Freezing of gait (FOG) is one of the most disabling and enigmatic symptoms in Parkinson's disease. Vascular lesions, observed in magnetic resonance imaging (MRI) scans, may produce or exacerbate this symptom.
Patients and methodsThe study includes 22 patients with Parkinson's disease subjects, 12 with freezing of gait and 10 without. All patients underwent an MRI scan and any vascular lesions were analysed using the modified Fazekas scale.
ResultsPatients with FOG scored higher on the modified Fazekas scale than the rest of the group. Although the two groups contained the same percentage of patients with vascular lesions (50% in both groups), lesion load was higher in the group of patients with FOG. Vascular lesions in the periventricular area and deep white matter seem to be the most involved in the development of FOG.
DiscussionVascular lesions may contribute to the onset or worsening of FOG in patients with PD. This study suggests that cerebral vascular disease should be considered in patients with FOG.
La congelación de la marcha (CDM) es uno de los fenómenos más incapacitantes y menos comprendido de la enfermedad de Parkinson idiopática (EPI). Las lesiones vasculares, objetivadas mediante resonancia magnética nuclear (RMN), podrían contribuir a la aparición o empeoramiento de este síntoma.
Pacientes y métodoSe estudió un grupo de 22 pacientes con EPI avanzada, 12 con episodios de CDM y 10 sin dichos episodios. Se realizó RMN en todos los pacientes y se analizaron, mediante la escala de Fazekas modificada, las lesiones vasculares existentes.
ResultadosLos pacientes con CDM obtuvieron puntuaciones superiores en la escala de Fazekas modificada. Aunque el porcentaje de pacientes que presentaban lesiones vasculares fue el mismo en ambos grupos (50% en los 2 grupos), la carga lesional fue superior en el grupo de pacientes con CDM. Las lesiones vasculares en la región periventricular y en la sustancia blanca profunda son las que parecen estar más implicadas en la aparición de la CDM.
ConclusionesLas lesiones vasculares podrían contribuir a la aparición o al empeoramiento de la CDM en los pacientes con EPI, con este estudio se sugiere que la afección vascular cerebral debe ser considerada en los pacientes con CDM.
Freezing of gait (FOG), a gait disorder in idiopathic Parkinson's disease (PD), began to be described in detail after 1970 when the benefits of levodopa for other motor symptoms of PD were shown to be less marked for this specific disorder.1
Different aspects of gait disorders, such as small steps, loss of postural reflexes, and festinating gait, were well known in the pre-levodopa era. Although FOG had also been mentioned in older literature, it seems to be much more noticeable after long-term treatment with levodopa.2
FOG is characterised by its brief duration and episodic onset (on starting to walk, encountering obstacles, turning, etc.). Patients describe the sensation as being “glued to the floor”.3
FOG may occur in early PD, but always in a mild form; in fact, early onset of this symptom is an alarm signal that may cast doubt on a diagnosis of idiopathic PD. This phenomenon is more typically observed after years of disease and treatment, when patients also present motor complications of long-term treatment including motor fluctuations and dyskinesias.4,5
FOG is not exclusive to idiopathic PD and may appear in other parkinsonian syndromes, including vascular parkinsonism (VP).5
VP is one of the most controversial concepts in neurology; classic literature referred to it as ‘arteriosclerotic parkinsonism’6 before the term ‘vascular parkinsonism’ came into use.7,8 Although VP and idiopathic PD constitute different clinical entities, it is sometimes difficult to determine if the patient has idiopathic PD, VP, or PD with an associated cerebrovascular disease that would somehow exacerbate any of the parkinsonian symptoms.9,10
Our study aims to analyse the potential relationship between cerebrovascular lesions and FOG episodes in a patient series with PD.
Patients and methodsThis study was conducted at Hospital General Universitario, Ciudad Real, in 2011. All patients signed an informed consent form before being included in the study.
The study included a total of 22 patients with advanced idiopathic PD and motor complications (motor fluctuations and dyskinesias); 12 experienced FOG episodes (group I) and 10 did not (group II).
Group I patients presented FOG during the ‘off’ state. In all patients, freezing disappeared or lessened in duration or in number of episodes during the ‘on’ state.
All patients underwent cognitive evaluation with the MMSE and clock-drawing test; patients whose evaluations revealed dementia were excluded from the study.
