precise moment in the course of the disease cytokine expression starts, and whether this response is protective or pathogenic.16
ALS is a relatively heterogeneous entity.4 The highly variable clinical expression, duration, and severity of the disease (as measured with the ALSFRS-R) may explain the lack of significant differences in MCP-1 and MIP-1β levels between patient subgroups. The correlation between levels of these cytokines and such variables as survival and clinical progression may help confirm the involvement of cytokine-mediated inflammation in ALS. Understanding the cytokine environment over the course of ALS and the role of cytokines in disease progression is essential for the development of reliable biomarkers and effective treatment strategies. Our study has several limitations, including the small size and the heterogeneity of our sample, the small number of controls, and the lack of data about serum MCP-1 and MIP-1β levels. Future studies should also include more objective functional assessment scales. In any case, our study is the first to detect elevated MIP-1β levels in patients with ALS and to suggest that MIP-1β may play a role in disease pathogenesis.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Dr Sergio Lozano-Rodríguez for reviewing the manuscript.
Please cite this article as: Martínez HR, Escamilla-Ocañas CE, Camara-Lemarroy CR, González-Garza MT, Moreno-Cuevas J, García Sarreón MA. Incremento de las citoquinas proteína quimiotáctica de monocitos-1 (MCP-1) y proteína inflamatoria macrofágica-1β (MIP-1β) en líquido cefalorraquídeo de pacientes con esclerosis lateral amiotrófica. Neurología. 2020;35:165–169.