Thrombectomy in the carotid artery territory was recently shown to be effective up to 24 hours after symptoms onset.
MethodsWe conducted a retrospective review of a prospective registry of patients treated at our stroke reference centre between November 2016 and April 2019 in order to assess the safety and effectiveness of mechanical thrombectomy performed beyond 6 hours after symptoms onset in patients with acute ischaemic stroke and large vessel occlusion in the carotid artery territory.
ResultsData were gathered from 59 patients (55.9% women; median age, 71 years). In 33 cases, stroke was detected upon awakening; 57.6% of patients were transferred from another hospital. Median baseline NIHSS score was 16, and median ASPECTS score was 8, with 94.9% of patients presenting > 50% of salvageable tissue. Satisfactory recanalisation was achieved in 88.1% of patients, beyond 24 hours after onset in 5 cases. At 90 days of follow-up, 67.8% were functionally independent; those who were not were older and presented higher prevalence of atrial fibrillation, greater puncture-to-recanalisation time, and higher NIHSS scores, both at baseline and at discharge.
ConclusionIn our experience, mechanical thrombectomy beyond 6 hours was associated with good 90-day functional outcomes. Age, NIHSS score, puncture-to-recanalisation time, and presence of atrial fibrillation affected functional prognosis. The efficacy of the treatment beyond 24 hours after onset merits study.
La eficacia de la trombectomía mecánica en territorio carotídeo en las primeras 24 horas se ha probado con trabajos publicados recientemente.
MétodosRevisión retrospectiva a partir de un registro prospectivo en nuestro centro de referencia de ictus para valorar la eficacia y seguridad del tratamiento endovascular realizado más allá de las 6 horas de evolución de los síntomas en pacientes con ictus isquémico agudo y oclusión de gran vaso en territorio carotídeo, entre noviembre de 2016 y abril de 2019.
ResultadosSe recopilaron datos de 59 pacientes (55,9% mujeres, mediana de edad 71 años). Treinta y tres pacientes fueron detectados al despertar. El 57,6% de los casos fueron traslados secundarios. La mediana de NIHSS basal fue 16. La mediana del ASPECTS fue 8 y el 94,9% de los pacientes presentó > 50% de tejido salvable. El 88,1% de los pacientes logró una recanalización satisfactoria, en 5 pacientes después de 24 horas de evolución. El 67,8% de los casos logró la independencia funcional a los 90 días de seguimiento. Los pacientes que no lograron la independencia funcional presentaban mayor edad, mayor proporción de fibrilación auricular, mayor tiempo punción-recanalización y mayor puntuación NIHSS, tanto basal como al alta.
ConclusiónEn nuestra experiencia la trombectomía mecánica después de las 6 horas se asoció con buenos resultados de funcionalidad a los 90 días. La edad, la puntuación NIHSS, el tiempo punción-recanalización y la prevalencia de fibrilación auricular fueron factores determinantes en el pronóstico funcional. La eficacia de este tratamiento por encima de las 24 horas merece ser estudiada.
The effectiveness of mechanical thrombectomy beyond 6 hours after onset of acute ischaemic stroke with large vessel occlusion in the carotid artery territory has been established in the DAWN and DEFUSE 3 clinical trials,1,2 published in 2018. Both trials showed the benefits of endovascular treatment administered within 24 and 16 hours, respectively, of symptom onset. Increasing the therapeutic window to 24 hours, in cases selected based on functional neuroimaging findings (perfusion MRI and CT), has expanded the treatment window.3–6 We present our experience with endovascular treatment administered beyond 6 hours after symptom onset in patients with carotid artery territory stroke attended between November 2016 and April 2019.
Material and methodsStudy designWe retrospectively reviewed data recorded between November 2016 and April 2019 in a prospective registry of our hospital, a stroke reference centre. All the patients included in the study were treated at our centre; patients or their legal representatives gave written informed consent for treatment. All authors participated in data collection. The study was approved by the local research ethics committee. This study received no funding of any kind.
PatientsWe selected patients meeting the following inclusion criteria: age older than 18 years, functional independence before stroke, ischaemic stroke in the carotid artery territory, and treatment with mechanical thrombectomy at our centre beyond 6 hours after the last time they were known to be asymptomatic (we included cases of witnessed stroke, unwitnessed stroke, and wake-up stroke). Functional independence was defined as a score of 0-2 on the modified Rankin Scale (mRS); this tool evaluates functional disability after stroke and is scored from 0 (no symptoms) to 6 (death).7 We also collected data on age, sex, vascular risk factors, drug habits (smoking, alcohol consumption), and history of atrial fibrillation or cerebrovascular disease.
All patients, including those transferred from other centres, underwent multimodal neuroimaging studies at our hospital (non-contrast head CT, CT angiography of the intra- and extracranial arteries, CT perfusion); these were performed with a multidetector CT scanner (Aquilion ONE, Toshiba; OLEA software) following the protocol established by the stroke care plan for the region of Madrid.8 Perfusion images were obtained in cine mode, with an acquisition time of 60 seconds and scan delay of 7 seconds, after injection of 50 mL of iodinated contrast agent (Ultravist 370) at a flow rate of 5 mL/s, followed by 50 mL of 0.9% saline solution at the same flow rate; the contrast agent was administered via cubital intravenous access with a power injector. Helical CT angiography (parameters: tube voltage 100 kVp, current 50-600 mA, slice thickness 0.5 mm, field of view 30 cm, matrix 256 × 256) was performed from the aortic arch to the top of the frontal sinuses following injection of 50 mL of iodinated contrast agent (Ultravist 370) at a flow rate of 5 mL/s, followed by 50 mL of saline solution at the same flow rate. Patients transferred from other centres underwent imaging studies at our hospital; in all cases, studies were interpreted by a neuroradiologist with over 5 years’ experience. All patients presented large vessel occlusion in the carotid artery territory (intracranial segment of the internal carotid artery [ICA] or middle cerebral artery [MCA]), a small initial infarct core, and a favourable ischaemic penumbra profile, with potentially salvageable tissue as shown in functional neuroimaging studies. An Alberta Stroke Program Early Computed Tomography Score (ASPECTS) ≥ 5 points was considered to indicate small initial infarct core. ASPECTS is a grading system that quantifies early ischaemic changes in the MCA territory on non-contrast head CT images; the maximum score is 10 points (indicating no early signs of ischaemia), with 1 point subtracted for every region showing early ischaemic changes.9,10 Large vessel occlusion in the carotid artery territory was defined as intracranial occlusion of the ICA or of the first or second segment of the MCA (M1 or M2, respectively). Tissue was considered potentially salvageable when there was a >20% mismatch between mean transit time and cerebral blood volume. Patients with acute ischaemic stroke of the posterior circulation were excluded.