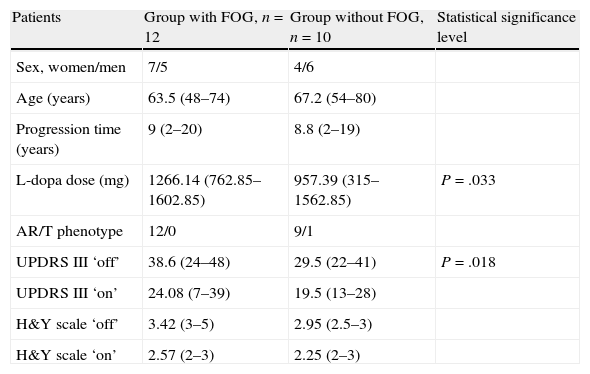
Patient characteristics are shown in Table 1.
Patient characteristics.
| Patients | Group with FOG, n=12 | Group without FOG, n=10 | Statistical significance level |
| Sex, women/men | 7/5 | 4/6 | |
| Age (years) | 63.5 (48–74) | 67.2 (54–80) | |
| Progression time (years) | 9 (2–20) | 8.8 (2–19) | |
| L-dopa dose (mg) | 1266.14 (762.85–1602.85) | 957.39 (315–1562.85) | P=.033 |
| AR/T phenotype | 12/0 | 9/1 | |
| UPDRS III ‘off’ | 38.6 (24–48) | 29.5 (22–41) | P=.018 |
| UPDRS III ‘on’ | 24.08 (7–39) | 19.5 (13–28) | |
| H&Y scale ‘off’ | 3.42 (3–5) | 2.95 (2.5–3) | |
| H&Y scale ‘on’ | 2.57 (2–3) | 2.25 (2–3) |
AR: akinetic-rigid; FOG: freezing of gait; H&Y: Hoehn and Yahr; T: tremor.
We calculated the levodopa equivalent daily dose for all levodopa doses in different formulations (levodopa-carbidopa, levodopa-benserazide, controlled-release levodopa, levodopa-carbidopa-entacapone). We also calculated the total levodopa daily equivalent dose for dopaminergic agonists according to earlier descriptions11 (100mg levodopa=130mg controlled-release levodopa=70mg levodopa+entacapone=1mg pramipexole=5mg rotigotine=5mg ropinirole=10mg apomorphine). Other antiparkinsonian drugs (amantadine, selegiline, rasagiline) were not included in this analysis.11
Since patients were studied after having spent 12hours without PD drugs and 1hour after taking them, we evaluated both ‘off’ and ‘on’ states. Motor functions were examined using UPDRS part III and the Hoehn and Yahr scale (H&Y) in both ‘off’ and ‘on’ states. We evaluated gait and quantified FOG episodes using a test in which patients had to walk a distance of 7 metres before returning to the starting point. On both the outbound and return trips they had to pass through a half-shut door and go round an obstacle. Patients had to carry a glass of water on the return trip. We measured the time needed to complete the test in ‘on’ and ‘off’ states and the number of FOG episodes experienced by patients.
Complete brain MRI scans were performed for all 22 patients. Two different neurologists analysed T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences to quantify lesions; one of the neurologists was blinded to patient identity. Concordance between results was 85%. In cases without concordance, the opinion of our hospital's neuroradiologist was provided as the final result.
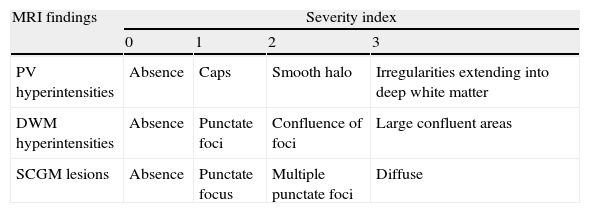
The modified Fazekas scale was used to analyse vascular disease. This scale describes the hyperintense MRI signals in 3 regions of the brain in ascending order of severity and frequency of findings. It examines periventricular hyperintensities (PVH), deep white-matter hyperintensities (DWMH) and lesions in subcortical grey-matter (SCGM),13 as shown in Table 2.
