This study was performed with the purpose of analysing the relationship between epileptological and surgical variables and post-operative memory performance, following surgery for refractory mesial temporal lobe epilepsy (MTLE) due to hippocampal sclerosis (HS).
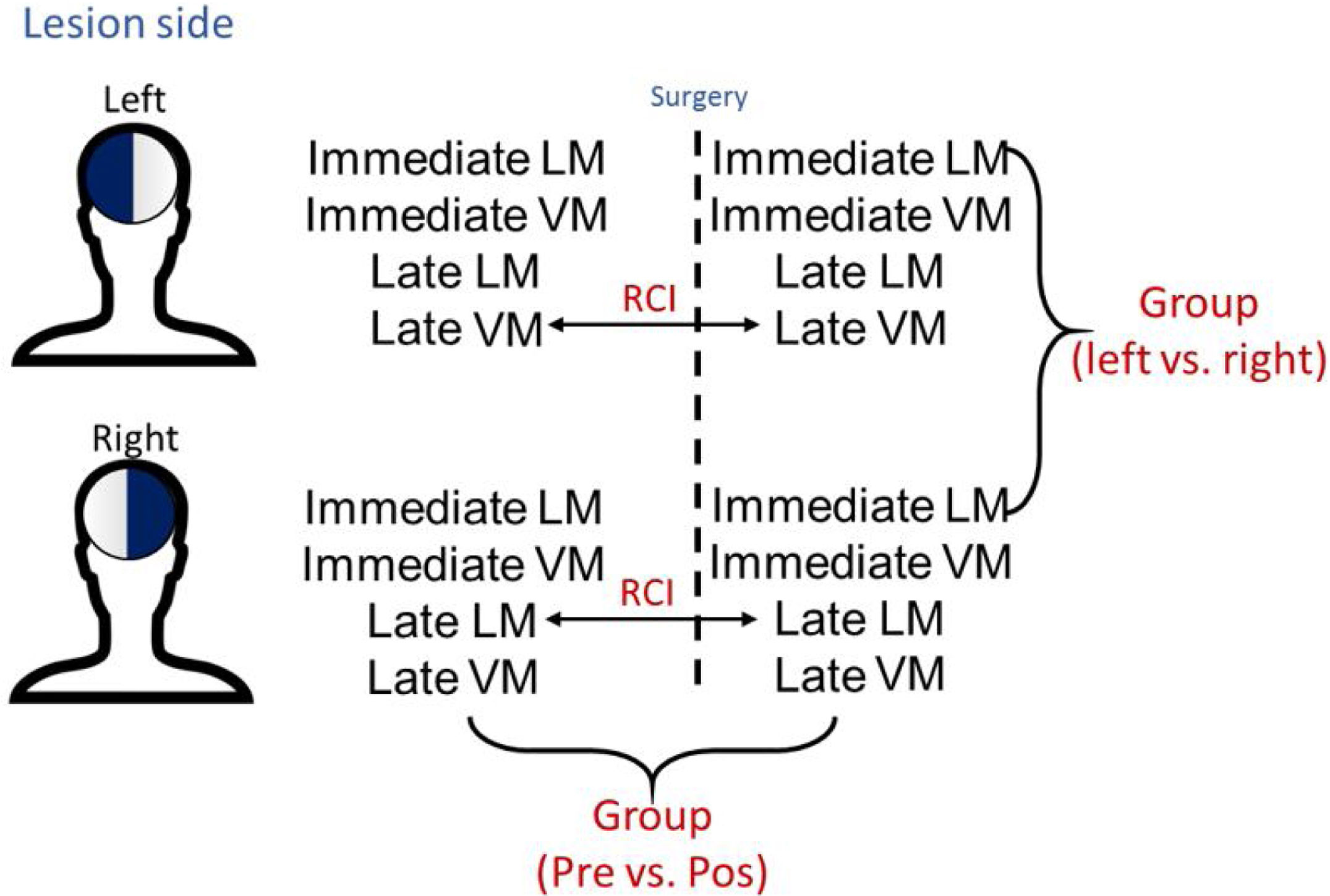
MethodsLogical memory (LM) and visual memory (VM) scores for immediate and late follow-up of 201 patients operated for MTLE/HS were reviewed. Scores were standardized with a control group of 54 healthy individuals matched for age and education. The Reliable Change Index (RCI) was calculated to verify individual memory changes for late LM and VM scores. A multiple linear regression analysis was carried out with the RCI, using LM and VM scores as well as the clinical variables.