TreatmentWe selected all patients undergoing mechanical thrombectomy in the carotid artery territory, whether or not they had previously been treated with alteplase, when clinically appropriate. The standard endovascular procedure used at our centre follows the endovascular treatment protocol for acute ischaemic stroke established by the stroke care plan for the region of Madrid8 and is performed by expert interventional neuroradiologists with over 15 years’ experience with endovascular treatment for cerebrovascular diseases; our hospital currently performs more than 90 endovascular procedures per year in patients with acute stroke. The protocol includes a diagnostic angiography study using the femoral approach to assess perfusion with the Thrombolysis in Cerebral Infarction (TICI) scale.11 The decision to use a guide catheter or a balloon catheter (pREset or Solitaire stent retriever during the last 3 years, direct aspiration with the ACE 068 system, or a combination of both techniques) was made by the neurointerventionist. Some patients with ipsilateral ICA stenosis underwent carotid artery angioplasty with or without stent placement. General anaesthesia and conscious sedation were used indistinctly, based on the patient’s clinical status. At the end of the procedure, an 8-F Angio-Seal femoral closure device was used; local compression was also applied in patients who had previously received anticoagulation therapy.
Clinical assessment and resultsStroke severity was assessed with the National Institutes of Health Stroke Scale (NIHSS).12 The procedure was considered effective when neuroimaging studies revealed successful recanalisation (TICI grade 2B-311); neuroimages were assessed by expert neuroradiologists. The primary efficacy outcome was functional independence (mRS score 0-2) at 90 days after stroke. Safety analysis evaluated in-hospital mortality and mortality at 90 days of follow-up, symptomatic intracranial haemorrhage (defined as an increase of at least 4 points on the NIHSS associated with intracranial haemorrhage),13 early neurological impairment (defined as an increase of at least 4 points on the NIHSS within 24 hours of stroke in the absence of intracranial haemorrhage), and complications of the procedure. Following our hospital’s protocol, all patients underwent a follow-up CT scan 24 hours after the procedure. All patients were evaluated at baseline, at discharge, and at follow-up consultations at 90 days, 6 months, and 12 months using the NIHSS and the mRS.
Statistical analysisThe Shapiro-Wilk test was used to test for normality. Categorical variables are presented as percentages, and continuous variables as means and standard deviations (SD) or medians and quartiles 1 and 3 (Q1-Q3), as appropriate. The chi square test was used to compare categorical variables, and the t test to compare continuous variables. Logistic regression was used to identify predictor variables; associations are presented as odds ratios (OR) and 95% confidence intervals (95% CI). Statistical significance was set at P < .05. Statistical analysis was performed with version 14 of the Stata software.
