Numerous invasive and non-invasive neuromodulation devices have been developed and applied to patients with headache and neuralgia in recent years. However, no updated review addresses their safety and efficacy, and no healthcare institution has issued specific recommendations on their use for these 2 conditions.
MethodsNeurologists from the Spanish Society of Neurology’s (SEN) Headache Study Group and neurosurgeons specialising in functional neurosurgery, selected by the Spanish Society of Neurosurgery (SENEC), performed a comprehensive review of articles on the MEDLINE database addressing the use of the technique in patients with headache and neuralgia.
ResultsWe present an updated review and establish the first set of consensus recommendations of the SEN and SENEC on the use of neuromodulation to treat headache and neuralgia, analysing the current levels of evidence on its effectiveness for each specific condition.
ConclusionsCurrent evidence supports the indication of neuromodulation techniques for patients with refractory headache and neuralgia (especially migraine, cluster headache, and trigeminal neuralgia) selected by neurologists and headache specialists, after pharmacological treatment options are exhausted. Furthermore, we recommend that invasive neuromodulation be debated by multidisciplinary committees, and that the procedure be performed by teams of neurosurgeons specialising in functional neurosurgery, with acceptable rates of morbidity and mortality.
En los últimos años han surgido numerosos dispositivos de neuromodulación, invasivos y no invasivos, que se han aplicado en pacientes con cefaleas y neuralgias sin que exista una revisión actualizada de su eficacia y seguridad, ni recomendaciones de ninguna institución sanitaria sobre su uso específico en cada entidad nosológica.
MétodosNeurólogos del Grupo de Cefaleas de la Sociedad Española de Neurología (SEN) y neurocirujanos expertos en neurocirugía funcional seleccionados por la Sociedad Española de Neurocirugía (SENEC), hemos realizado una revisión exhaustiva en el sistema Medline sobre neuromodulación en cefaleas y neuralgias.
ResultadosPresentamos una revisión actualizada y establecemos por primera vez unas recomendaciones consensuadas entre la SEN y la SENEC sobre el uso de la neuromodulación en cefaleas y neuralgias, adjudicando niveles de evidencia sobre su eficacia actual, específicamente en cada entidad nosológica.
ConclusionesLos resultados actuales de los estudios proporcionan evidencias para la indicación de técnicas de neuromodulación en casos refractarios de cefaleas y neuralgias (sobre todo en migraña, cefalea en racimos y neuralgia del trigémino), seleccionados por neurólogos expertos en cefaleas, tras comprobar el agotamiento de las opciones farmacológicas. Adicionalmente, en el caso de la neuromodulación invasiva, se recomienda que los casos sean debatidos en comités multidisciplinarios y la cirugía sea realizada por equipos de neurocirujanos expertos en neurocirugía funcional y con una morbimortalidad aceptable.
Numerous neuromodulation devices have been developed in recent years that act on the central and peripheral nervous systems to treat headache and craniofacial neuralgia refractory to pharmacological treatment. The underlying principle in neuromodulation is the blockade or controlled, reversible modification of the nociceptive system by stimulating peripheral nerves, the vagus nerve, the cervical spinal cord, or cortical or deep brain regions.
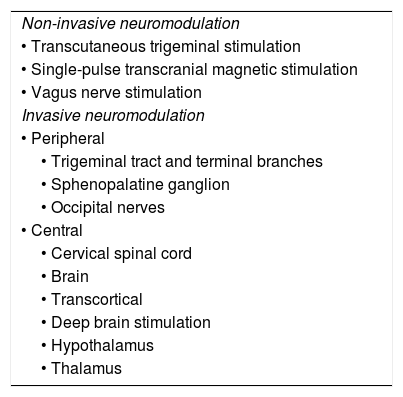
Some devices allow self-administration with external applicators (non-invasive) whereas others require surgical implantation (invasive) (Table 1). These guidelines are therefore divided into 2 main sections:
- •
Non-invasive neuromodulation
- •
Invasive neuromodulation
Types of neuromodulation techniques.
| Non-invasive neuromodulation |
| • Transcutaneous trigeminal stimulation |
| • Single-pulse transcranial magnetic stimulation |
| • Vagus nerve stimulation |
| Invasive neuromodulation |
| • Peripheral |
| • Trigeminal tract and terminal branches |
| • Sphenopalatine ganglion |
| • Occipital nerves |
| • Central |
| • Cervical spinal cord |
| • Brain |
| • Transcortical |
| • Deep brain stimulation |
| • Hypothalamus |
| • Thalamus |
Invasive neuromodulation involves the use of complex technologies, whose management, implantation, and monitoring requires a high level of specialisation in neurology and neurosurgery. Furthermore, given the very limited availability of these devices due to their high cost, many professionals are not familiar with them.
In the light of evidence from multiple studies demonstrating the safety and effectiveness of neuromodulation, there is a need for an official position on the recommendation and use of these techniques; however, it should be noted that indication of neuromodulation must always be established by neurologists specialising in headache and neuralgia.
ObjectivesThese guidelines, the first to address neuromodulation as a treatment for refractory headache and neuralgia, were developed through a partnership between the Spanish Society of Neurology and the Spanish Society of Neurosurgery, and have 2 objectives:
- •
To describe the available neuromodulation devices and techniques.
- •
To analyse the current level of evidence on their safety and efficacy and make recommendations on their indication.
We performed a comprehensive systematic review and analysis of articles published on the MEDLINE database addressing neuromodulation in patients with headache and craniofacial neuralgia. No filters were applied, and we analysed papers published between 1967, when Shealy et al.1 implanted the first neuromodulation system, to May 2019. Levels of evidence and grades of recommendation were established according to the criteria of the Spanish Society of Neurology’s 2015 clinical practice guidelines for headache (Table 2).2
Criteria employed for therapeutic interventions in the Spanish Society of Neurology’s 2015 clinical practice guidelines for headache.2 Level of evidence and grade of recommendation.
