HIV-associated cognitive impairment occurs even in the early stages of infection. Short-term memory, psychomotor speed, attention, and executive functioning are the main capacities affected. Controversy exists regarding whether highly active antiretroviral therapy (HAART) is helpful in combating this process. The objective of the present study is to determine the association between cognitive impairment and HAART in HIV-infected patients from Hospital Regional de Huacho.
MethodsProspective study of HIV patients meeting criteria to start HAART. Twenty-one HIV-positive patients were recruited between April and July 2011. Researchers administered a standardised neuropsychological test battery before and 4 weeks after onset of HAART. Psychomotor speed, executive function, short term memory (visual and verbal), attention, and visuospatial performance were evaluated.
ResultsNineteen patients completed the study (14 males and 5 females). In the pre-HAART evaluation, most patients scored below average on the executive function and psychomotor speed subtests. Psychomotor speed and immediate visual memory improved significantly after four months of treatment with HAART.
ConclusionsSome degree of cognitive decline may present even in the early and asymptomatic stages of HIV infection. The benefits of antiretroviral treatment for cognitive performance can be detected after only a few weeks of follow-up.
La presencia de deterioro cognitivo asociado al virus de la inmunodeficiencia humana (VIH) se presenta desde estadios tempranos de la infección. Las principales funciones comprometidas son la memoria a corto plazo, la velocidad psicomotriz, la atención y la función ejecutiva. Existe controversia sobre la disminución de esta complicación con la terapia antirretroviral de gran actividad (TARGA). El presente trabajo tiene como objetivo determinar la asociación entre la alteración cognitiva y la terapia antirretroviral en pacientes con VIH del Hospital Regional de Huacho.
MétodosEstudio prospectivo de pacientes con VIH y con criterios para ingresar a TARGA. A 21 pacientes VIH +, enrolados desde abril hasta julio del 2011, se les administró una batería neuropsicológica estandarizada antes y 4 meses después del inicio de la TARGA. Las funciones cognitivas evaluadas fueron la velocidad psicomotriz, la función ejecutiva, la memoria a corto plazo (visual y verbal), la atención y las habilidades visuoespaciales.
ResultadosDiecinueve pacientes terminaron el estudio (14 varones y 5 mujeres). En la evaluación pre-TARGA se encontró que la mayoría de los pacientes obtenían puntuaciones menores a los promedios en las pruebas de función ejecutiva y velocidad psicomotriz. Luego de 4 meses de iniciar TARGA, la velocidad psicomotriz y la memoria inmediata visual mejoraron significativamente.
ConclusionesEl compromiso cognitivo en algún grado puede presentarse desde las fases tempranas y asintomáticas de la infección por el VIH. El beneficio del tratamiento antirretroviral sobre el rendimiento cognitivo se puede observar aun con pocas semanas de seguimiento.
Cognitive impairment is a well-documented neurological complication among patients with acquired immune deficiency syndrome (AIDS). It may evolve into a subcortical type of dementia that is caused by multiple factors.1–4 This complication may also present in early stages of infection with human immunodeficiency virus (HIV).5,6 The main features of cognitive disorders are loss of memory, attention, and executive function, with psychomotor retardation and impairment of visuospatial abilities.4,7 A wide array of instruments are available for measuring these changes: the Trail Making Test, digit span, Rey Auditory Verbal Learning Test, category and letter verbal fluency tests, and others. They are indicated by the World Health Organization (WHO) for different geographical areas and sociocultural settings.8
Highly active antiretroviral therapy (HAART) increases cognitive function because of its ability to reach high concentrations in nervous tissue and its effectiveness against the HIV infection within lymphocytes, macrophages, and microglia.4,9–12 Nevertheless, some articles show that despite HAART treatment, cognitive impairment increases as patients age.13 This is due to other factors, such as low drug penetrance, sustained inflammation, amyloid deposition, and presence of comorbidities.14 However, other articles mention that cognitive function may improve when patients discontinue treatment.15
The wide variety of seemingly contradictory results in international publications and the lack of data in our setting led us to undertake this study. Its aim is to determine the association between antiretroviral therapy and cognitive function in HIV-positive patients in a Peruvian population.