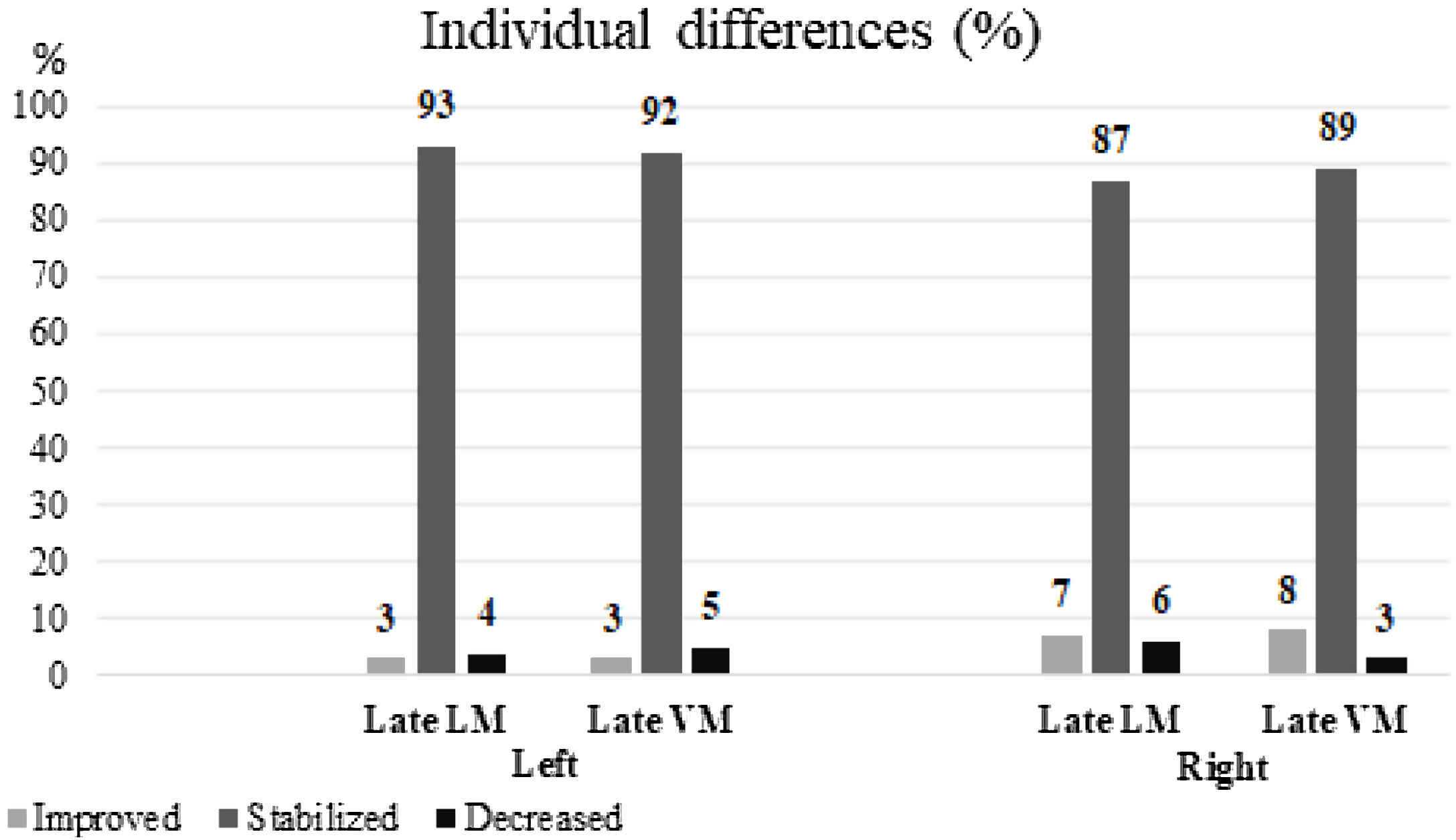
ResultsA total of 112 (56%) patients had right HS. The RCI of the right HS group demonstrated that 6 (7%) patients showed improvement while 5 (6%) patients showed decreased scores in late LM; for late VM, 7 (8%) patients presented improvement, and 2 (3%) patients showed poorer scores. RCI of the left HS group showed that 3 (3%) individuals showed improved scores, while scores of 5 (4%) patients worsened for late LM; for late VM, 3 (3%) patients presented higher scores and 6 (5%) showed lower scores. Left HS and advanced age at onset of the first epileptic seizure were predictors of late LM loss (p<.05).