| Level 1 | Controlled prospective clinical trials with masked outcome assessment in a representative populationSystematic reviews of controlled clinical trials carried out in a representative populationBoth types require the following characteristics:a) Randomised samplingb) Clearly defined objectivesc) Clearly defined exclusion/inclusion criteriad) Acceptable accounting for drop-outse) Baseline characteristics of patients are explicitly described in the text and similar between groups, or any differences have been statistically adjusted. |
| Level 2 | Prospective cohort studies in a representative population with masked outcome assessment and meeting all criteria from a) to e)Prospective controlled clinical trials with masked outcome assessment in a representative population, but not meeting one of the criteria from a) to e) |
| Level 3 | All other controlled trials in a representative population in which outcome assessment was independent from the treatment administered |
| Level 4 | Uncontrolled trials, case series, case reports, or expert opinions |
| Grade A | Recommendation definitively effective, ineffective, or dangerousRequires at least one conclusive level 1 study or 2 consistent level 2 studies |
| Grade B | Recommendation likely to be effective, ineffective, or dangerousRequires at least one conclusive level 2 study or several level 3 studies |
| Grade C | Recommendation that may be effective, ineffective, or dangerousRequires at least 2 conclusive level 3 studies |
The Roman physician Scribonius Largus described the first case of neuromodulatory treatment in the first century AD, when he found that accidental contact with a black torpedo fish, capable of delivering shocks of up to 220 V when hunting and as a defence mechanism, greatly improved symptoms of gout in Anteros, an official in the court of emperor Tiberius.3,4 While this fish was used by ancient Roman, Greek, and Islamic physicians to treat headaches and rectal prolapse, this experience did not attract further medical interest until electricity was developed in the 18th century.3 Benjamin Franklin himself studied the effect of electrical stimulation on muscle contraction.3
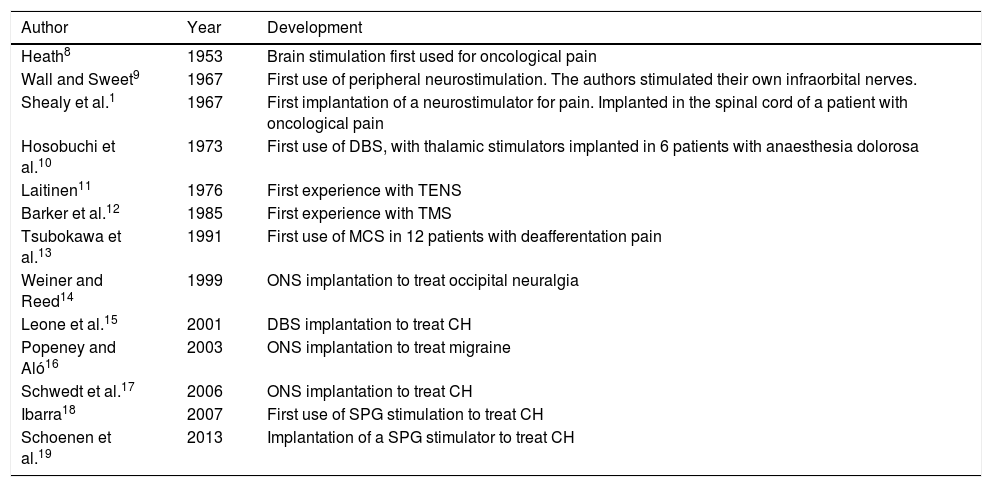
In 1874, the American Robert Bartholomew5 was the first to stimulate the human cortex, causing muscle contraction, in a controversial experiment in which the patient died. Years later, in 1908, Horsley and Clark6 developed stereotactic surgery. Lastly, the “gate control” theory of pain, proposed by Melzack and Wall7 in 1965, represented the final impulse in the development of neuromodulation.3Table 3 summarises the main developments in the history of neuromodulation, specifically in the treatment of pain.1,8–19 Numerous neuromodulation devices have since been developed that act on the central and peripheral nervous systems to treat refractory headache and craniofacial neuralgia.20
Main developments in the history of neuromodulation for craniofacial pain.
| Author | Year | Development |
|---|---|---|
| Heath8 | 1953 | Brain stimulation first used for oncological pain |
| Wall and Sweet9 | 1967 | First use of peripheral neurostimulation. The authors stimulated their own infraorbital nerves. |
| Shealy et al.1 | 1967 | First implantation of a neurostimulator for pain. Implanted in the spinal cord of a patient with oncological pain |
| Hosobuchi et al.10 | 1973 | First use of DBS, with thalamic stimulators implanted in 6 patients with anaesthesia dolorosa |
| Laitinen11 | 1976 | First experience with TENS |
| Barker et al.12 | 1985 | First experience with TMS |
| Tsubokawa et al.13 | 1991 | First use of MCS in 12 patients with deafferentation pain |
| Weiner and Reed14 | 1999 | ONS implantation to treat occipital neuralgia |
| Leone et al.15 | 2001 | DBS implantation to treat CH |
| Popeney and Aló16 | 2003 | ONS implantation to treat migraine |
| Schwedt et al.17 | 2006 | ONS implantation to treat CH |
| Ibarra18 | 2007 | First use of SPG stimulation to treat CH |
| Schoenen et al.19 | 2013 | Implantation of a SPG stimulator to treat CH |
CH: cluster headache; DBS: deep brain stimulation; MCS: motor cortex stimulation; ONS: occipital nerve stimulation; SPG: sphenopalatine ganglion; TENS: transcutaneous electrical nerve stimulation; TMS: transcranial magnetic stimulation.
Non-invasive neuromodulation involves the transcutaneous electrical stimulation of the supraorbital or the vagus nerve, or transcranial magnetic stimulation, using devices that are not implanted (Table 3). Non-invasive neuromodulation can be self-administered and avoids the need for surgical procedures and the associated complications and cost.
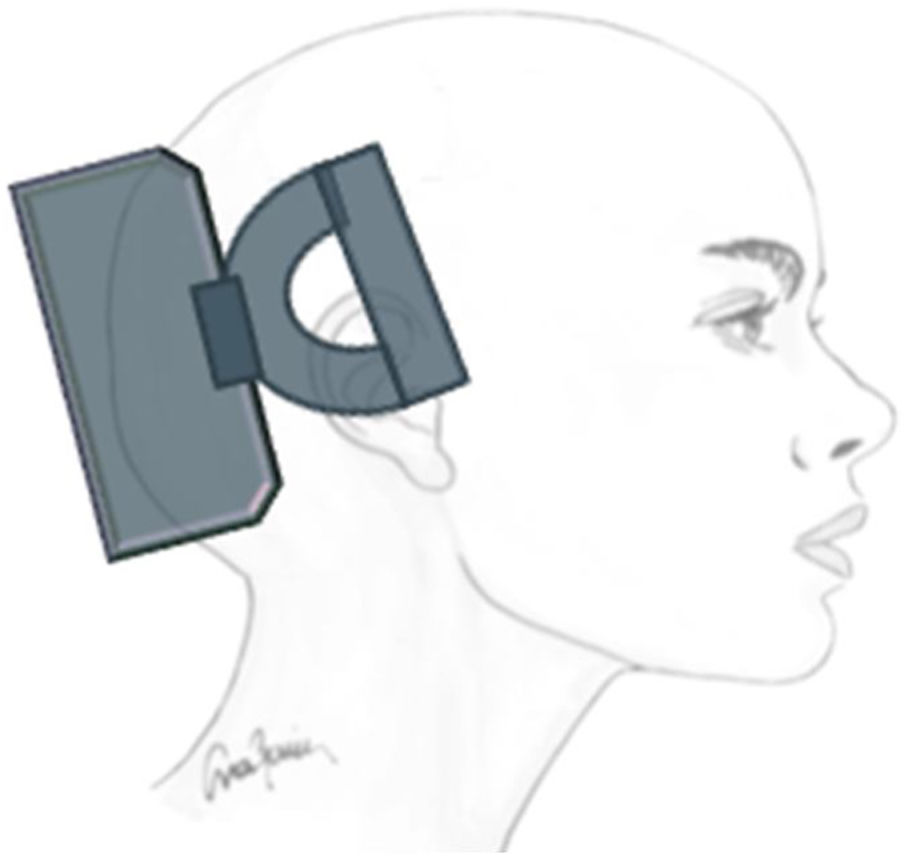
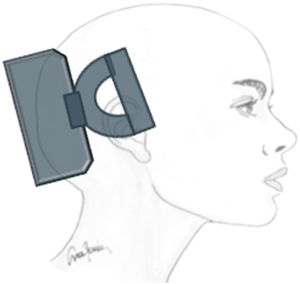
Transcutaneous trigeminal stimulation: supraorbital nerveThis technique modulates nociceptive activity at the level of the trigeminal ganglion and may act on the anterior cingulate cortex (Fig. 1).21 In migraine, the technique has been assessed for:
Preventive treatment. In the PREMICE trial, patients with migraine presenting at least 2 attacks per month were randomly assigned to receive 20 minutes/day of either active stimulation (n = 34) or sham stimulation (n = 33).22 Three months later, the treatment group showed a significant reduction in the number of migraine days compared to controls, with no adverse reactions. Thirty-eight percent of patients receiving active treatment and 12% of those receiving sham stimulation showed a > 50% reduction in the number of attacks. The excellent safety and tolerability of the treatment are also reported in the results of a survey of 2313 patients, 4% of whom presented adverse reactions (which were mild in all cases); only 2% dropped out.23 A subsequent open-label trial included 23 patients with chronic migraine who used the device for 20 minutes/day for 4 months; the number of migraine days only decreased in 8 patients.24 Further open-label studies of patients with chronic and episodic migraine suggest that the treatment is efficacious, safe, and well tolerated.25,26 In an open-label study of 7 patients with chronic migraine, simultaneous stimulation of the supraorbital nerve (SON) and occipital nerve provided additional benefits.27
Symptomatic treatment. A recent double-blind randomised controlled trial evaluated symptomatic treatment of migraine attacks with active stimulation of the SON (n = 52) and sham stimulation (n = 54).28 The endpoint measured was the reduction of pain intensity one hour after stimulation; a significant reduction was observed in the active treatment group.