Patients and methodsStudy designAnalytic, prospective study.
SubjectsWe recruited 21 HIV-positive patients aged 18 years and older who were treated at the infectious disease department at Hospital Regional de Huacho, Lima, between April and July 2011. Patients were selected according to the following inclusion criteria: first-time treatment with HAART, at least 4 years of schooling, no history of opportunistic infections of the nervous system, no consumption of drugs or alcohol in the past 6 months, and no other causes of cognitive impairment or alteration of consciousness. We excluded patients for whom viral load data or CD4 count was not available before HAART was started. Effectiveness of HAART was measured based on viral load and CD4 count values. Two patients discontinued treatment during the study, so the study analysis only examines data from 19 patients.
Neuropsychological testsAll subjects were assessed with a thorough clinical neurological examination performed by the researcher, after which they underwent a neuropsychological evaluation conducted by a psychologist with experience in evaluating HIV-positive patients. The evaluation consisted of the tests listed here. The Trail Making Test parts A and B is a test in which the subject connects letters and numbers printed on a worksheet in the shortest possible time. Times ranged from 22 to 57seconds for part A, and from 48 to 145seconds for part B, depending on age and years of schooling. The digit/symbol coding test, used to evaluate immediate visual memory, involves filling in the maximum number of boxes on a printed sheet within 120seconds. Each completed box will contain a number from 1 to 9 and correct answers are scored with 1 point, with 133 being the highest possible score. The Rey Auditory Verbal Learning Test evaluates deferred verbal memory. Here, subjects recall a list of 15 words after an interval of several minutes; the maximum score is 15. The digit-span task was used to measure attention based on the patient's ability to retain a sequence of digits, repeat the sequence, and then provide it in reverse order. The maximum score on this test is 30. Lastly, in the complex figure test, the patient was asked to copy a geometric figure as accurately as possible. The copy is scored according to previously defined criteria, and the highest possible score is 15. These tests were repeated when the patients had completed 4 months of antiretroviral treatment. Results were adjusted for age and educational level based on normative data16–19 in order to determine success or failure on each of the tests. The Beck Depression Inventory was also used to evaluate the patient's mood.
Statistical analysisCategorical variables were presented as absolute and relative frequencies; quantitative variables were presented as measures of central tendency (mean and median) and measures of dispersion (standard deviation and interquartile range). The dependent variable (cognitive function) was measured as a discrete variable (expressed by the score obtained on each of the 6 tests). We used the Wilcoxon signed rank sum test to compare results for each variable during pre- and post-HAART intervals. For purposes of contrasting hypotheses, values of P<.05 were considered statistically significant. Analyses were performed using SPSS statistical software for Windows, version 17.0.
Ethical considerationsThe study protocol was approved by the ethics committees and research committees at Universidad Nacional Mayor de San Marcos (UNMSM) and at Hospital de Huacho. Patients and their legal representatives gave their written consent to participate in the study.
ResultsNineteen patients, comprising 14 men (73.7%) and 5 women (26.3%), completed the study. Their mean age±standard deviation was 33.5±7.1 years. Disease duration, defined as the time between diagnosis of HIV and the study date, ranged from 1 to 240 months; mean duration was 37.8±61.6 months. The mean number of years of schooling was 10.4±3.8.
Seventeen patients (89.5%) were treated with a regimen of zidovudine/lamivudine/efavirenz (AZT-3TC-EFV) and only 2 patients (10.5%) were treated with zidovudine/lamivudine/lopinavir-ritonavir (AZT-3TC-LPV/r). Mean CD4 count in patients before beginning HAART was 142±120.2 and the mean viral load was 4.9±1.2log. Mean CD4 count after 4 months on HAART was 283±186.3 and the mean viral load was 2±1.2log.