ConclusionLeft MTLE/HS and seizure onset at advanced ages were predictive factors for the worsening of late LM. We observed poorer baseline LM function in the left HS group and improvement of LM in some patients who had resection of the right MTL. Patients in the right HS group showed a higher percentage of reliable post-operative improvement for both VM and LM scores.
Este estudio analiza la relación entre las variables epileptológicas y quirúrgicas y el rendimiento de la memoria tras el tratamiento quirúrgico del síndrome de epilepsia temporal medial con esclerosis del hipocampo (ETM-EH).
MétodosRevisamos las puntuaciones en memoria lógica (ML) y visual (MV) obtenidas por 201 pacientes operados por ETM-EH durante el postoperatorio inmediato y tardío. Dichas puntuaciones se estandarizaron con un grupo control de 54 individuos sanos de edad y nivel educativo similares. Aplicamos el índice de cambio fiable (ICF) para comprobar si existían cambios individuales en las puntuaciones en ML y MV durante el postoperatorio tardío. Para el análisis de regresión lineal múltiple, utilizamos el ICF e incluimos las puntuaciones en ML y MV así como las variables clínicas.
ResultadosUn total de 112 (56%) pacientes presentaban esclerosis del hipocampo (EH) derecho. En el grupo con EH derecho, el ICF mostró que 6 (7%) pacientes presentaron mejoría mientras que 5 (6%) pacientes obtuvieron una peor puntuación en ML durante el postoperatorio tardío; en el caso de la MV, 7 (8%) pacientes obtuvieron mejores puntuaciones y 2 (3%) tuvieron peores durante el postoperatorio tardío. En el grupo con EH izquierdo, 3 (3%) pacientes presentaron una mejoría en la puntuación en ML mientras que 5 (4%) pacientes obtuvieron peores puntuaciones; en el caso de la MV, las puntuaciones mejoraron en 3 (3%) pacientes y empeoraron en 6 (5%). EH izquierdo y una edad avanzada al inicio de la primera crisis epiléptica predijeron una pérdida de ML en el postoperatorio tardío (p<0,05).
ConclusiónEl síndrome de ETM-EH izquierdo y una edad avanzada al inicio de las crisis fueron factores predictores de una peor ML en el postoperatorio tardío. Observamos un decremento en el rendimiento inicial de la ML en el grupo de EH izquierdo y una mejor ML en algunos pacientes sometidos a resección del lóbulo temporal medial derecho. Los pacientes del grupo de EH derecho presentaron un porcentaje mayor de mejoría en las puntuaciones en MV y ML tras la cirugía.
Mesial temporal lobe epilepsy, frequently due to hippocampal sclerosis (MTLE/HS), is the most prevalent epileptic syndrome which is both refractory to medication and surgically-remediable.1,2 Despite high rates of seizure control, resections of the mesial temporal structures visibly impinge upon memory circuits, and therefore post-operative memory decline is a major concern.2
Neuropsychological assessment, combined with a clinical interview, has the potential to identify an individual's basal cognitive functioning, the hemispheric lateralization of cognitive dysfunction, and the functional performance of the region to be removed.3 Also, when an evaluation is performed pre- and post-operatively, the impact of the procedure on an individual's cognition can be assessed.3 However, despite numerous studies, recognizing patients at a higher risk of significant memory decline following resection is still difficult.4 For instance, although there is enough evidence linking left lateralization of the disease and verbal logical memory, it is difficult to determine on an individual basis, the risk of significant decline, even taking into consideration the presence of HS and preoperative functionality.4,5 Thus, a degree of uncertainty often compromises decisions on the best surgical strategy, and research is certainly needed on prognostic factors for decline.6
Predictive factors for post-operative memory changes vary widely among studies and include surgical technique, age at surgery, preoperative cognitive status, and seizure freedom.7 Selective resection of mesial structures in patients who already have significant memory abnormalities, being seizure-free, and early age of surgery appear to be protective factors against a significant decline.7–10 However, these findings are not universal7,11 and such discrepancies prevent firm decision-making on an individual basis. The main reasons for heterogeneous results were the fact that most studies recruited patients with a broad range of pathologies impinging upon different structures of the MTL and employed different methodologies to identify post-operative changes in memory.12 The Reliable Change Index (RCI) is considered the gold standard to evaluate cognitive alterations after any kind of intervention. This method is efficient in determining the differences in practice, learning, and measurement errors in the postoperative evaluation.13 Herein, this paper presents the correlation of a large number of clinical, epileptological and surgical variables with reliable memory changes after one to five years following resection of a single, homogeneous pathology in the MTL. Restricting the analyses to patients with HS, the most prevalent subtype of MTLE, allowed the authors to provide a more reliable perspective of predictors for changes in the memory performance.