The most significant adverse reactions include paraesthesia, local pain, and skin problems in the area of stimulation.21–24 The device is not recommended for patients with metal implants, implanted nerve stimulators in the head, pacemakers, or defibrillators.
Single-pulse transcranial magnetic stimulationThe main action mechanism of transcranial magnetic stimulation (TMS) in the treatment of migraine is the modulation of electrical activity in the cortex and thalamus29; inhibition of cortical spreading depression is thought to control aura and pain.30
Symptomatic treatment. In a multicentre controlled trial, 2 magnetic pulses were applied 30 seconds apart at the onset of aura.31 A non-invasive portable TMS system (Fig. 2) was used, and the trial included 82 patients in the active stimulation group and 82 in the sham stimulation group. Pain resolved within 2 hours in 39% of patients in the treatment group and 22% of patients in the sham group.31
Preventive treatment. An open-label study evaluated the safety and efficacy of TMS at 3 months in 59 patients with episodic and 131 patients with chronic migraine.32 The study reported 62% efficacy for pain relief, with no adverse reactions. The number of migraine days decreased from 12 to 9 in patients with episodic migraine and from 24 to 16 in those with chronic migraine.32 The ESPOUSE study recently analysed 132 patients with migraine (episodic migraine in the majority of patients).32 Patients applied 2 pulses every 12 hours as preventive treatment, and 3 consecutive pulses to treat attacks. Forty-six percent of patients presented ≥ 50% reduction in headache days and decreased symptomatic medication use and disability. Only 9 patients dropped out due to adverse reactions. TMS should not be used in patients with epilepsy, cranial bone defects, metal plates in the head or neck, or pacemakers or other stimulators.
Non-invasive vagus nerve stimulationVagus nerve stimulation (VNS) presents a multifactorial action mechanism: it inhibits cortical spreading depression,33 acts on the trigemino-cervical complex,34 and inhibits parasympathetic pathways35 (Fig. 3). For these reasons, it has been used to treat migraine and cluster headache (CH).
Migraine. Symptomatic treatment. Twenty-seven patients included in an open-label study self-administered 2 90-second doses of stimulation at 15-minute intervals upon onset of pain.36 Pain resolved within 2 hours in 22% of patients in the treatment group, and 43% of patients reported an improvement. Forty-six percent of patients reported adverse reactions; none of these were severe.36 In the PRESTO study, a controlled clinical trial, 243 patients were randomly assigned to receive VNS or sham stimulation in the first 20 minutes after pain onset; they were instructed to apply a second dose 15 minutes later if pain did not improve.37 A significantly higher percentage of patients were pain-free at 30 minutes in the treatment group than in the sham group (12% vs 4%); however, this was not the case at 120 minutes (30% vs 19%). VNS was well tolerated.37
Preventive treatment. The EVENT study evaluated the efficacy of VNS (30 patients) against sham stimulation (29 patients) in patients with chronic migraine.38 Stimulation was administered in 2 90-second doses at a 5-10 minute interval every 8 hours. Eleven percent of patients achieved a > 50% reduction in headache days at 2 months, 25% at 4 months, and 38% at 6 months. In the 8-month open-label phase, the number of headache days decreased by 7. The treatment was well tolerated, with adherence greater than 95% and no severe adverse reactions.38 The PREMIUM study was recently completed, and analysed 332 patients, finding no significant differences.39 Post hoc analysis did identify differences in the reduction of the number of migraine days.
Cluster headache. Symptomatic treatment. The ACT1 study included 85 patients with episodic CH and 48 with chronic CH.40 In episodic CH, VNS was associated with a greater clinical improvement than sham stimulation in the first 15 minutes (34% vs 10%) and better sustained response (15-60 minutes), with good tolerance. However, this benefit was not observed in patients with chronic CH.40 The ACT2 study followed a similar design, including 27 patients with episodic CH and 65 with chronic CH; similarly, benefits were only observed for episodic CH.41
Preventive treatment. An open-label study evaluated the safety and efficacy of VNS for pain prevention in 8 patients with episodic CH and 11 with chronic CH.42 Stimulation was applied twice daily, with additional doses to treat attacks. Fifteen of the 19 patients reported improvements of up to 60%, with good tolerance. Most patients reduced symptomatic medication use and showed very good tolerance.42
In the PREVA study, 97 patients with chronic CH were randomly assigned to receive standard treatment plus VNS (n = 48) or standard treatment only (n = 49).43 Patients in the treatment group applied 2 90-second doses of stimulation 5-10 minutes apart, twice daily, and were instructed to administer further doses in the event of acute attacks. After 4 weeks of treatment (randomised phase), a significant reduction was observed between patients receiving VNS and those receiving standard treatment in the number of weekly attacks and in the use of symptomatic medication. Treatment was generally well tolerated.43
The VNS device is not recommended for patients with pacemakers or other stimulators, for patients with carotid atherosclerosis, or for patients undergoing surgery to the neck, with potential lesions to the vagus nerve.
Invasive neuromodulationInvasive peripheral neuromodulationNeuromodulation of terminal branches of the trigeminal nerveTechnique. Stimulation is applied to the SON and the infraorbital nerve. The subcutaneous electrode is implanted in the appropriate nerve exit point in the facial skeleton; efficacy is checked before placement of the generator, which is connected by a subcutaneous lead (wireless devices also exist).44
Effectiveness. The literature includes reports of 40 patients undergoing this procedure, with a heterogeneous group of refractory pain syndromes, including post-herpetic, post-surgical, and post-traumatic neuralgia45–47; supraorbital neuralgia48; non-specific facial pain49,50; and trigeminal autonomic cephalalgia.51 Different variables were used to analyse pain in these patients (30 prospective and 10 retrospective); patients reported pain relief of 50%-100% and a reduction in symptomatic medication use.45–53 Finally, up to 30% of patients presented complications, with infection and electrode migration being the most common.