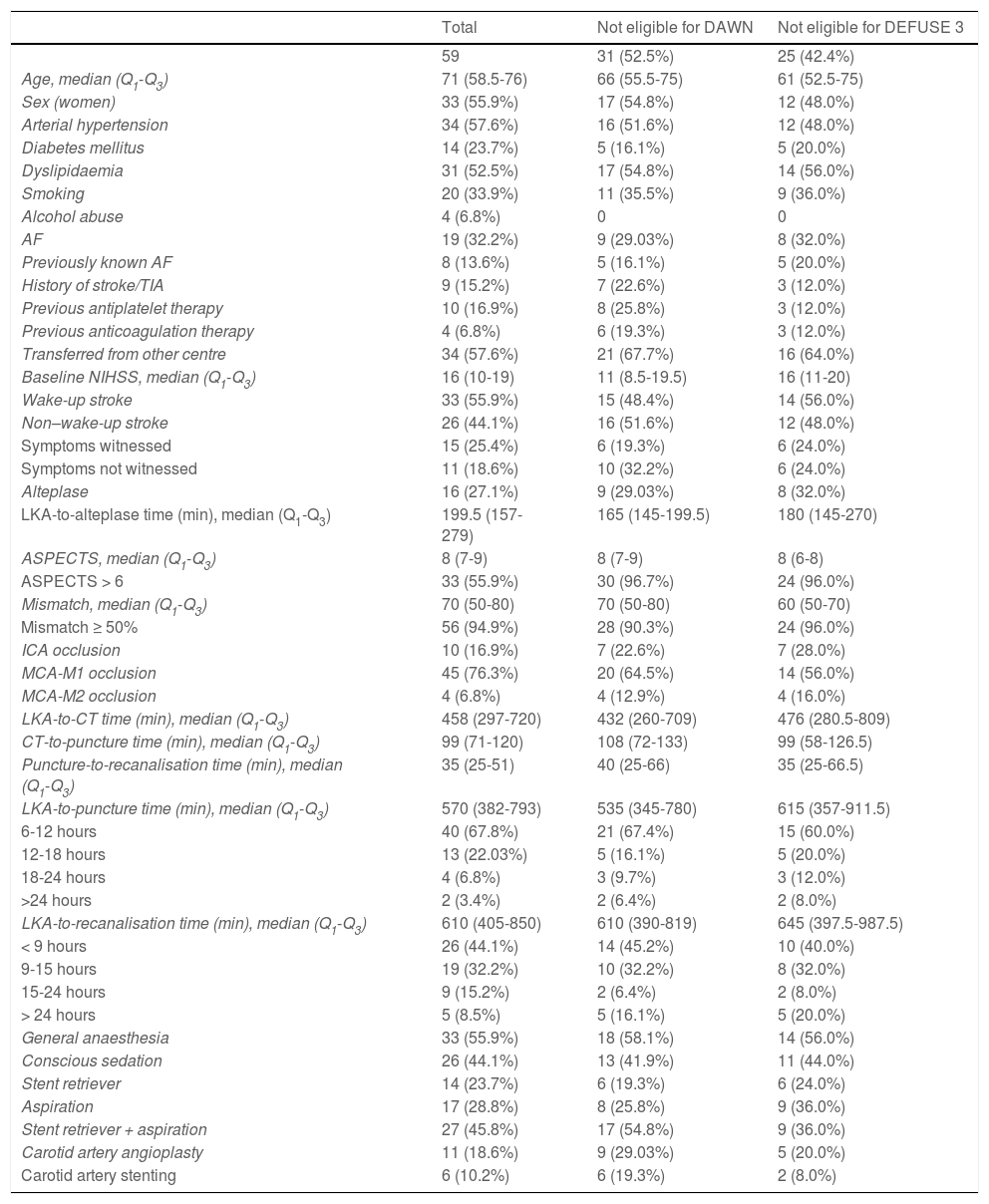
ResultsData were gathered from 59 patients undergoing mechanical thrombectomy due to large vessel occlusion in the carotid artery territory. Patients’ baseline characteristics are summarised in Table 1. Of the total sample, 28 patients (47.5%) met the DAWN criteria and 34 patients (57.6%) met the DEFUSE 3 criteria. Women accounted for 55.9% of the sample (n = 33); median age (Q1-Q3) was 71 years (58.5-76). A total of 33 patients (55.9%) presented wake-up ischaemic stroke, and 34 (57.6%) were transferred from other centres. The median NIHSS score at baseline was 16 points (10-19); all patients scored ≥ 6 points, except for one patient who scored 4 points due to disabling aphasia.
Baseline characteristics of our sample.
| Total | Not eligible for DAWN | Not eligible for DEFUSE 3 | |
|---|---|---|---|
| 59 | 31 (52.5%) | 25 (42.4%) | |
| Age, median (Q1-Q3) | 71 (58.5-76) | 66 (55.5-75) | 61 (52.5-75) |
| Sex (women) | 33 (55.9%) | 17 (54.8%) | 12 (48.0%) |
| Arterial hypertension | 34 (57.6%) | 16 (51.6%) | 12 (48.0%) |
| Diabetes mellitus | 14 (23.7%) | 5 (16.1%) | 5 (20.0%) |
| Dyslipidaemia | 31 (52.5%) | 17 (54.8%) | 14 (56.0%) |
| Smoking | 20 (33.9%) | 11 (35.5%) | 9 (36.0%) |
| Alcohol abuse | 4 (6.8%) | 0 | 0 |
| AF | 19 (32.2%) | 9 (29.03%) | 8 (32.0%) |
| Previously known AF | 8 (13.6%) | 5 (16.1%) | 5 (20.0%) |
| History of stroke/TIA | 9 (15.2%) | 7 (22.6%) | 3 (12.0%) |
| Previous antiplatelet therapy | 10 (16.9%) | 8 (25.8%) | 3 (12.0%) |
| Previous anticoagulation therapy | 4 (6.8%) | 6 (19.3%) | 3 (12.0%) |
| Transferred from other centre | 34 (57.6%) | 21 (67.7%) | 16 (64.0%) |
| Baseline NIHSS, median (Q1-Q3) | 16 (10-19) | 11 (8.5-19.5) | 16 (11-20) |
| Wake-up stroke | 33 (55.9%) | 15 (48.4%) | 14 (56.0%) |
| Non–wake-up stroke | 26 (44.1%) | 16 (51.6%) | 12 (48.0%) |
| Symptoms witnessed | 15 (25.4%) | 6 (19.3%) | 6 (24.0%) |
| Symptoms not witnessed | 11 (18.6%) | 10 (32.2%) | 6 (24.0%) |
| Alteplase | 16 (27.1%) | 9 (29.03%) | 8 (32.0%) |
| LKA-to-alteplase time (min), median (Q1-Q3) | 199.5 (157-279) | 165 (145-199.5) | 180 (145-270) |
| ASPECTS, median (Q1-Q3) | 8 (7-9) | 8 (7-9) | 8 (6-8) |
| ASPECTS > 6 | 33 (55.9%) | 30 (96.7%) | 24 (96.0%) |
| Mismatch, median (Q1-Q3) | 70 (50-80) | 70 (50-80) | 60 (50-70) |
| Mismatch ≥ 50% | 56 (94.9%) | 28 (90.3%) | 24 (96.0%) |
| ICA occlusion | 10 (16.9%) | 7 (22.6%) | 7 (28.0%) |
| MCA-M1 occlusion | 45 (76.3%) | 20 (64.5%) | 14 (56.0%) |
| MCA-M2 occlusion | 4 (6.8%) | 4 (12.9%) | 4 (16.0%) |
| LKA-to-CT time (min), median (Q1-Q3) | 458 (297-720) | 432 (260-709) | 476 (280.5-809) |
| CT-to-puncture time (min), median (Q1-Q3) | 99 (71-120) | 108 (72-133) | 99 (58-126.5) |
| Puncture-to-recanalisation time (min), median (Q1-Q3) | 35 (25-51) | 40 (25-66) | 35 (25-66.5) |