MethodsSubjectsData were collected from medical records of 201 patients in the age range of 16–60 years, evaluated and operated on for MTLE/HS at the Epilepsy Surgery Program of Hospital São Lucas of Pontifícia Universidade Católica do Rio Grande do Sul, between 1996 and 2016. MRI findings suggestive of HS were independently confirmed by an experienced neuroradiologist and by the surgical team. Individuals were then subdivided into two groups according to the hippocampal sclerosis lateralization (left or right) and then also by the type of surgery: anterior temporal lobectomy (ATL) or selective amygdalohypocampectomy (SAH). The type of surgery was determined according to the partient's extent and type of injury on the MRI scan. As this is a study carried out with medical records, all researchers signed a data usage agreement. We used the recommendations published by Witt and Helmstaedter for a neuropsychological retest in the surgical context of epilepsy.14
Data collectionSociodemographic, clinical, and neuropsychological data were collected for analysis. The Engel scale15 was utilized after surgery to evaluate the impact of the surgical procedure on the frequency of seizures. All individuals underwent extensive neuropsychological testing, including the Wechsler Memory Scale – Revised,16 before and after the neurosurgical procedure within a 5-year interval. Memory scores of each individual were collected utilizing the logical memory and visual memory recall tests (LM and VM, respectively). In the LM test, two stories were read to the patient and the patient was asked to recall the reading immediately (iLM) and 30min later (lLM). The VM test comprises four cards with geometric figures presented for 10s each – one at a time. The patients were then asked to draw, after each presentation, whatever they remembered of the images immediately (iVM) and 30min after the presentation, corresponding to the late score (lVM). The survey was then carried out according to instructions.
Statistical analysisBoth group and individual analyses were performed in this study. Parametric test (t-test paired) were used to compare the socio-demographic and memory scores between the groups and within the groups before and after surgery (Fig. 1). The individual analyses were performed as per the Reliable Change Index (RCI), a method developed by Jacobsen and Traux13,17 and later modified by other psychometrists18–21 (Fig. 1). For calculations, the control population of 54 healthy individuals from the WMS-R16,22 manual was used to normalize scores in standard deviation (SD), the deficit was estimated using a value equal to or less than −1 SD.18,20,21 For RCI, only late LM and late LV data were used, considering the importance of temporal mesial structures for this type of function.23 The results of the RCI are presented as percentages according to a previous study,20 classified with a confidence interval of 90%: considering an improvement (RCI>+1.645), stability (−1.645<RCI<1.645), or worsening (RCI<−1.645) of function. Multiple linear regressions were also performed to find out associations of memory changes. All statistical analyses were performed with the RStudio program (v1.0.136),24 and p <0.05 was considered statistically significant. All patients had the same underlying disease and this index would be used to decrease the possibilities of biases and measurement errors concerning memory change.