Modified Fazekas scale.
| MRI findings | Severity index | |||
| 0 | 1 | 2 | 3 | |
| PV hyperintensities | Absence | Caps | Smooth halo | Irregularities extending into deep white matter |
| DWM hyperintensities | Absence | Punctate foci | Confluence of foci | Large confluent areas |
| SCGM lesions | Absence | Punctate focus | Multiple punctate foci | Diffuse |
PV: periventricular; DWM: deep white matter; SCGM: subcortical grey matter.
The Evans index was also used to rule out the presence of hydrocephalus in all patients.
For statistical inference of the results, we applied the t-test for normally distributed quantitative variables and the Mann-Whitney test for quantitative ordinal variables and those without a normal distribution. Proportions were compared using the Pearson chi-square test. When conditions were not met, we used likelihood ratios and the Fisher exact test.
ResultsAll 22 patients scored above 24 on the MMSE and performed adequately on the clock-drawing test.
Patients in group I had a mean age of 63.5 years (range, 48–74; SD 8.67); mean time since PD onset was 9 years (range, 2–20; SD 5.56). The mean levodopa dose was 1266.14mg (range, 762.85–1602.85mg; SD 253.81). Mean H&Y stage was 3.42 during ‘off’ state (range, 3–5; SD 0.66) and 2.57 during ‘on’ state (range, 2–3; SD 0.53). Mean score on UPDRS part III was 38.6 during ‘off’ state (range, 24–48; SD 9.34) and 24.08 during ‘on’ state (range, 7–39; SD 11.1). All patients in this group presented motor complications and displayed the akinetic-rigid phenotype (Table 1).
Patients in group II had a mean age of 67.2 years (range, 54–80; SD 7.65); mean time since PD onset was 8.8 years (range, 2–19; SD 7.65). The mean levodopa dose was 957.39mg (range, 315–1562.85mg; SD 377.05). Mean H&Y stage was 2.95 in ‘off’ (range, 2.5–3; SD 0) and 2.25 in ‘on’ (range, 2–3; SD 0.5). Mean score on UPDRS part III was 29.5 in ‘off’ (range, 22–41; SD 6.78) and 19.5 in ‘on’ (range, 13–28; SD 4.99). All patients presented motor fluctuations and 90% of the patients displayed the akinetic-rigid phenotype (Table 1).
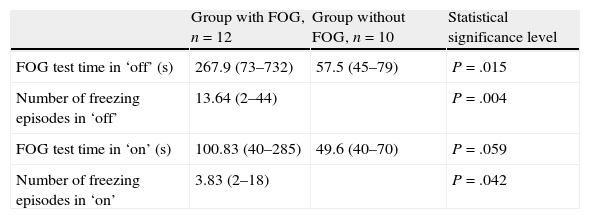
For group I, the mean time in seconds to complete the gait test in ‘off’ was 267.9 (range, 73–732, SD 237.11) vs 100.83 in ‘on’ (range, 40–285, SD 83.72). In ‘off’, patients presented a mean of 13.64 freezing episodes (range, 2–44; SD 12.11). In ‘on’, patients presented a mean of 3.83 freezing episodes (range, 2–18; SD 5.76) (Table 3).
Freezing of gait test.
| Group with FOG, n=12 | Group without FOG, n=10 | Statistical significance level | |
| FOG test time in ‘off’ (s) | 267.9 (73–732) | 57.5 (45–79) | P=.015 |
| Number of freezing episodes in ‘off’ | 13.64 (2–44) | P=.004 | |
| FOG test time in ‘on’ (s) | 100.83 (40–285) | 49.6 (40–70) | P=.059 |
| Number of freezing episodes in ‘on’ | 3.83 (2–18) | P=.042 |
FOG: freezing of gait.
For group II, the mean time in seconds to complete the gait test in ‘off’ was 57.5 (range, 45–79, SD 10.96) vs 49.6 in ‘on’ (range, 40–70, SD 10.34) (Table 3).