| LKA-to-puncture time (min), median (Q1-Q3) | 570 (382-793) | 535 (345-780) | 615 (357-911.5) |
| 6-12 hours | 40 (67.8%) | 21 (67.4%) | 15 (60.0%) |
| 12-18 hours | 13 (22.03%) | 5 (16.1%) | 5 (20.0%) |
| 18-24 hours | 4 (6.8%) | 3 (9.7%) | 3 (12.0%) |
| >24 hours | 2 (3.4%) | 2 (6.4%) | 2 (8.0%) |
| LKA-to-recanalisation time (min), median (Q1-Q3) | 610 (405-850) | 610 (390-819) | 645 (397.5-987.5) |
| < 9 hours | 26 (44.1%) | 14 (45.2%) | 10 (40.0%) |
| 9-15 hours | 19 (32.2%) | 10 (32.2%) | 8 (32.0%) |
| 15-24 hours | 9 (15.2%) | 2 (6.4%) | 2 (8.0%) |
| > 24 hours | 5 (8.5%) | 5 (16.1%) | 5 (20.0%) |
| General anaesthesia | 33 (55.9%) | 18 (58.1%) | 14 (56.0%) |
| Conscious sedation | 26 (44.1%) | 13 (41.9%) | 11 (44.0%) |
| Stent retriever | 14 (23.7%) | 6 (19.3%) | 6 (24.0%) |
| Aspiration | 17 (28.8%) | 8 (25.8%) | 9 (36.0%) |
| Stent retriever + aspiration | 27 (45.8%) | 17 (54.8%) | 9 (36.0%) |
| Carotid artery angioplasty | 11 (18.6%) | 9 (29.03%) | 5 (20.0%) |
| Carotid artery stenting | 6 (10.2%) | 6 (19.3%) | 2 (8.0%) |
AF: atrial fibrillation; ASPECTS: Alberta Stroke Programme Early CT Score; ICA: internal carotid artery; LKA: last time known to be asymptomatic; MCA: middle cerebral artery; NIHSS: National Institutes of Health Stroke Scale; Q1-Q3: quartile 1-quartile 3; TIA: transient ischaemic attack.
Median ASPECTS was 8 points; 56 patients (94.9%) presented ASPECTS ≥ 6 points, and 3 scored 5 points. Fifty-six patients (94.9%) presented an ischaemic penumbra of ≥ 50% on perfusion CT images. Ten patients (16.9%) presented proximal occlusion of the intracranial ICA and 49 (83.1%) presented occlusion of the MCA (45 patients [76.3%] in M1 and the remaining 4 [6.8%] in M2). We detected a total of 6 tandem occlusions. Three of the patients with proximal occlusions of the ICA also presented a thrombus in the MCA, and 3 patients with proximal MCA-M1 occlusions also presented a thrombus in MCA-M2.
Sixteen patients were treated with IV alteplase at the standard dose (0.9 mg/kg); 10 of these had been transferred from other centres, where this treatment had been started. At our centre, alteplase was administered according to the standard protocol, within 4.5 hours of symptom onset; however, in selected cases, alteplase was administered beyond that time window if the penumbra profile and other imaging findings were favourable. Three patients received alteplase beyond 4.5 hours after symptom onset due to favourable findings in perfusion imaging studies.
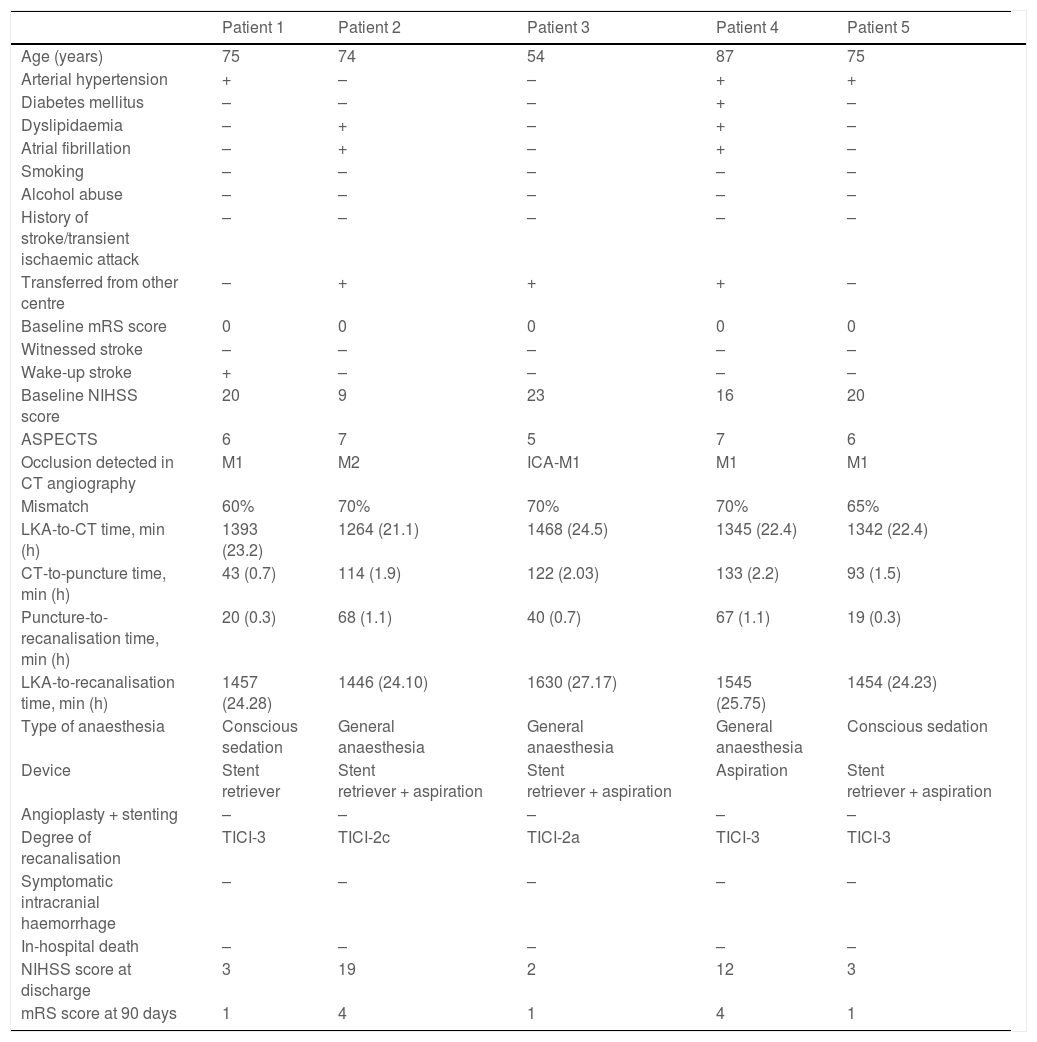
The median time from the moment when the patient was last known to be asymptomatic to recanalisation was 610 minutes (405-850). In 38 patients (64.4%), recanalisation was achieved at 6-12 hours after symptom onset, and in 5 patients (8.5%), recanalisation was achieved at 24 hours. Table 1 shows management times in our sample, and Table 2 presents information on the patients achieving recanalisation beyond 24 hours after symptom onset.