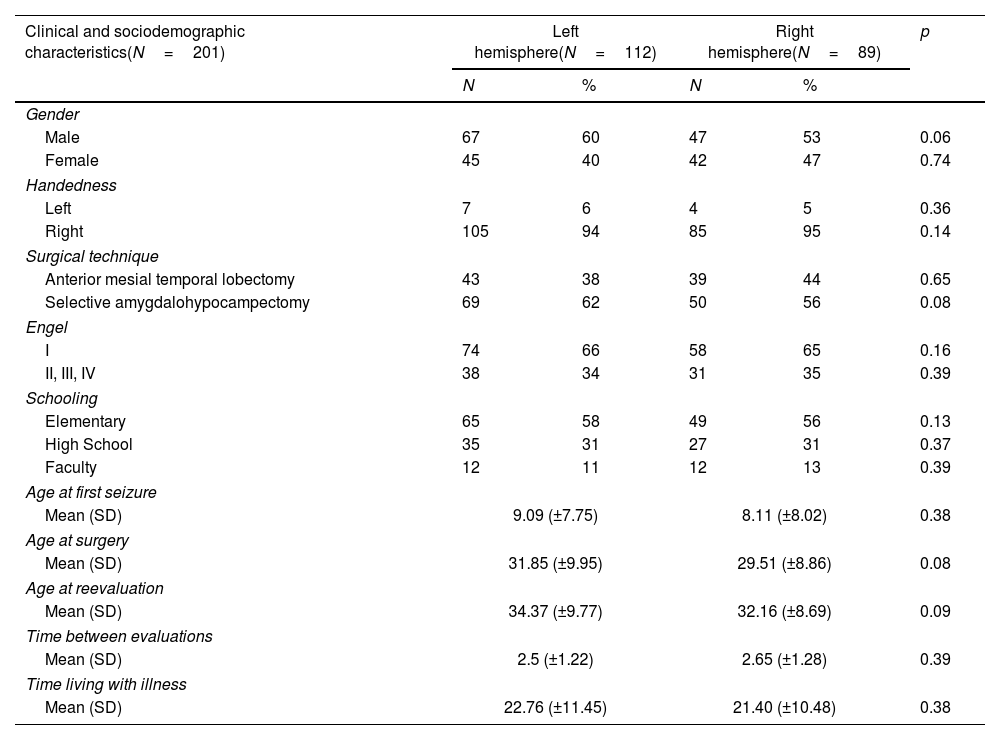
ResultsSample characteristicsBoth left HS and right HS groups shared similar socio-demographic characteristics according to age, sex, and education (Table 1). The left HS group showed a lower baseline in late LM scores as compared to the right HS group in the pre-operative stage (13.06±8.10 vs. 16.03±7.9), p<0.01) (Table 2). Regarding late VM, no significant differences were found (p>0.05). Both groups shared similar levels of education, time for the first seizure and time for living with the disease (p>0.05 for all measures) (Table 1).
Sociodemographic, clinical, and neuropsychological characteristics of the patients.
| Clinical and sociodemographic characteristics(N=201) | Left hemisphere(N=112) | Right hemisphere(N=89) | p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender | |||||
| Male | 67 | 60 | 47 | 53 | 0.06 |
| Female | 45 | 40 | 42 | 47 | 0.74 |
| Handedness | |||||
| Left | 7 | 6 | 4 | 5 | 0.36 |
| Right | 105 | 94 | 85 | 95 | 0.14 |
| Surgical technique | |||||
| Anterior mesial temporal lobectomy | 43 | 38 | 39 | 44 | 0.65 |
| Selective amygdalohypocampectomy | 69 | 62 | 50 | 56 | 0.08 |
| Engel | |||||
| I | 74 | 66 | 58 | 65 | 0.16 |
| II, III, IV | 38 | 34 | 31 | 35 | 0.39 |
| Schooling | |||||
| Elementary | 65 | 58 | 49 | 56 | 0.13 |
| High School | 35 | 31 | 27 | 31 | 0.37 |
| Faculty | 12 | 11 | 12 | 13 | 0.39 |
| Age at first seizure | |||||
| Mean (SD) | 9.09 (±7.75) | 8.11 (±8.02) | 0.38 | ||
| Age at surgery | |||||
| Mean (SD) | 31.85 (±9.95) | 29.51 (±8.86) | 0.08 | ||
| Age at reevaluation | |||||
| Mean (SD) | 34.37 (±9.77) | 32.16 (±8.69) | 0.09 | ||
| Time between evaluations | |||||
| Mean (SD) | 2.5 (±1.22) | 2.65 (±1.28) | 0.39 | ||
| Time living with illness | |||||
| Mean (SD) | 22.76 (±11.45) | 21.40 (±10.48) | 0.38 | ||
N: sample; SD: standard deviation; p: p-value.