Neuromodulation of the trigeminal tractTechnique. Two approaches have been described. The first, developed by Shelden et al.54 in 1967, resembles the percutaneous ablation techniques used to treat trigeminal neuralgia (TN). A stimulation needle, typically a monopolar cylindrical electrode, is inserted with fluoroscopic guidance through the foramen ovale to access the trigeminal cave. In the second technique, described by Meyerson and Håkansson55 in 1980, a subtemporal craniotomy is performed and a pad with 2 electrodes is sutured to the dura mater surrounding the Gasserian ganglion; the electrodes are connected by a lead to a generator placed at the subclavicular level.
Effectiveness. The literature includes 5 studies and small series, including a total of 365 patients.54–66 A review including 233 of these patients showed an initial > 50% reduction in pain in 80%-92% of patients, but with a reduction to 48% after over 4 years of follow-up.65 Given the poor effectiveness, stimulation of the trigeminal tract is not indicated for the treatment of TN. Complications, generally infections, have been reported in 30% of patients.
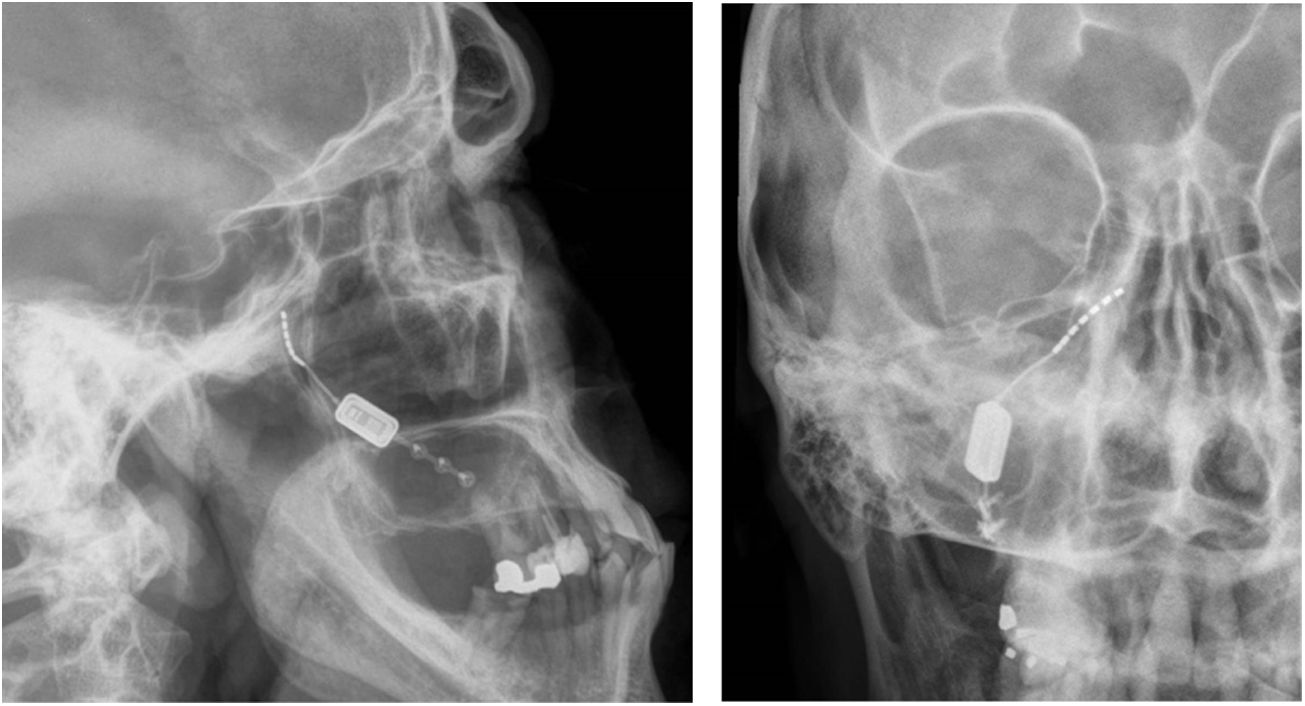
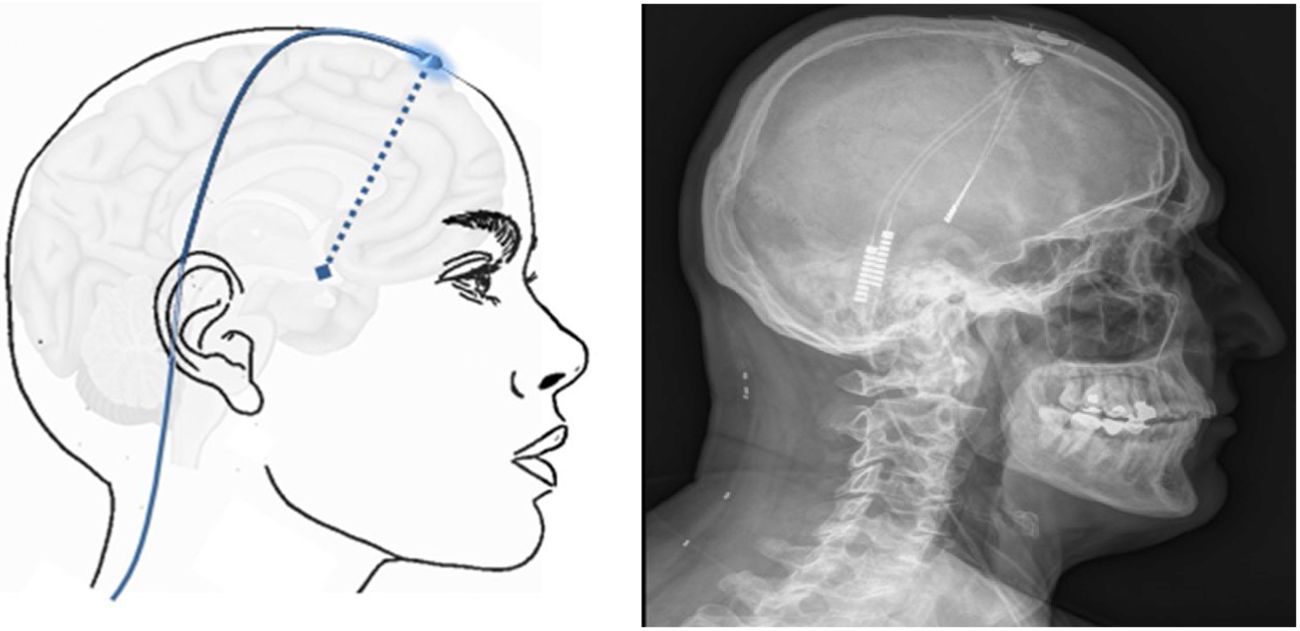
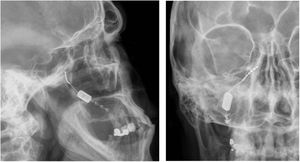
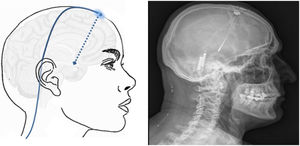
Neuromodulation of the ipsilateral sphenopalatine ganglionInitial experience with the use of non-implanted electrodes to stimulate the sphenopalatine ganglion (SPG) ipsilateral to CH pain shows that the treatment is effective in 61% of patients.17,67 A microstimulator has since been designed that can be implanted in the SPG following CT imaging analysis of the pterygopalatine fossa68 and antibiotic prophylaxis. The device is placed transorally with a minimally invasive gingival approach, with the patient under general anaesthesia and admitted to hospital for 24 hours.69 Intraoperative navigation techniques may be used, and a simple radiography is used to verify the position of the stimulator (Fig. 4).70
Profile and anteroposterior radiography images showing a wireless neurostimulation device implanted in the pterygopalatine fossa of a patient with chronic refractory cluster headache in the ipsilateral side. Image courtesy of Dr José Miguel Láinez of Hospital Clínico Universitario de Valencia (Spain).