Patients undergoing mechanical thrombectomy beyond 24 hours after symptom onset.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age (years) | 75 | 74 | 54 | 87 | 75 |
| Arterial hypertension | + | – | – | + | + |
| Diabetes mellitus | – | – | – | + | – |
| Dyslipidaemia | – | + | – | + | – |
| Atrial fibrillation | – | + | – | + | – |
| Smoking | – | – | – | – | – |
| Alcohol abuse | – | – | – | – | – |
| History of stroke/transient ischaemic attack | – | – | – | – | – |
| Transferred from other centre | – | + | + | + | – |
| Baseline mRS score | 0 | 0 | 0 | 0 | 0 |
| Witnessed stroke | – | – | – | – | – |
| Wake-up stroke | + | – | – | – | – |
| Baseline NIHSS score | 20 | 9 | 23 | 16 | 20 |
| ASPECTS | 6 | 7 | 5 | 7 | 6 |
| Occlusion detected in CT angiography | M1 | M2 | ICA-M1 | M1 | M1 |
| Mismatch | 60% | 70% | 70% | 70% | 65% |
| LKA-to-CT time, min (h) | 1393 (23.2) | 1264 (21.1) | 1468 (24.5) | 1345 (22.4) | 1342 (22.4) |
| CT-to-puncture time, min (h) | 43 (0.7) | 114 (1.9) | 122 (2.03) | 133 (2.2) | 93 (1.5) |
| Puncture-to-recanalisation time, min (h) | 20 (0.3) | 68 (1.1) | 40 (0.7) | 67 (1.1) | 19 (0.3) |
| LKA-to-recanalisation time, min (h) | 1457 (24.28) | 1446 (24.10) | 1630 (27.17) | 1545 (25.75) | 1454 (24.23) |
| Type of anaesthesia | Conscious sedation | General anaesthesia | General anaesthesia | General anaesthesia | Conscious sedation |
| Device | Stent retriever | Stent retriever + aspiration | Stent retriever + aspiration | Aspiration | Stent retriever + aspiration |
| Angioplasty + stenting | – | – | – | – | – |
| Degree of recanalisation | TICI-3 | TICI-2c | TICI-2a | TICI-3 | TICI-3 |
| Symptomatic intracranial haemorrhage | – | – | – | – | – |
| In-hospital death | – | – | – | – | – |
| NIHSS score at discharge | 3 | 19 | 2 | 12 | 3 |
| mRS score at 90 days | 1 | 4 | 1 | 4 | 1 |
–: no; +: yes; ASPECTS: Alberta Stroke Program Early Computed Tomography Score; CT: computed tomography; ICA: internal carotid artery; LKA: last time known to be asymptomatic; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; TICI: Thrombolysis in Cerebral Infarction scale.
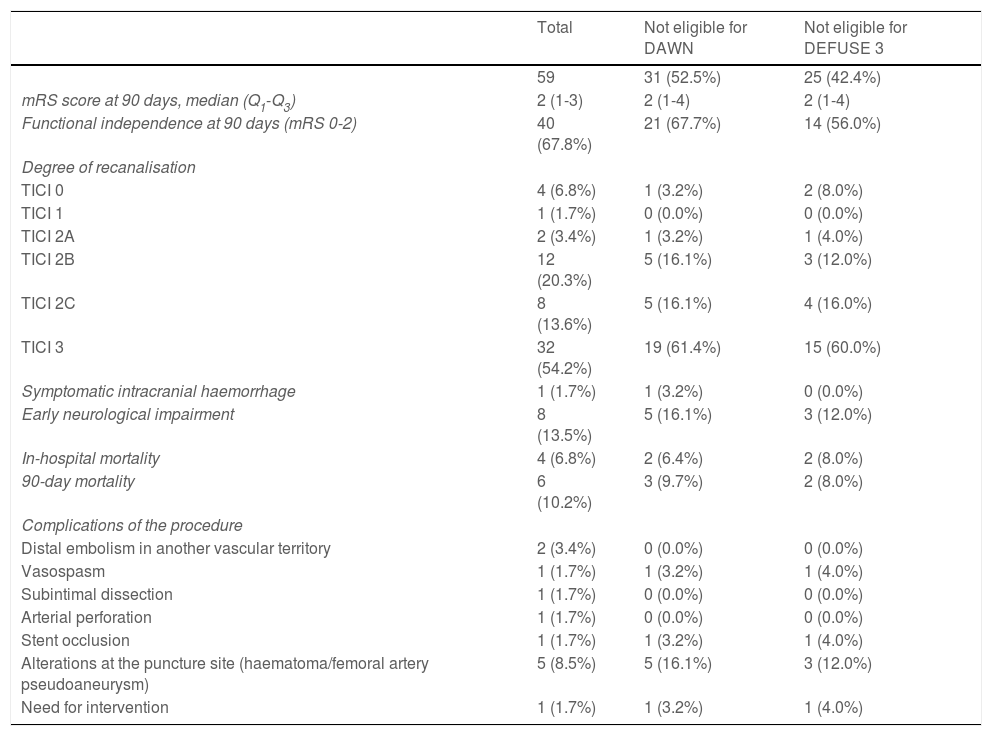
Successful recanalisation was achieved in 52 patients (TICI 2B: 20.3%; TICI 2C: 13.6%; TICI 3: 54.2%). A total of 33 patients (55.9%) underwent general anaesthesia and the remaining 26 (44.1%) underwent conscious sedation. Stent retrievers (Solitaire or pREset) were used in 23.7% of patients, aspiration (ACE 068 system) was performed in 28.8% of patients, and a combination of both techniques was used in 45.8% of cases. A total of 11 patients (18.6%) underwent carotid artery angioplasty; stent placement was required in 6 of these patients. Regarding complications of the procedure, 2 patients (3.4%) presented distal embolism in another vascular territory; one patient (1.7%) presented carotid artery vasospasm, which resolved with nimodipine and was not associated with neurological impairment; another patient presented non-occlusive subintimal carotid artery dissection with no associated neurological symptoms; and another patient displayed laceration of the MCA, resulting in sulcal subarachnoid haemorrhage, with worsening of neurological symptoms. One patient (1.7%) presented carotid stent occlusion, with no clinical consequences, and required reintervention. A total of 5 patients (8.5%) presented alterations at the puncture site: 3 patients presented groin haematoma, one presented occlusion of the ipsilateral popliteal artery, and another patient developed a pseudoaneurysm; only the latter required surgical treatment. Efficacy and safety results are summarised in Table 3.