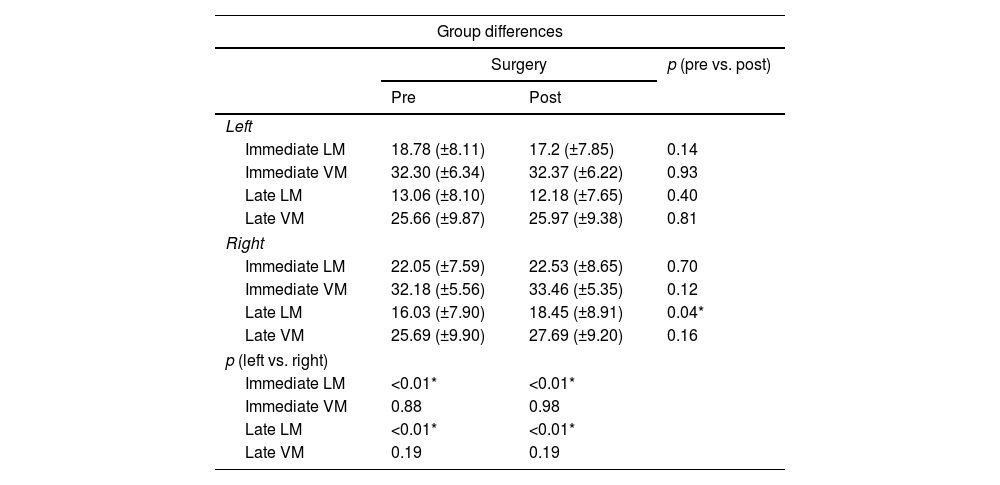
Neuropsychological results and the comparison between the performance of the groups.
| Group differences | |||
|---|---|---|---|
| Surgery | p (pre vs. post) | ||
| Pre | Post | ||
| Left | |||
| Immediate LM | 18.78 (±8.11) | 17.2 (±7.85) | 0.14 |
| Immediate VM | 32.30 (±6.34) | 32.37 (±6.22) | 0.93 |
| Late LM | 13.06 (±8.10) | 12.18 (±7.65) | 0.40 |
| Late VM | 25.66 (±9.87) | 25.97 (±9.38) | 0.81 |
| Right | |||
| Immediate LM | 22.05 (±7.59) | 22.53 (±8.65) | 0.70 |
| Immediate VM | 32.18 (±5.56) | 33.46 (±5.35) | 0.12 |
| Late LM | 16.03 (±7.90) | 18.45 (±8.91) | 0.04* |
| Late VM | 25.69 (±9.90) | 27.69 (±9.20) | 0.16 |
| p (left vs. right) | |||
| Immediate LM | <0.01* | <0.01* | |
| Immediate VM | 0.88 | 0.98 | |
| Late LM | <0.01* | <0.01* | |
| Late VM | 0.19 | 0.19 | |
LM: logical memory; VM: visual memory; Pre: preoperative; Post: postoperative; vs.: versus; p: p-value.
Both groups shared similar types of surgery, Engel scores and time for reevaluation (p>0.05 for all measures) (Table 1). As compared to the left HS group, the right HS group showed significantly higher scores in the immediate LM test in the baseline (22.05±7.59 vs. 18.78±8.11, p<0.01) and after surgery (22.53±8.65 vs. 17.2±7.85, p<0.01). The right HS group also showed increased late LM scores compared to the left HS subjects before (16.03±7.9 vs. 13.06±8.10, p<0.01) and after surgery (18.45±8.91 vs. 12.1±7.65, p<0.01) (Table 2 and Fig. 1). However, the VM scores did not differ between groups, either before or after the surgical procedure (p>0.05 for immediate VM and late VM measures).
Regarding within-group differences, the right HS group showed a borderline increase in late LM scores, when the scores before and after surgery (16.03±7.9 vs. 18.45±8.91, p=0.04) were compared. All other LM and VM scores did not show a significant difference in the within-group analysis, when before and after surgery scores were compared either for left HS or right HS groups (p>0.05 in all other measures) (Table 2).
In the left HS group, decreased scores were observed for late LM in 55 (49%) subjects during baseline and 61 (54%) subjects on follow-up as compared to the normative data from the population. Besides, decreased late VM scores were seen in 37 (33%) individuals during baseline and in 37 (33%) subjects on follow-up. In patients with right HS, when comparing the scores of this group of patients with a healthy control population, 40 (45%) individuals presented a poorer performance as compared to late LM in the previous evaluation and 30 (34%) individuals in the posterior evaluation. Considering late VM, the deficits were observed in 31 (35%) subjects in the pre-operative testing and 21 (23%) subjects in the post-operative testing.
Individual memory changesRCI of the late LM scores of the left HS group revealed that the majority of individuals were stable after surgery (104 subjects, 93%); while 3 (3%) subjects showed an improvement, 5 (4%) subjects showed worsening of symptoms. For the late VM scores of this group, the majority of individuals were found to be stable after surgery (103 subjects, 92%), although 3 showed improvement and 6 showed worsening of symptoms during this period (3% and 5%, respectively) (Fig. 2).