The stimulator is powered and activated by a wireless remote controller held to the patient’s cheek; therefore, it does not require leads or a subcutaneous generator, and surgical procedures to change batteries are not necessary.
Effectiveness. The stimulator was originally designed for abortive treatment of CH attacks, but was subsequently observed to also have a protective effect.
Effectiveness as symptomatic treatment. The Pathway CH-1 study recruited 28 patients with CH according to consensus selection criteria.19,71 A long-term 2-year follow-up study (Pathway CH-2) was subsequently published,72,73 and a registry (Pathway R-1) has been created.73
In the Pathway CH-1 trial, patients were randomly allocated to 3 treatment groups and received full, sub-perception, or sham stimulation.19 Twenty-eight patients completed the study, with a mean stimulation time of 11 minutes. Pain decreased at 15 minutes in 67% of patients in the full stimulation group (complete resolution in 34%), 7% in the sub-perception stimulation group, and 7% in the sham stimulation group. This is a similar level of efficacy to that reported for subcutaneous sumatriptan, the first-line drug for CH attacks. In fact, a one-year follow-up study of 71 patients observed a 51% reduction in symptomatic medication use and a 41% reduction in preventive medication consumption; this represents an annual cost saving of €7484.75
Effectiveness as preventive treatment. CH attack frequency decreased by more than 50% at one month in 43% of patients. Symptomatic medication use also decreased, and scores on quality of life scales improved. In both 2-year follow-up studies, in which over 18 000 CH attacks were treated, the effectiveness of the device was 65%-68% for symptomatic treatment and 55% for prevention.72–74 The results of the Pathway CH-2 study were presented at the 2018 Annual Scientific Meeting of the American Headache Association, but had not been published by the time these guidelines were completed. The study, including 99 patients, confirmed the effectiveness data described in the Pathway CH-1 study.
Safety. A total of 128 adverse reactions were recorded in the Pathway CH-1 study, 92% of which were mild.18 The device had to be removed or revised in 8 cases (16%) due to maxillary nerve involvement, migration of the stimulator, incorrect positioning of the electrode, or surgical infection.
The total reoperation rate was 18%. This figure has considerably improved in subsequent studies.18,71–85 Sensory symptoms (paraesthesia, dysaesthesia) were the most frequent (81% of patients), and resolved by one year in over 60%. Less frequent adverse reactions were trismus, dry eyes, conjunctivitis, local infections, swelling, and haematoma. Despite this, 92% of patients said that they would undergo the procedure again.71–85 The 2 subsequent studies to the Pathway CH-1 study reported a similar rate of adverse reactions, but no cases of stimulator migration; only 8 patients (10%) underwent a further surgical revision.71–74
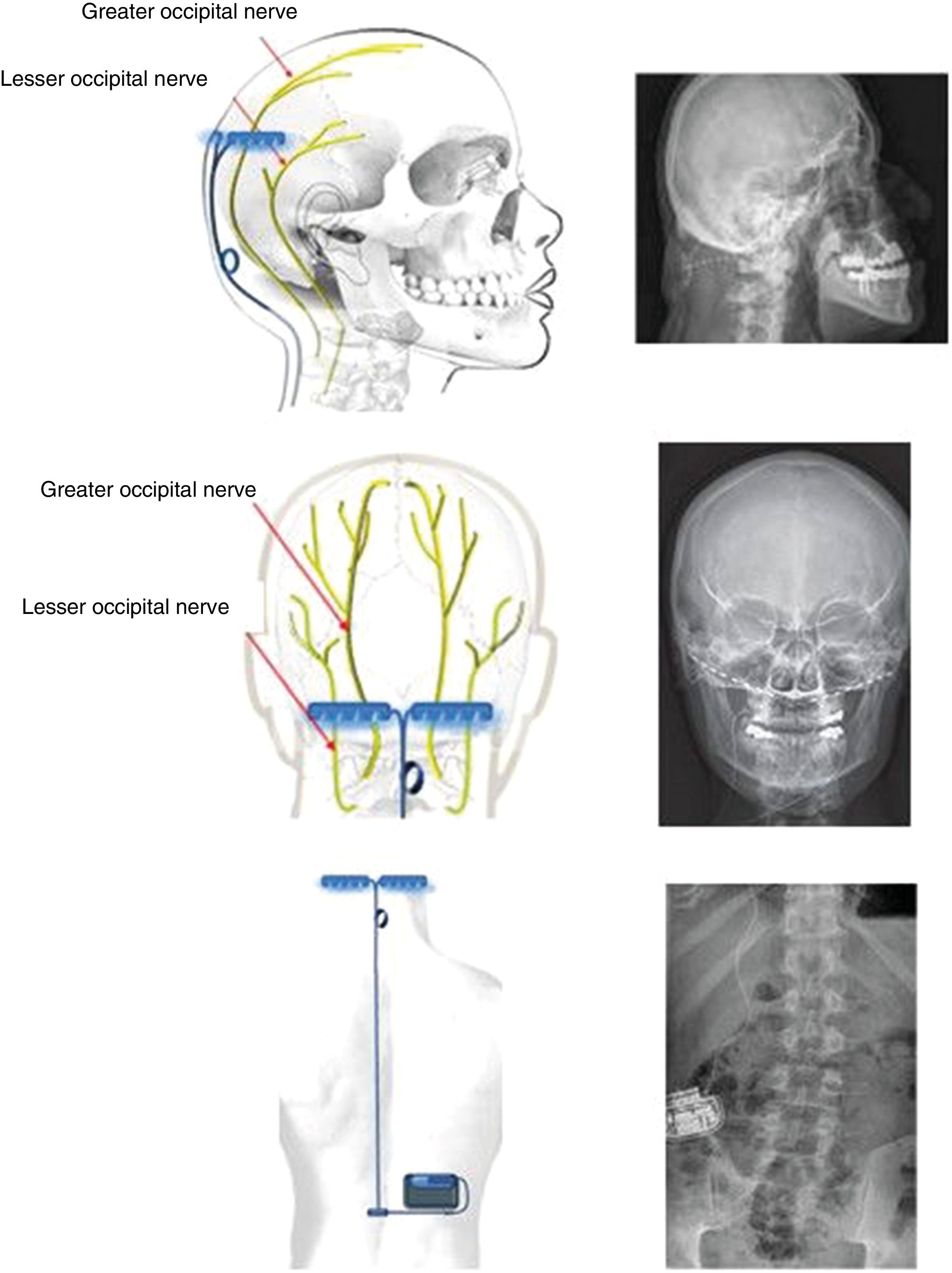
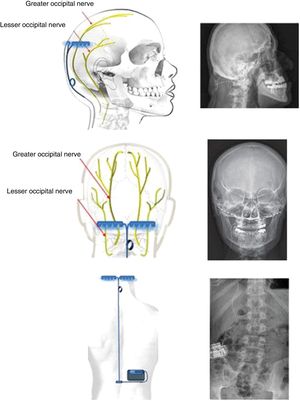
Bilateral occipital nerve stimulationIt may seem striking that implantation of electrodes in the occipital nerves should be therapeutic in CH, which clearly affects ocular/periocular (ie, anterior) regions, and is of central origin. However, studies in animals and humans have demonstrated a convergence of afferent fibres of the pars caudalis of the trigeminal nucleus and the nucleus of the greater occipital nerve (GON) at the cervical level, specifically at C2. This theory had already been proposed by Kerr and Olafson86 in 1961 (“The trigeminal and cervical volleys”), and the area is today known as the trigemino-cervical complex. Specifically, animal studies have shown that stimulation of the GON causes increased metabolism in the trigemino-cervical complex.87 Furthermore, blockade of the GON reduces the R2 response in the human trigeminal blink reflex.88 PET studies performed before and after implantation of the GON stimulator in patients with CH show that multiple areas of hypermetabolism normalise after implantation, with one exception, the hypothalamus.89 Therefore, the device has a relevant role in prevention of CH attacks, but not in their treatment. Given that this is an extracranial surgery, it is acceptable to place an additional electrode on the contralateral GON, as there is a possibility of pain switching sides over the course of the disease (described in 36% of patients).90,91
Technique. With the patient in the prone decubitus position, a vertical incision is made from 1 cm above to 1 cm below the occipital protuberance, along the midline. Subsequently, vertical incisions are made 4 cm either side of the midline, and the muscle fascia is exposed; electrodes are inserted extracranially at the level of the occipital bone and fixed using horizontal plates (Fig. 4). The electrodes are placed above the GON and lesser occipital nerve, stimulating both. Leads are placed by subcutaneous tunnelling along the midline to a middle thoracic level. From there, the lead is run to the upper buttock, where the generator is placed subcutaneously. The length of the lead is adapted to the patient’s anatomy, and its course includes a loop to prevent disconnection of the lead during forced cervical postures (Fig. 5). The electrodes may also be placed percutaneously above the occipitalis muscle fascia. Various techniques and systems have been described, with different types, directions, and numbers of electrodes and different anatomical placements of the generator.