Efficacy and safety outcomes.
| Total | Not eligible for DAWN | Not eligible for DEFUSE 3 | |
|---|---|---|---|
| 59 | 31 (52.5%) | 25 (42.4%) | |
| mRS score at 90 days, median (Q1-Q3) | 2 (1-3) | 2 (1-4) | 2 (1-4) |
| Functional independence at 90 days (mRS 0-2) | 40 (67.8%) | 21 (67.7%) | 14 (56.0%) |
| Degree of recanalisation | |||
| TICI 0 | 4 (6.8%) | 1 (3.2%) | 2 (8.0%) |
| TICI 1 | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) |
| TICI 2A | 2 (3.4%) | 1 (3.2%) | 1 (4.0%) |
| TICI 2B | 12 (20.3%) | 5 (16.1%) | 3 (12.0%) |
| TICI 2C | 8 (13.6%) | 5 (16.1%) | 4 (16.0%) |
| TICI 3 | 32 (54.2%) | 19 (61.4%) | 15 (60.0%) |
| Symptomatic intracranial haemorrhage | 1 (1.7%) | 1 (3.2%) | 0 (0.0%) |
| Early neurological impairment | 8 (13.5%) | 5 (16.1%) | 3 (12.0%) |
| In-hospital mortality | 4 (6.8%) | 2 (6.4%) | 2 (8.0%) |
| 90-day mortality | 6 (10.2%) | 3 (9.7%) | 2 (8.0%) |
| Complications of the procedure | |||
| Distal embolism in another vascular territory | 2 (3.4%) | 0 (0.0%) | 0 (0.0%) |
| Vasospasm | 1 (1.7%) | 1 (3.2%) | 1 (4.0%) |
| Subintimal dissection | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) |
| Arterial perforation | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) |
| Stent occlusion | 1 (1.7%) | 1 (3.2%) | 1 (4.0%) |
| Alterations at the puncture site (haematoma/femoral artery pseudoaneurysm) | 5 (8.5%) | 5 (16.1%) | 3 (12.0%) |
| Need for intervention | 1 (1.7%) | 1 (3.2%) | 1 (4.0%) |
mRS: modified Rankin Scale; TICI: Thrombolysis in Cerebral Infarction scale.
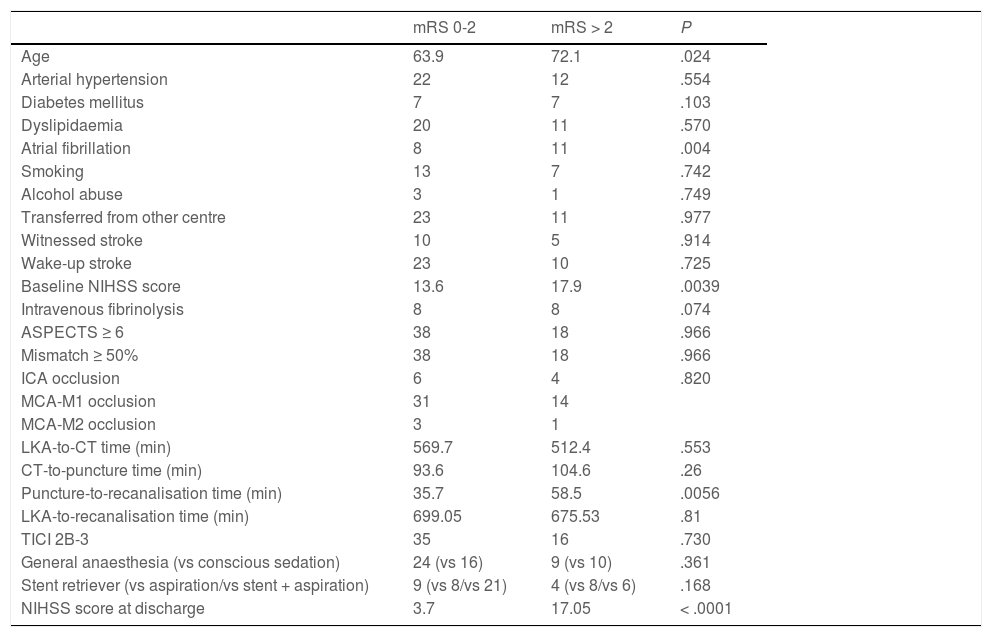
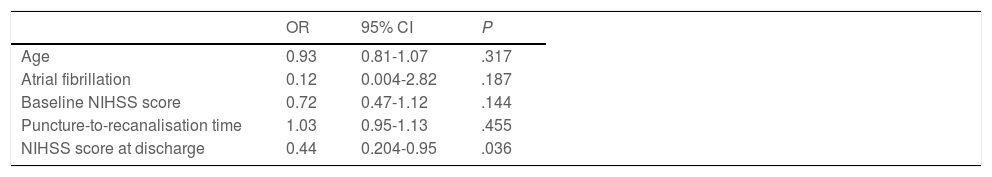
Forty patients (67.8%) were functionally independent at 90 days. Among these were 2 of the 3 patients receiving alteplase beyond 4.5 hours after symptom onset; the third patient presented complications during the procedure (MCA laceration associated with subarachnoid haemorrhage). Only one patient (1.7%) presented symptomatic intracranial haemorrhage after the procedure, and 6 patients (10.2%) died during follow-up. No significant differences in the rates of complications and symptomatic intracranial haemorrhage were observed between patients with and without functional dependence at 90 days. Likewise, no significant differences in functional outcomes were observed between patients undergoing general anaesthesia or conscious sedation (P = .361), between patients undergoing recanalisation with aspiration devices or stent retrievers (P = .168), or between patients who did and those who did not achieve successful recanalisation (P = .73). None of the patients in our series presented malignant cerebral infarction. The variables associated with poorer functional prognosis were age (P = .024), atrial fibrillation (P = .004), high NIHSS scores both at admission (P = .0039) and at discharge (P < .0001), and longer puncture-to-recanalisation time (P = .0056) (Table 4). Logistic regression analysis showed that NIHSS score at discharge was the most powerful prognostic variable (OR: 0.44; 95% CI, 0.204-0.950; P = .036) (Table 5).