Using the method of RCI, it was evidenced on the right HS that approximately 6 (7%) of all the patients presented improvement, 78 (87%) patients showed stability, and 5 (6%) patients presented with worsened symptoms in the late LM scores. In the same group, 7 patients (8%) showed an improvement, 80 (89%) patients showed stability, and 2 (3%) patients showed worsened conditions concerning late VM (Fig. 2).
Multiple linear regression analysis for memoryInitially, a univariate linear regression was executed to identify the most suitable variables for the model. Subsequently, multiple linear regression was calculated. The linear model showed a significant positive relationship between RCI of the late LM and the left hemisphere that underwent surgery (p=0.01), and a significant negative negative relationship between RCI of the late LM and the age of onset of seizures (p=0.04) in a model that also accounted for sex, handedness, Engel scores, surgical technique and education (overall model R-squared and p). Concerning late VM no factor, among all studied obtained statistical significance to be defined as a predictor for RCI.
Late logical memoryThe predictive factors for reliable change of late LM included the left operated hemisphere and age of onset of seizures.
Late visual memoryNo factor, among all studied, obtained statistical significance to be defined as a predictor for reliable change of late visual memory.
DiscussionWhen we divided our sample according to the lateralization of the disease and did not find statistically significant differences between the sociodemographic variables, we considered a paired sample with greater reliability in the identification of neuropsychological outcomes, as socio-educational factors, for example, could interfere with baseline cognitive and memory outcomes, as previously described.25 This division was based on a large number of studies and scientific consensus that each cerebral hemisphere is responsible for specific primary brain functions, especially for distinct memory functions.26,27
The first main finding of the present study is: the patients with an epileptogenic focus in the left hemisphere were found to show significantly lower preoperative and postoperative scores for immediate and late LM, compared to the group of patients with a focus in the right hemisphere. According to the literature, this finding reinforces the knowledge that most right-handed individuals have cerebral hemispheric dominance for LM and language functions in the left brain.28 The study sample in the present research work was substantially constituted by right-handed individuals, which supports this phenomenon. On the other hand, patients with the disease on the right side did not present deficient baseline results for VM, which shows either a lower hemispheric dominance for this type of function or the importance of non-mesial-temporal structures, coinciding with the results of a previous study.27,29 It is noteworthy that right-handed and left-handed patients are included with no knowledge of the language domain of the latter group, which is a limitation of this study.
The second important finding in this research is that the group of patients who underwent resection in the right temporal lobe showed a statistically significant increase in the late LM scores, typically associated with left hemisphere,30 after surgery. This indicates an improvement in the contralateral cognition opposite to that of the resected hippocampus. However, patients who operated for the left hemisphere did not present a statistically significant improvement in either of the two types of memory evaluation performed in this study. Alpherts et al.,27 in a long-term investigation on memory course in patients with MTL epilepsy, found similar results. In their study, the patients who were operated on for the disease in the right hemisphere showed an improvement in memory within a short time, although this improvement did not last for six years of follow-up. However, the decline observed in these patients who underwent resection of the left temporal lobe progressed only in the first two years after resection; later, the memory was stabilized. This result was confirmed by another study, in which the memory decline occurred only in the first two years after surgery, and a re-evaluation after ten years showed a cognitive stability.31 The mean evaluation time in the present study, between the pre- and post-operative periods, was two and a half years. Following the reasoning of these studies,31,32 it can be assumed that the course of memory concerning the exposed changes could also be attenuated. Nevertheless, this hypothesis could only be reliably confirmed from a reassessment of memory in further follow-ups.
The RCI method is understood by psychometrics, as the most reliable one to measure real changes after an intervention.19 An evident improvement for both late LM and VM in some patients belonging to the right hippocampal sclerosis group was observed, with a minor decline in late VM scores. Similar results were also presented by Shah,33 who used the same method in Indian patients with similar mesial temporal pathology as the sample in the present study: the individuals presented a significant improvement in LM when operated on the right hemisphere. Gul34 demonstrated that the group of patients with right hippocampal sclerosis also showed a statistically significant improvement in VM; however, these findings were not replicated in the present study. However, according to RCI, the proportion of patients who underwent neurosurgery for right hippocampal sclerosis and obtained a reliable improvement in late LM and VM scores was larger in relation to the number of cases that worsened. This phenomenon transpired differently in patients who were operated for the LH: the percentage of worsening symptoms for both LM and VM was slightly higher than that of improvements.