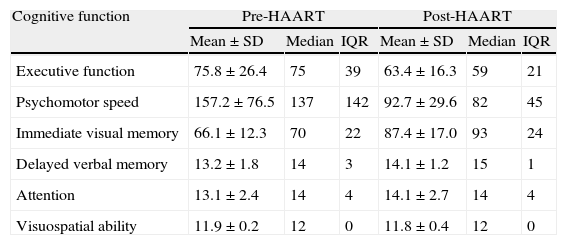
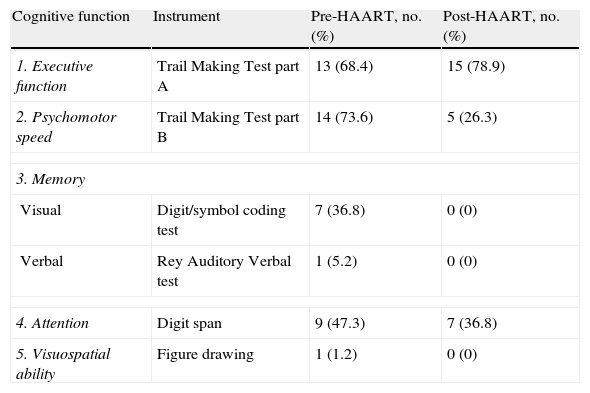
Table 1 lists patients’ scores on neuropsychological tests. Higher scores on executive function or psychomotor speed tests are indicative of poorer cognitive performance. ‘Failure’ on a test was defined as scores above normative values (for the Trail Making Test parts A and B) or (for all other tests) below normative values adjusted for age and years of schooling. According to these criteria, we found that most patients failed tests assessing executive function and psychomotor speed during the first evaluation; on the final evaluation, executive function remained the most impaired area (Table 2). Comparison of baseline scores for each cognitive function to scores 4 months after HAART onset revealed that psychomotor speed and immediate visual memory had improved significantly (P<.05). Executive function, deferred verbal memory, and attention showed non-significant trends toward improvement. Lastly, the test evaluating visuospatial ability revealed no changes; mean values reached before (11.9) and after (11.8) beginning antiretroviral treatment did not show statistically significant variance. On the other hand, 18 of the 19 patients (94.7%) performed the test correctly.
Patients’ scores on the neuropsychological tests before and after starting HAART.
| Cognitive function | Pre-HAART | Post-HAART | ||||
| Mean±SD | Median | IQR | Mean±SD | Median | IQR | |
| Executive function | 75.8±26.4 | 75 | 39 | 63.4±16.3 | 59 | 21 |
| Psychomotor speed | 157.2±76.5 | 137 | 142 | 92.7±29.6 | 82 | 45 |
| Immediate visual memory | 66.1±12.3 | 70 | 22 | 87.4±17.0 | 93 | 24 |
| Delayed verbal memory | 13.2±1.8 | 14 | 3 | 14.1±1.2 | 15 | 1 |
| Attention | 13.1±2.4 | 14 | 4 | 14.1±2.7 | 14 | 4 |
| Visuospatial ability | 11.9±0.2 | 12 | 0 | 11.8±0.4 | 12 | 0 |
SD: standard deviation; IQR: interquartile range.
Percentage of patients failing neuropsychological tests.
| Cognitive function | Instrument | Pre-HAART, no. (%) | Post-HAART, no. (%) |
| 1. Executive function | Trail Making Test part A | 13 (68.4) | 15 (78.9) |
| 2. Psychomotor speed | Trail Making Test part B | 14 (73.6) | 5 (26.3) |
| 3. Memory | |||
| Visual | Digit/symbol coding test | 7 (36.8) | 0 (0) |
| Verbal | Rey Auditory Verbal test | 1 (5.2) | 0 (0) |
| 4. Attention | Digit span | 9 (47.3) | 7 (36.8) |
| 5. Visuospatial ability | Figure drawing | 1 (1.2) | 0 (0) |
Three patients (15.7%) had moderate depression and 7 (36.8%) had mild depression according to Beck Inventory results. The mean scores for each test and differences between results from the two testing periods in the study did not vary significantly in an analysis that excluded the 3 patients presenting moderate depression.