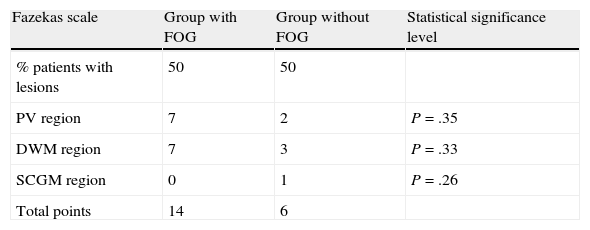
Analysis of MRI scans using the modified Fazekas scale revealed vascular lesions in 50% of patients in both groups. The lesion load (referring to the number and size of the lesions) was larger in group I patients, but no statistically significant differences were found. Lesions are listed in Tables 4–6.
Summary of scores from MR images according to Fazekas scale.
| Fazekas scale | Group with FOG | Group without FOG | Statistical significance level |
| % patients with lesions | 50 | 50 | |
| PV region | 7 | 2 | P=.35 |
| DWM region | 7 | 3 | P=.33 |
| SCGM region | 0 | 1 | P=.26 |
| Total points | 14 | 6 |
FOG: freezing of gait; PV: periventricular; DWM: deep white matter; SCGM: subcortical grey matter.
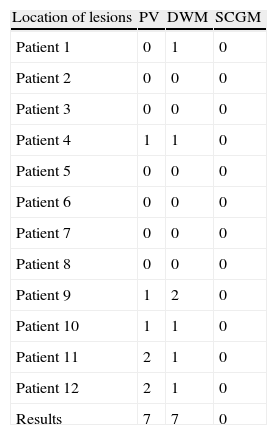
Lesions detected in MR image according to Fazekas scale in group with FOG.
| Location of lesions | PV | DWM | SCGM |
| Patient 1 | 0 | 1 | 0 |
| Patient 2 | 0 | 0 | 0 |
| Patient 3 | 0 | 0 | 0 |
| Patient 4 | 1 | 1 | 0 |
| Patient 5 | 0 | 0 | 0 |
| Patient 6 | 0 | 0 | 0 |
| Patient 7 | 0 | 0 | 0 |
| Patient 8 | 0 | 0 | 0 |
| Patient 9 | 1 | 2 | 0 |
| Patient 10 | 1 | 1 | 0 |
| Patient 11 | 2 | 1 | 0 |
| Patient 12 | 2 | 1 | 0 |
| Results | 7 | 7 | 0 |
FOG: freezing of gait; PV: periventricular; DWM: deep white matter; SCGM: subcortical grey matter.
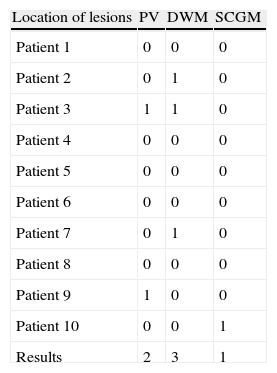
Lesions detected in MR scan and rated on the Fazekas scale in group without FOG.
| Location of lesions | PV | DWM | SCGM |
| Patient 1 | 0 | 0 | 0 |
| Patient 2 | 0 | 1 | 0 |
| Patient 3 | 1 | 1 | 0 |
| Patient 4 | 0 | 0 | 0 |
| Patient 5 | 0 | 0 | 0 |
| Patient 6 | 0 | 0 | 0 |
| Patient 7 | 0 | 1 | 0 |
| Patient 8 | 0 | 0 | 0 |
| Patient 9 | 1 | 0 | 0 |
| Patient 10 | 0 | 0 | 1 |
| Results | 2 | 3 | 1 |
FOG: freezing of gait; PV: periventricular; DWM: deep white matter; SCGM: subcortical grey matter.
An Evans index of less than 0.3 was used to rule out the presence of normal pressure hydrocephalus in all patients in both groups, as well as presence of any other lesions that could contribute to freezing of gait in the patient.
DiscussionRisk factors linked to FOG include the following: time since PD onset,12 PD severity,13–17 age,18 motor complications,13,14 cognitive impairment,19,20 long-term levodopa treatment,21–24 akinetic-rigid phenotype,13,14 and associated vascular disease.25–27
We found no significant differences in our patients regarding time since PD onset.
Nevertheless, UPDRS part III and H&Y scores were higher for group I. This supports the hypothesis that disease severity is the most important risk factor for developing FOG and that onset of FOG is associated with deficits in postural balance.
Regarding age effects, the oldest patients in our study are those without FOG, and furthermore, 2 of the 4 patients experiencing more than 20 episodes were, at ages 48 and 51, the youngest in the group.