Diagrams and radiography images showing the bilateral extracranial implantation of electrodes on the occipital bone, with a subcutaneous lead connecting the electrodes to a subcutaneous generator implanted in the right lumbar fossa of a patient with chronic refractory cluster headache.
Effectiveness. The literature currently includes over 200 reports of patients with CH treated with these devices; electrodes were placed bilaterally in 85% of cases.14,19,92–108 Between 65% and 78% of patients report a > 50% reduction in attack frequency, with 10%-40% of patients presenting conversion from chronic to episodic CH. Up to 60% of patients are even reported to experience long pain-free periods.92–108 However, none of these studies includes a control group treated with sham stimulation or pharmacological treatment, as the stimulator causes paraesthesia when connected, alerting the patient to the fact that the device is functioning. A meta-analysis of 8 open-label studies including a total of 96 patients found a response rate of 34%-71%, and a 29% reduction in weekly attacks at 1-3 years of follow-up.109
In long-term follow-up studies, 80%-100% of patients continued using prophylactic drugs, although 66% no longer required corticosteroids.103–108
Predictors of poor response seem to include severe anxiety/depression and occipital pain at the C2-C3 level prior to implantation.110,111 Anaesthetic block of the GON has been proposed as a predictor of treatment effectiveness; however, 3 studies have found that this is not a reliable selection criterion.112
The ICON study is a randomised, double-blind, international, multicentre study that is currently underway; it includes parallel groups in order to assess the efficacy of the occipital stimulator in the prevention of CH.113
In addition to the patients with CH, the procedure was performed in 81 patients with other trigeminal autonomic cephalalgias (60 with SUNCT, 18 with hemicrania continua, and 3 with SUNA). In SUNCT, the technique is reported to have 77% efficacy as a preventive treatment at 44 months of follow-up, with pain intensity decreasing by 4 points.114–116 Occipital nerve stimulation has also been used to treat migraine and neuralgia of the GON.
A total of 263 patients with migraine have been included in 3 randomised controlled trials117–119; the results showed an improvement in most secondary endpoints, but not the primary endpoint. Therefore, the procedure’s effectiveness for migraine prevention has not been reliably demonstrated, despite the positive findings of non-controlled open-label studies.15,120 As a result, there is currently no evidence to support recommending its use in patients with migraine.
A total of 107 cases of occipital neuralgia treated with GON stimulation have been reported in case reports and series, the largest of which included 76 patients; according to these studies, the procedure has 50%-85% efficacy for controlling pain. In a meta-analysis evaluating 9 studies with this indication, a level of evidence of 3 was established.13,121–127
As is the case for SPG stimulation, a small, wireless neurostimulator has been developed that can be implanted in the occipital region; the device has shown promising results for CH, migraine, and hemicrania continua.114,128–130 Preliminary studies are ongoing. Unfortunately, development of the prototype has been suspended.
Safety. The adverse reactions reported include paraesthesia in the scalp (100%) and infections (5%).92–108,131 Two studies with follow-up periods longer than 10 years show good tolerance to adverse reactions when these are persistent. Only 25% of the paraesthesias described were sufficiently severe for stimulation parameters to be modified.92–108,131 The vast majority of paraesthesias resolved after parameters were modified. Only 2 cases have been reported in which the device had to be reported for this reason.
An American MAUDE registry analysed safety in an estimated sample of 11 000 devices implanted over 10 years.132 Surgical complications were recorded in less than 3% of cases, and no deaths were reported. Complications were reported in 11% of cases, with electrode migration being the most frequent, followed by infection of the device, malfunctioning, breakage, and disconnection of the electrode.
Patients must inevitably undergo further procedures to change batteries, if they are not rechargeable (battery life will vary according to the frequency and intensity of stimulation required to control pain); 65% of patients underwent surgery to change stimulator batteries over a 6-year follow-up period.92–108 All patients reported symptom worsening when batteries failed. Finally, 66%-100% of patients would recommend the system to another patient.
Invasive central nervous system neuromodulationSpinal cord neuromodulationTechnique. An incision is made over the midline in the cervical region, exposing the vertebrae. Electrodes are inserted through vertebral laminectomy and implanted in the upper cervical epidural space (C2-C3) with fluoroscopic guidance. The placement of the lead and generator is similar to that described for occipital nerve stimulators.
Effectiveness. A total of 76 cases have been reported in the literature. Of these, 8 had CH, with 71% reporting a > 50% reduction in pain,133,134 and 35 had migraine, with 50%-71% effectiveness.134–136 The treatment has also been used successfully in 17 cases of painful trigeminal neuropathy,137–139 7 cases of occipital neuralgia,139 5 cases of post-herpetic neuralgia,139 2 cases of SUNA,134 and 2 cases of cervicogenic headache.140,141
Safety. Cases have been reported of infection, electrode migration, and, in 3% of cases, cerebrospinal fluid leak.