Differences in prognostic factors between patients with and without functional dependence at 90 days.
| mRS 0-2 | mRS > 2 | P | |
|---|---|---|---|
| Age | 63.9 | 72.1 | .024 |
| Arterial hypertension | 22 | 12 | .554 |
| Diabetes mellitus | 7 | 7 | .103 |
| Dyslipidaemia | 20 | 11 | .570 |
| Atrial fibrillation | 8 | 11 | .004 |
| Smoking | 13 | 7 | .742 |
| Alcohol abuse | 3 | 1 | .749 |
| Transferred from other centre | 23 | 11 | .977 |
| Witnessed stroke | 10 | 5 | .914 |
| Wake-up stroke | 23 | 10 | .725 |
| Baseline NIHSS score | 13.6 | 17.9 | .0039 |
| Intravenous fibrinolysis | 8 | 8 | .074 |
| ASPECTS ≥ 6 | 38 | 18 | .966 |
| Mismatch ≥ 50% | 38 | 18 | .966 |
| ICA occlusion | 6 | 4 | .820 |
| MCA-M1 occlusion | 31 | 14 | |
| MCA-M2 occlusion | 3 | 1 | |
| LKA-to-CT time (min) | 569.7 | 512.4 | .553 |
| CT-to-puncture time (min) | 93.6 | 104.6 | .26 |
| Puncture-to-recanalisation time (min) | 35.7 | 58.5 | .0056 |
| LKA-to-recanalisation time (min) | 699.05 | 675.53 | .81 |
| TICI 2B-3 | 35 | 16 | .730 |
| General anaesthesia (vs conscious sedation) | 24 (vs 16) | 9 (vs 10) | .361 |
| Stent retriever (vs aspiration/vs stent + aspiration) | 9 (vs 8/vs 21) | 4 (vs 8/vs 6) | .168 |
| NIHSS score at discharge | 3.7 | 17.05 | < .0001 |
ASPECTS: Alberta Stroke Program Early Computed Tomography Score; CT: computed tomography; ICA: internal carotid artery; LKA: last time known to be asymptomatic; MCA: middle cerebral artery; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; TICI: Thrombolysis in Cerebral Infarction scale.
Logistic regression analysis of the variables showing a statistically significant association with poorer functional prognosis.
| OR | 95% CI | P | |
|---|---|---|---|
| Age | 0.93 | 0.81-1.07 | .317 |
| Atrial fibrillation | 0.12 | 0.004-2.82 | .187 |
| Baseline NIHSS score | 0.72 | 0.47-1.12 | .144 |
| Puncture-to-recanalisation time | 1.03 | 0.95-1.13 | .455 |
| NIHSS score at discharge | 0.44 | 0.204-0.95 | .036 |
Regarding stroke aetiology, 18.6% of patients presented atherothrombotic stroke, 37.3% had cardioembolic stroke, 40.7% presented embolic stroke of unknown source, and the remaining 3.4% had stroke of other determined origin. Among patients with cardioembolic stroke, 36.4% had known history of atrial fibrillation. Two of these patients were receiving acenocoumarol and had an INR within the therapeutic range, one patient was receiving low doses of a direct-acting anticoagulant despite normal renal function, one patient was receiving anticoagulation therapy with enoxaparin following recent surgery, another patient was taking acetylsalicylic acid, and 3 patients were not receiving antiplatelet or anticoagulation therapy. The remaining patients with atrial fibrillation were diagnosed with this cardiac alteration during hospitalisation or in subsequent follow-up assessments. One patient presented recurrent atrial flutter, which had previously been treated with ablation. Rare causes of stroke included one case of carotid artery dissection associated with a thrombus in the MCA and another case of amyloidosis associated with multiple myeloma.