Although all patients in the study had fluctuating PD, we should point out that half of the individuals in group I had not yet presented any severe motor complications and only displayed mild wearing-off phenomena. In some patients, there was no clear correlation between motor fluctuation intensity and number of episodes of FOG; these findings support the hypothesis that these two phenomena have different pathophysiological explanations.
Our patients did not present dementia, but numerous authors have advanced the possibility that damage leading to apraxia may underlie the development of FOG, which would point to a frontal lobe dysfunction component in FOG onset.20 This study did not include a more detailed cognitive evaluation that would allow us to draw any such conclusions from our patient group.
FOG episodes decreased significantly with levodopa treatment in most of our patients. However, 2 of our patients presented the same number of FOG episodes before and after levodopa, despite showing improvement on both motor scales, faster completion of the gait test, and decreased duration of FOG episodes after treatment.
Researchers recently discussed the different pathophysiological mechanisms underlying FOG episodes appearing in ‘off’ and episodes appearing in ‘on’; the mechanism in the latter case exacerbates when medication is increased, causing the patient to enter a ‘supra-on’ state.21–24 In our series, no patients experienced exacerbations after medication time, but all were following their normal drug regimens with no dose increases.
As seen in the literature,13,14 the akinetic-rigid phenotype and not the tremor phenotype was dominant in the FOG group (100%), although almost all of our patients without FOG also presented that phenotype (90%).
On the other hand, brain MRI scans revealed vascular lesions in 50% of our patient total, with a higher Fazekas scale score in group I. These lesions may contribute to, or exacerbate, FOG phenomena.
According to a recent anatomical pathology study, the term VP denotes presence of a parkinsonian syndrome that is chronologically related to the presence of acute or chronic vascular encephalopathy. It can be demonstrated by computed tomography (CT) or MRI and absence of other causes that might explain a parkinsonian syndrome.25The prevalence of vascular encephalopathy in patients with PD ranges from 6% to 44% according to different series.26–28
It is clear that the FOG phenomenon is complex. While vascular risk factors are more common in VP (81%) than in idiopathic PD (32%),29 the two entities may be concomitant.