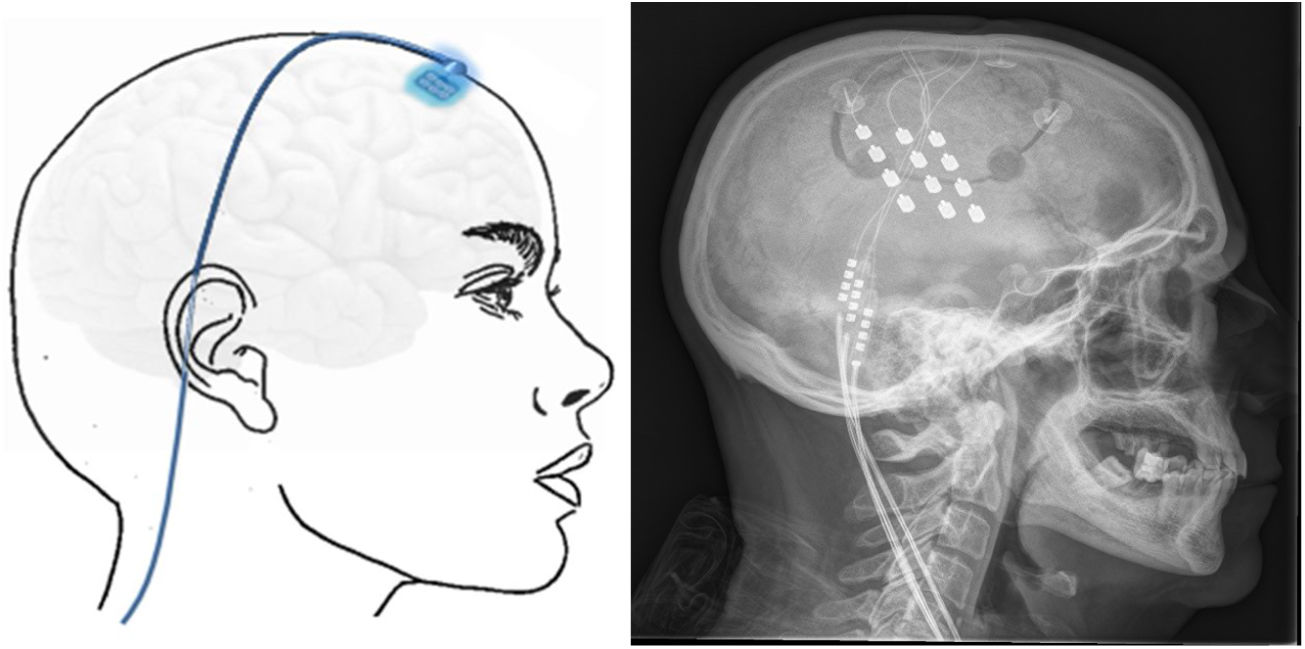
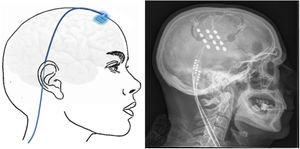
Transcortical neuromodulationTechnique. MRI is used to locate the area of facial pain, anterior to the Rolandic fissure at the level of the inferior frontal sulcus. With this information, neuronavigation-guided craniotomy is performed with the patient under local or general anaesthesia. The cortical area representing the hand is located using intraoperative evoked potentials. The electrodes (generally 4) were initially implanted subdurally, but epidural placement is now used to avoid complications (Fig. 6). After verifying the proper placement and functioning of the device, the electrodes are connected to a subclavicular generator with a lead placed via subcutaneous tunnelling.142–145
Left: electrodes implanted in the cortical motor area through a frontal burr hole with a subcutaneous lead. Right: head radiography image showing the electrodes implanted in the motor cortex of a patient with contralateral refractory trigeminal neuralgia secondary to multiple sclerosis.
Effectiveness. A total of 106 cases have been reported in 6 studies, of which the majority were retrospective and none included more than 20 patients. The included patients presented a range of refractory conditions: TN refractory to surgery or secondary to multiple sclerosis or trauma, painful trigeminal neuropathy, central neuropathic facial pain, and deafferentation pain. Effectiveness is estimated at 50%-100% at 3-40 months of follow-up.142–149
Safety. Besides surgical complications, other complications include seizures related to the intensity of stimulation (12%) and cognitive alterations.142–149
Deep brain neuromodulationHypothalamic neuromodulationHypothalamic neuromodulation is a surgical deep brain stimulation (DBS) technique used to treat patients with refractory CH. An electrode is placed in the inferior posterior hypothalamus ipsilateral to pain and connected by a lead to a generator placed in the subclavicular or periumbilical area.
Brain fMRI and PET studies conducted in the late 1990s showed that the ipsilateral posterior hypothalamus is specifically activated during CH attacks,150 whereas hypothalamic activation is not observed during migraine or TN attacks. Furthermore, MRI post-processing studies have shown that grey matter in the inferior posterior hypothalamus presents increased density and volume.151 For these reasons, the hypothalamus is the current target for DBS in the treatment of CH.152–154 New targets have also been proposed, including the ventral tegmental area of the midbrain, the lateral wall of the third ventricle, and floating electrodes that stimulate the floor of the third ventricle.155–160
Technique. The procedure is performed on patients selected according to strict criteria.161 A stereotactic frame is fitted, and a head CT scan is performed to mark the stereotactic location of the hypothalamus. The CT images are combined with the previous MRI study in the neuronavigation system to identify the best entry point and trajectory to guide the electrode to the target. The electrode is inserted through a frontal burr hole (Fig. 7) and subcutaneous tunnelling is performed to run the lead from the burr hole to the subclavicular or periumbilical area where the generator will be implanted.
Effectiveness. Over 100 cases have been reported of DBS implantation to treat CH. The largest study (19 patients)161–164 reported 70% effectiveness in reducing pain days by > 50%, with a mean follow-up period of nearly 9 years; pain fully resolved in 30% of patients and the stimulator could be switched off in almost all of them, and only 29%-34% of patients did not improve, with bilateral CH explaining most cases of ineffectiveness, in up to 80% of patients.14,108,161–180 Only one study, including 11 patients, compared pain frequency with the stimulator switched on and off, reporting 60% efficacy over 10 months of follow-up.177
DBS has also been used to treat other pain disorders in 9 patients: 3 with SUNCT, one with paroxysmal hemicrania, one with secondary CH, and 5 with TN secondary to multiple sclerosis.181–188
Safety. One patient (1%) died during surgery due to cerebral haemorrhage.14,108,161–188 Adverse reactions associated with the stimulator include incorrect positioning or migration of the electrode, non-lethal third ventricle haemorrhage, and infection; the latter was the most frequent complication. Rarer adverse events included seizures, diplopia, anxiety, transient ischaemic attack, tremor, dystonia, changes in thirst and appetite, and syncope. Unlike DBS surgery for other diseases, no cognitive/behavioural alterations have been described in patients with CH.189
Thalamic neuromodulationNinety-one cases of DBS targeting the thalamus have been reported, usually in patients with TN secondary to multiple sclerosis, post-herpetic painful trigeminal neuropathy, central post-stroke pain, facial pain secondary to deafferentation, and persistent idiopathic facial pain. Other reports mention untreatable TN, but do not specify the previous pharmacological or surgical treatments attempted. The typical target is the ventral posteromedial nucleus of the thalamus; on occasion, the periventricular or periaqueductal grey matter is targeted, and it is possible to stimulate both (dual stimulation). In pain involving the first branch of the trigeminal nerve, the posterior thalamus can be targeted, as with CH. Despite the heterogeneity of the diseases, procedures, targets, and follow-up periods reported, we may conclude that the procedure presents 37%-85% effectiveness for reducing pain at 1-30 months of follow-up.190–194
ConclusionsNeuromodulation techniques must be strictly reserved to patients whose headache or craniofacial neuralgia is refractory to pharmacological treatment; therefore, before these techniques are indicated, a neurologist specialising in headache and neuralgia must ensure that the patient has exhausted the available pharmacological treatments, administered at optimal doses and for the necessary duration.