DiscussionFour months after starting HAART treatment, patients displayed significant improvements in the areas of immediate visual memory and psychomotor speed. Similar results have been reported by different studies. In the United States, 25 patients were evaluated before and 8 weeks after starting HAART, and they displayed improvements in executive function, immediate visual memory, and psychomotor speed.11 Similar results were also found in 48 patients in a 6-month follow-up study after starting HAART,20 a 6-month follow-up study of 23 HIV-positive South African patients,21 and a 5-year follow-up study of 101 patients in Australia.22 Psychomotor speed increased after HAART, or after monotherapy with agents with good central nervous system penetrance, in a cohort of 58 patients evaluated by researchers at Johns Hopkins University.10 Paradoxically, other authors have found improvements in cognitive areas (executive function, psychomotor speed, and immediate visual memory) in patients whose treatment was suspended; this could be explained by the neurotoxicity of some antiretroviral drugs.15
Many studies conduct an analysis based on the total score for each patient. Therefore, if they claim that the patient displays less cognitive impairment, we do not know if there has been improvement in all cognitive areas, or if only a few functions have improved. One valuable contribution provided by this study is its breakdown of the variable ‘cognitive function’ into several dimensions so that we can better understand the behaviour of each dimension over time. As such, we observe that executive function, attention, and verbal memory did not improve significantly; this may be due to the short follow-up time. On the other hand, the complex figure drawing test was not sensitive enough to reveal changes in visuospatial abilities. It should therefore be replaced by more difficult tests in later studies so as to avoid the phenomenon known in statistics as the ‘ceiling effect’.
Cognitive alterations due to HIV infection have gained in recognition ever since the first descriptions of AIDS were published in 1981. These manifestations may even arise as mild cognitive impairment in early stages of the disease, and they can be detected in otherwise asymptomatic patients if appropriate neuropsychological tests are used.5,6 Nevertheless, not all studies deliver similar results. Selnes et al.23 performed an 18-month follow-up study on a cohort of 258 patients and controls. Their results did not support gradual cognitive impairment during the asymptomatic phases of infection with HIV, and similar results were found by 2 Italian studies of 328 and 165 patients.24,25 Disparities between results may be due to factors other than HIV infection, including risk factors such as drug use26 and mood,27 protective factors including years of schooling and cognitive reserve,28 and genetic factors.29 This study was based on follow-up in 19 patients soon to start HAART and who manifested no neurological symptoms. All were evaluated with a cognitive test battery before starting treatment, and results showed that most already demonstrated deficits in the executive function, psychomotor speed, immediate memory, and attention tests. Cognitive impairment in early stages of the infection has also been thoroughly documented by multiple researchers.3,5,24 To this end, they used the Trail Making Test parts A and B and similar tasks, and selected subtests from the Wechsler Adult Intelligence Scale (WAIS) and NEUROPSI. These instruments, which have been validated in Spanish-speaking populations in South America and Mexico,18,19 are the same ones used in our study. In conclusion, all patients diagnosed with HIV should complete a baseline neuropsychological evaluation and undergo periodic retesting to determine if he/she is developing cognitive impairment and detect any coadjuvant factors. These steps will help doctors develop strategies for preventing this neurological complication.