The third important finding was that left hippocampal sclerosis and the age of onset of the first seizure were important predictors for reliable changes in LM after surgery. Patients who underwent left hemisphere surgery showed a lower rate of reliable change, that is, a tendency of decline in memory, which is already widely described in international literature specializing on the subject and can now be reproduced for the first time within the Brazilian population. Two studies performed on patients who underwent surgery for MTLE demonstrated that age at the time of procedure is a predictor of cognitive change: the younger the individual, the better the prognosis to the course of memory.4,35 However, these results were not evidenced in the present research, not even during the time at which the individual lived with the disease before the neurosurgical procedure. However, the age of onset of the first seizure was a predictive factor: this occurrence could be explained by the theory of neural plasticity and cerebral reorganization,36 since a younger brain would have a greater potential for the reorganization of cognitive functions when exposed to a situation of disorganization for the first time. Thus, the lower the age of onset of seizures, the higher the score toward reliable memory improvement is seen. Other determinants already known by literature about the postsurgical neuropsychological outcome are the functional integrity of the resected tissues37; the reserve capacities and functional plasticity of the brain, in this case, the contralateral temporomesial structures in particular38; the postsurgical seizure outcome8 and the decrease of the antiepileptic drug load and withdraw of antiepileptic agents with unfavorable cognitive side effects can also enhance the postsurgical cognitive status.39
Approximately 65% of the patients in this study remained free of seizures at the time after re-evaluation. The findings of the present study coincide with the results of Helmstaedter,8 in which 63% of the studied individuals had Engel I scores after the surgical procedure was performed to treat MTL epilepsy. However, unlike the findings of this author, the present study showed that being free of seizures is not a predictor for reliable improvement in memory. Similarly, other investigations31,32 also point in the same direction as we observed. Alvim et al.40 demonstrated, in a structural neuroimaging study, that even after neurosurgery performed for the removal of epileptogenic focus, atrophy and progressive loss of gray matter existed, indicating that a mechanism underlying the pathology could be responsible for the decrease in brain volume, even in patients free of seizures and in absence of cognitive decline. The investigations suggest that the occurrence of the epileptic spectrum continues even after the seizures have been eradicated.40,41
Since this is a retrospective cohort study, some limitations were observe, such as the loss of participants due to inadequate information on neuropsychological test protocols: out of 413 verified protocols, only 201 could be included. No parallel versions of memory tests were used, which is considered an important restriction in the interpretation of the results. Further, the re-evaluation of memory was done in a short time (on average 2½ years) and the follow-up period involves up to 5 years, in which changes toward the mean may occur. In terms of future perspectives, long term follow-up of patients operated, as well as the use of RCI to verify other cognitive functions in patients with mesial temporal lobe epilepsy and other types of epilepsy must be considered.
ConclusionThis study indicates the left hemispheric dominance for the functions of late logical memory in patients with refractory MTLE, with HS as the underlying disease. Also, a statistically significant improvement in both immediate LM and late LM, in a group of patients who underwent resection for the epileptogenic focus on the right hemisphere, was observed. Patients with right hippocampal sclerosis had a higher percentage of reliable improvement in both, VM and LM scores. The epileptogenic focus in the left hemisphere and late-onset age of the first seizure was found to be predictive factors for a reliable worsening of LM. Thus, surgical treatment can be understood as an extremely effective alternative in MTLE patients with HS, since most of them become free of disabling seizures after the procedure and only a few experience changes in memory. The prognosis is even more positive when the disease occurs in the right cerebral hemisphere, where the functions of logical memory tend to be better in the first year after surgery.
Ethical considerationsThis research complies with all the norms established by Law 466/2012 regarding Human Studies, according to the opinions issued by the Research Ethics Committees from Universidade Federal do Rio Grande do Sul (UFRGS) and PontifíciaL Universidade Católica do Rio Grande do Sul (PUCRS), respectively under the numbers: 2,471,665 and 2,492,372. The authors also state that this research was conducted in accordance with the principles of the World Medical Association Declaration of Helsinki.
Authors’ contributionsDesign, data collection, management, analysis and interpretation of the data, as well as preparation, review and approval of the manuscript were under the control and responsibility of the authors.
Conflict of interestNone of the authors have relationships that might lead to a perceived conflict of interest.
The authors thank all the professionals who work in the Epilepsy Surgery Program of the São Lucas Hospital of PUCRS and the statistical advice of the same institution. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil – CAPES (Coordination of Improvement of Higher Education Personnel) – Finance Code 001.