DiscussionMechanical thrombectomy is widely used in the treatment of acute ischaemic stroke and large vessel occlusion in the carotid artery territory within 6 hours of stroke onset; this approach is recommended in numerous clinical practice guidelines3,4 and supported by evidence from clinical trials.14–19 Furthermore, meta-analyses including data from these clinical trials have shown that the likelihood of achieving functional independence following stroke decreases with every one-hour delay in groin puncture20,21; this underscores the time-dependent and dynamic nature of stroke. In 2018, however, the DAWN and DEFUSE 3 trials1,2 demonstrated the efficacy of mechanical thrombectomy in patients treated beyond 6 hours after symptom onset, in the context of favourable functional neuroimaging results, which widens the therapeutic window in patients with carotid artery ischaemic stroke. This has given rise to the late window paradox.22
Real-life results from our centre are consistent with findings from the clinical trials and meta-analyses published. Most patients in our series presented wake-up stroke (55.9%); although a small minority of strokes were witnessed (25.4%), this percentage was higher than those reported in the DAWN and DEFUSE 3 trials (10% and 14%, respectively). The rate of successful recanalisation was also higher in our study (88.1%, vs 84% in DAWN and 76% in DEFUSE 3), as was the rate of functional independence at 90 days (67.8%, vs 49% in DAWN and 45% in DEFUSE 3).
Although randomised clinical trials are regarded as the gold standard of clinical evidence, applicability of their results in clinical practice may be limited by excessively restrictive inclusion criteria. In our series, approximately half of patients would not have been eligible for endovascular treatment according to the inclusion criteria of the DAWN and DEFUSE 3 trials. However, our rates of successful recanalisation and functional independence are similar to those reported by these trials. Our results are also consistent with those of previous studies into real-life experience with mechanical thrombectomy in the context of wider time windows for patients not meeting trial inclusion criteria.23–28
Mechanical thrombectomy at 6-24 hours after symptom onset has been found to be cost-effective in patients with large vessel occlusion in the carotid artery territory,29 which has given rise to changes in emergency acute stroke care protocols. More than half of our patients were transferred from other hospitals, although this did not result in higher rates of complications or functional disability. We therefore believe that patients with favourable neuroimaging findings can benefit from mechanical thrombectomy at a reference stroke unit despite delays in performing the procedure.30 In this context, advanced neuroimaging techniques, particularly perfusion neuroimaging, have been shown to be useful as they provide relevant information on the extension of potentially salvageable tissue and therefore on whether the patient may benefit from reperfusion therapy.10,31–33 However, optimal recanalisation does not always result in satisfactory functional outcomes; this is known as futile recanalisation.34 In our study, no significant differences were observed in the rate of successful recanalisation between patients who did and who did not achieve functional independence at 90 days. However, patients scoring ≥ 2 points on the mRS were older, scored higher on the NIHSS both at admission and at discharge, and presented longer puncture-to-recanalisation times; these variables have been associated with higher risk of futile recanalisation.35,36 Low ASPECTS has also been regarded as a predictor of futile recanalisation.37 However, in our series, no significant differences were observed in neuroimaging findings between patients who were functionally independent at 90 days and those who were not. In fact, 2 of the 3 patients with an ASPECTS of 5 were functionally independent at 90 days, and the remaining patient scored 3 points on the mRS.
Regarding technical aspects, clinical practice guidelines for mechanical thrombectomy recommend using stent retrievers.4 In our series, nearly half of patients underwent mechanical thrombectomy with combined use of a stent retriever and aspiration; this did not affect safety or functional outcomes. Previous studies including samples where nearly half of patients did not meet the DAWN and DEFUSE 3 selection criteria report high rates of successful recanalisation with manual aspiration exclusively, with no significant differences in the rates of functional independence or symptomatic intracranial haemorrhage.28 With regard to anaesthesia, some studies suggest that conscious sedation may be associated with shorter puncture-to-reperfusion times and greater likelihood of functional independence.38 In our series, general anaesthesia and conscious sedation were used in similar percentages of patients, with no significant differences in functional outcomes between groups.
In patients presenting symptoms of over 24 hours’ progression, insufficient evidence is available to support the effectiveness and safety of mechanical thrombectomy for carotid artery territory occlusion; while some studies show comparable results to those of the DAWN trial, using similar inclusion criteria with the exception of symptom progression time,39 others report no clinical benefit.28 In our sample, 5 patients underwent mechanical thrombectomy beyond 24 hours after the last time they were known to be asymptomatic, with 3 achieving functional independence at 90 days, going beyond the late window paradox.
Intravenous thrombolysis is also claiming its place in this late window scenario. The EXTEND trial40 demonstrated the efficacy and safety of this treatment when administered within 9 hours of symptom onset in patients with favourable perfusion imaging profiles. In our series, 3 patients with favourable penumbra profiles received alteplase beyond 4.5 hours after symptom onset. The only patient who was not functionally independent at 90 days experienced complications during the procedure that had an impact on clinical status. However, this subgroup is very small, preventing us from drawing robust conclusions; in any case, this was not the main objective of the study.
Our study has several limitations, including its retrospective, single-centre design and the relatively small size of the sample. Furthermore, progression times may have been shorter than estimated in some cases classified as unwitnessed stroke; this possibility has been pointed out in previous studies.1,2,39 In any case, our low rate of severe adverse events suggests that mechanical thrombectomy is safe beyond 6 hours after symptom onset.
ConclusionIn our experience, patients with acute ischaemic stroke secondary to large vessel occlusion in the carotid artery territory may benefit from endovascular treatment within 24 hours after the last time they were known to be asymptomatic (this goes beyond the inclusion criteria of clinical trials) if they meet specific functional neuroimaging requirements. This underscores the critical role of functional neuroimaging in assessing patient eligibility in cases of long progression time or unknown symptom onset. In these cases, the procedure is associated with good safety and functional outcomes. Uncertainty remains regarding patients not meeting the eligibility criteria established in the currently available guidelines, such as those with longer symptom progression times (> 24 hours) or with low ASPECTS (but with favourable functional neuroimaging profiles); this constitutes a promising line for future research. Although the late window approach has revolutionised the field of revascularisation therapy for acute ischaemic stroke, additional randomised clinical trials are needed to confirm these findings and to establish the optimal time window for mechanical thrombectomy.
Conflicts of interestThe authors have no conflicts of interest to declare.