In theory, any vascular lesion may cause parkinsonism if it is extensive enough or if it affects key structures. The ability of a vascular lesion to produce or exacerbate parkinsonian symptoms is explained by how it interrupts pathways that connect the basal ganglia and the motor cortex.30,31 In the same way, vascular lesions that affect the basal ganglia and the thalamus may slow brain metabolism in the ipsilateral frontal lobe due to deafferentation. This may increase frontal lobe dysfunction in patients with parkinsonism,32,33 consequently affecting cognitive performance, gait, etc.
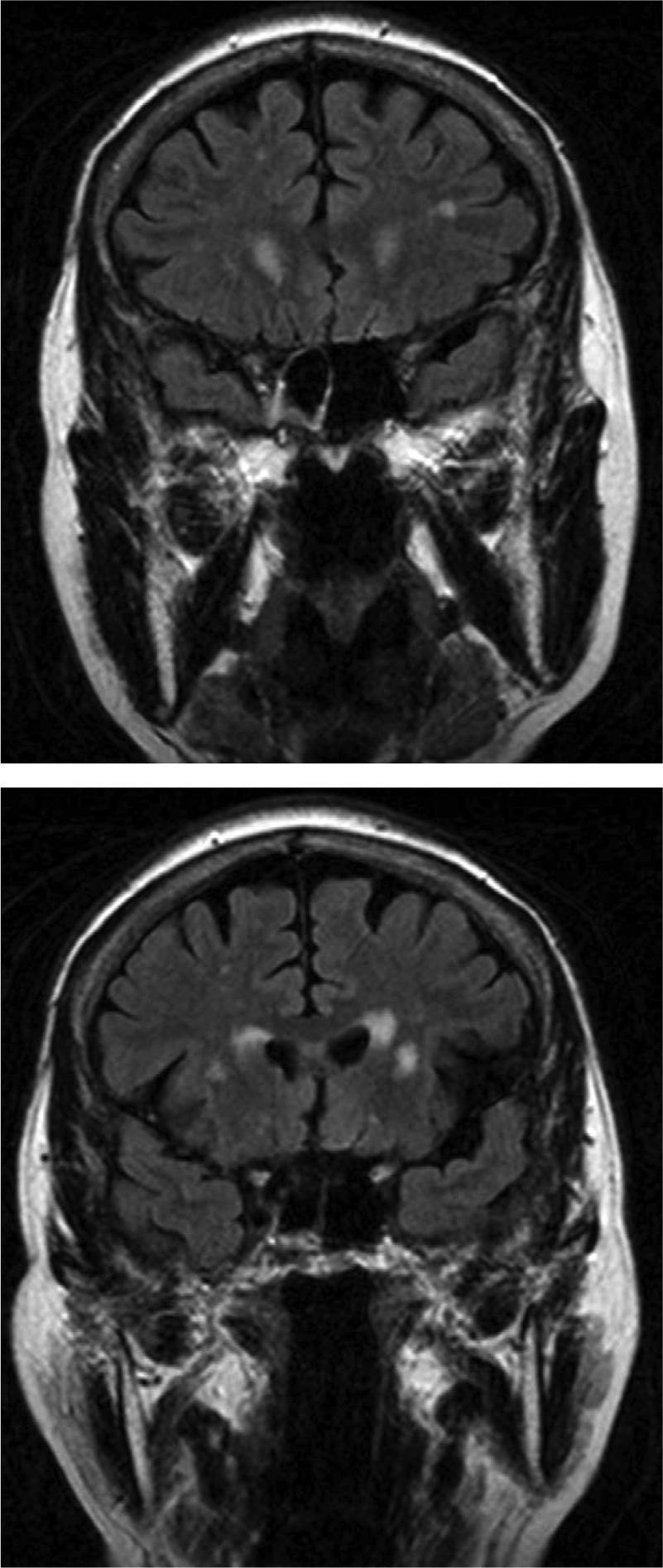
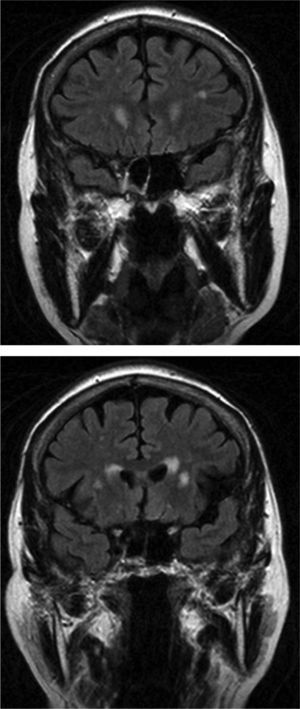
In group I, MR scans in 6 of our 12 patients revealed significant lesions; vascular lesions were limited to the periventricular and deep white-matter regions. Analysis of the 3 patients with the largest lesion load detected by MRI and scores of 3 on the Fazekas scale showed that only 2 showed slight improvements in the number of freezing episodes after treatment. Most lesions in these patients were in the periventricular region (Fig. 1); however, levodopa treatment resolved freezing of gait in the third patient, who scored 3 on the Fazekas scale and whose lesions mainly affected deep white matter. These findings indicate that periventricular lesions may contribute more to the appearance of FOG. We did not observe vascular lesions in the brainstem that would be consistent with damage to the pedunculopontine nucleus, a structure that seems to be involved in FOG pathophysiology.34
Although there are studies on the prevalence of cerebrovascular disease in PD,26,28 none have focused on the impact of this entity on FOG development or exacerbation in patients with parkinsonism. The fact that we observed a larger vascular lesion load in the patient group with FOG suggests that cerebrovascular disease should be considered in parkinsonian patients with FOG. In this process, doctors should analyse the possible pathophysiological impact and the new treatment targets for this disorder which is both difficult to manage and damaging to quality of life.35,36
Study limitationsOur study is limited by its small sample size; a higher number of patients are needed in order to obtain significant results.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study was presented in poster format at the 2011 Annual Meeting of the SEN.
Please cite this article as: Gallardo MJ, Cabello JP, Pastor C, Muñoz-Torrero JJ, Carrasco S, Ibañez R, et al. Pacientes con enfermedad de Parkinson avanzada con y sin congelación de la marcha. Análisis comparativo de lesiones vasculares en resonancia magnética cerebral. Neurología. 2014;29:218–223