Multiple articles have described the impact of antiretroviral drug use. This treatment gained importance after HAART appeared in 1996 and researchers found that the three drug combination elicited reductions in mortality, morbidity, and the appearance of opportunistic infections, thereby increasing quality of life in patients who managed to adhere to the treatment.30–32 In addition to these benefits, it was later discovered that HAART has an effect on neurological impairment, despite evidence from some studies showing that AIDS dementia complex was not lessened due to poor central nervous system penetrance by these drugs.13 On the other hand, a study recently published by Winston et al.33 demonstrated the beneficial effect of different antiretroviral combinations on cognitive impairment in 28 neurologically asymptomatic patients over a follow-up period of 48 weeks. In patients receiving HAART, cognitive impairment is associated not only with decreased quality of life and a poorer prognosis but also with poorer treatment adherence, which further debilitates the patient's condition.4
The high incidence and prevalence rates of HIV in Latin America, especially in Peru, with 22549 cases of AIDS and 32932 cases of HIV infection yielding an incidence rate of 2.65 per 100000 inhabitants,34 require neurologists to investigate topics like the ones raised in this study. However, few published studies have addressed cognitive impairment in Peruvian populations.35 The city of Huacho is home to a large population of HIV-positive patients, but there have been no studies relating to this precise problem.
This study has several limitations, especially its small sample size. Additionally, there was no follow-up on cerebrospinal fluid viral load, which is an important determinant of the degree of neurological impairment.2 Patients with CD4 counts below 200 have poorer results on psychometric tests,24 although some studies have shown that viral load and CD4 lymphocyte count are not associated with cognitive changes.22 Follow-up time was short, but sufficient to detect significant changes; however, a longer study period could help us gain a more in-depth understanding of the behaviour of each cognitive function. Since amelioration of cognitive performance may be attributed to other factors, results from a control group on placebo might support our findings if they were poorer than results from a treatment group. Nevertheless, this is not possible for medical and ethical reasons; HAART cannot be postponed in patients needing treatment. Furthermore, cognitive performance tends to decrease over time in patients with HIV,3,5 even in those in early stages of immune deficiency.14 This argument supports the idea that the cognitive benefits shown here are secondary to starting antiretroviral treatment. It is difficult to rule out a potential practice effect in this type of study when the same patient is able to complete the task more efficiently in the second evaluation. Here, we cannot compare the effect of different combinations of antiretroviral drugs on cognitive function since most of our patients were treated with the standard regimen in Peru (AZT-3TC-EFV) and only two also received protease inhibitors. Regarding this last point, the literature indicates that zidovudine and protease inhibitors are better than other drugs at improving performance on cognitive tests.11
Most of the available data on HIV and cognitive impairment has been gathered in non-Peruvian studies. Cultural diversity and language differences make it difficult to extrapolate these results. On this basis, researchers must design studies in our populations using cognitive evaluation instruments that have been validated in Spanish. Spanish is the native language of almost 10% of the world's population. With this in mind, a group of researchers validated the NEUROPSI battery in a Mexican population,19 and another group of researchers developed the HUMANS battery for Spanish-speaking patients with HIV.36
ConclusionsIn the course of a few weeks, antiretroviral treatment may improve cognitive dysfunction in patients with previously detected deficits. By evaluating cognitive function broken down into its separate components, we find significant improvement in the dimensions of immediate visual memory and psychomotor speed. There was a tendency toward improvement in other dimensions, but differences were not significant. Our study population displays diminished cognitive performance from the earliest stages of HIV infection, even in patients who do not manifest neurological symptoms.
Conflicts of interestThe authors have no conflicts of interest to declare.
Dr Miguel Angel Estela, infectious disease specialist at Hospital Regional de Huacho.
Nila Pajuelo, psychologist at Hospital Regional de Huacho.
Dr Eddy Segura, epidemiologist, Universidad Peruana Cayetano Heredia.
Please cite this article as: Guevara-Silva EA. Deterioro cognitivo y tratamiento antirretroviral en pacientes con virus de la inmunodeficiencia humana en una población peruana. Neurología. 2014;29:224–229.