In the case of migraine and CH, consensus criteria are available to establish whether pain is refractory to pharmacological treatment; this is not the case for other headache disorders.195,196 Fortunately, few patients are refractory to pharmacological treatment: 5% for migraine,197 10% for CH,198 and 12% for TN.199 For this reason, sample sizes in studies into neuromodulation are small in absolute terms, but not in relative terms. For instance, the minimum sample size required to extrapolate study results with statistical power is 10 times greater for migraine than for CH, as migraine affects 12% of the population, compared to just 0.1% for CH.200,201
It should also be noted that for patients with such severe, disabling pain, it is ethically controversial to perform studies comparing connected and disconnected stimulators. Given all these considerations, studies into neuromodulation mainly provide level 3 or 4 evidence (Table 4). Nonetheless, drug studies present the same limitations for such conditions as CH or TN, with practically no controlled trials having been performed.
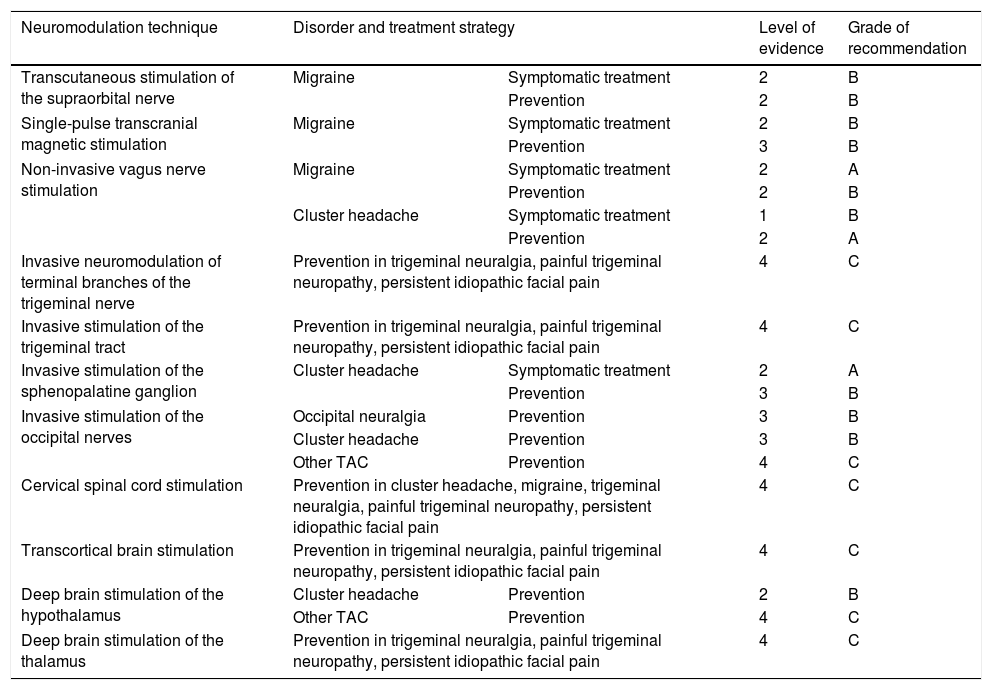
Levels of evidence and grades of recommendation for neuromodulation for the treatment of different types of headache and craniofacial neuralgias, according to the criteria employed for therapeutic interventions in the Spanish Society of Neurology’s 2015 clinical practice guidelines for headache.20.
| Neuromodulation technique | Disorder and treatment strategy | Level of evidence | Grade of recommendation | |
|---|---|---|---|---|
| Transcutaneous stimulation of the supraorbital nerve | Migraine | Symptomatic treatment | 2 | B |
| Prevention | 2 | B | ||
| Single-pulse transcranial magnetic stimulation | Migraine | Symptomatic treatment | 2 | B |
| Prevention | 3 | B | ||
| Non-invasive vagus nerve stimulation | Migraine | Symptomatic treatment | 2 | A |
| Prevention | 2 | B | ||
| Cluster headache | Symptomatic treatment | 1 | B | |
| Prevention | 2 | A | ||
| Invasive neuromodulation of terminal branches of the trigeminal nerve | Prevention in trigeminal neuralgia, painful trigeminal neuropathy, persistent idiopathic facial pain | 4 | C | |
| Invasive stimulation of the trigeminal tract | Prevention in trigeminal neuralgia, painful trigeminal neuropathy, persistent idiopathic facial pain | 4 | C | |
| Invasive stimulation of the sphenopalatine ganglion | Cluster headache | Symptomatic treatment | 2 | A |
| Prevention | 3 | B | ||
| Invasive stimulation of the occipital nerves | Occipital neuralgia | Prevention | 3 | B |
| Cluster headache | Prevention | 3 | B | |
| Other TAC | Prevention | 4 | C | |
| Cervical spinal cord stimulation | Prevention in cluster headache, migraine, trigeminal neuralgia, painful trigeminal neuropathy, persistent idiopathic facial pain | 4 | C | |
| Transcortical brain stimulation | Prevention in trigeminal neuralgia, painful trigeminal neuropathy, persistent idiopathic facial pain | 4 | C | |
| Deep brain stimulation of the hypothalamus | Cluster headache | Prevention | 2 | B |
| Other TAC | Prevention | 4 | C | |
| Deep brain stimulation of the thalamus | Prevention in trigeminal neuralgia, painful trigeminal neuropathy, persistent idiopathic facial pain | 4 | C | |
TAC: trigeminal autonomic cephalalgia.
While neurologists can indicate non-invasive neuromodulation, indication of invasive neuromodulation should ideally be discussed by a multidisciplinary committee including neurologists specialising in headache and neuralgia, neurosurgeons, neuroradiologists, and anaesthesiologists from the pain unit. It is also very difficult for a surgical team to achieve optimal rates of morbidity and mortality by operating exclusively on patients with refractory headache or craniofacial neuralgia. Rather, invasive neuromodulation techniques should be performed by surgical teams who have surpassed the learning curve in neuromodulation through experience accumulated in treating other diseases (epilepsy, movement disorders, psychiatric disorders). Finally, the European Headache Federation202 recommends that a sequential approach be followed in the management of patients refractory to pharmacological treatment,108 beginning with the least risky surgical techniques and considering DBS as the last procedure to try.
FundingThese guidelines have received no public or private funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Eva García Fernández for contributing illustrations for these guidelines.
Please cite this article as: Belvís R, Irimia P, Seijo-Fernández F, Paz J, García-March G, Santos-Lasaosa S, et al. Neuromodulación en cefaleas y neuralgias craneofaciales: Guía de la Sociedad Española de Neurología y de la Sociedad Española de Neurocirugía. Neurología. 2021;36:61–79.