Non-ergoline dopamine agonists (DA) are effective treatments for Parkinson's disease (PD). This review presents the pharmacology, evidence of efficacy and safety profile of pramipexole, ropinirole, and rotigotine, and practical recommendations are given regarding their use in clinical practice.
ResultsExtended-release formulations of pramipexole and ropinirole and transdermal continuous delivery rotigotine patches are currently available; these may contribute to stabilising of plasma levels.
In early PD, the three drugs significantly improve disability scales, delay time to dyskinesia and allow a later introduction of levodopa. In late PD they reduced total ‘off’-time, improved Unified Parkinson's Disease Rating Scale (UPDRS) in both ‘on’ and ‘off’ state and allowed a reduction in total levodopa dosage. A significant improvement in quality of life scales has also been demonstrated. Extended-release formulations have proved to be non-inferior to the immediate release formulations and are better tolerated (ropinirole). Despite a generally good safety profile, serious adverse events, such as impulse control disorder and sleep attacks, need to be routinely monitored. Although combination therapy has not been addressed in scientific literature, certain combinations, such as apomorphine and another DA, may be helpful. Switching from one DA to another is feasible and safe, although in the first days an overlap of dopaminergic side effects may occur. When treatment with DA is stopped abruptly, dopamine withdrawal syndrome may present. Suspending any DA, especially pramipexole, has been linked to onset of apathy, which may be severe.
ConclusionsNew non-ergotine DAs are a valuable option for the treatment of both early and late PD. Despite their good safety profile, serious adverse effects may appear; these effects may have a pathoplastic effect on the course of PD and need to be monitored.
Los agonistas dopaminérgicos no ergóticos (AD) son tratamientos útiles en la Enfermedad de Parkinson (EP). Revisamos la farmacología, el grado de evidencia en cuanto a eficacia y tolerabilidad de pramipexol, ropinirol y rotigotina, y proponemos algunas recomendaciones para su uso en la práctica clínica.
DesarrolloEn el momento actual se dispone de formas de liberación prolongada (LP) de pramipexol y ropinirol y de administración transdérmica de rotigotina, que contribuyen a una mayor estabilidad plasmática de los niveles del fármaco. En la EP inicial los tres fármacos mejoran de forma significativa las escalas de incapacidad de los pacientes, retrasan la aparición de discinesias y permiten retrasar la introducción de levodopa. En la EP avanzada reducen el tiempo off, mejoran la UPDRS en on y en off y permiten reducir la dosis total de levodopa. Además, los tres han sido capaces de inducir una mejoría significativa en las escalas de la calidad de vida relacionada con la salud. Las fórmulas de LP han demostrado la no inferioridad frente a las de liberación inmediata, e incluso una mejor tolerabilidad (ropinirol). A pesar de su buen perfil de seguridad, entre los efectos adversos graves cabe destacar el trastorno de control de impulsos, cuya aparición puede ser precoz, y los accesos de sueño (sleep attacks). Aunque la terapia combinada no ha sido estudiada específicamente, algunas asociaciones (como la de apomorfina y otros AD) pueden ser beneficiosas. El cambio de un AD a otro es factible de un día para otro, aunque en los primeros días puede haber una sumación de efectos adversos dopaminérgicos que debe tenerse en cuenta. La suspensión brusca del tratamiento con AD puede inducir un síndrome de deprivación dopaminérgica. La retirada de cualquier AD, en particular pramipexol, se ha asociado a aparición de apatía que puede ser grave.
ConclusionesLos nuevos AD no ergóticos constituyen una opción válida de tratamiento de la EP tanto inicial como avanzada. A pesar de su buen perfil de tolerabilidad, no están exentos de efectos adversos graves, que pueden tener un efecto patoplástico en la EP y que deben monitorizarse.
Levodopa remains the most effective symptomatic treatment for Parkinson's disease (PD). However, its relationship to the onset of motor complications (fluctuations and dyskinesia) places some limits on its use, especially in patients younger than 70 with mild PD symptoms. Dopaminergic agonists (DA) provide a safe and effective alternative to levodopa in younger patients and using these drugs is associated with a lower incidence of motor complications at the 5-year mark. They are effective both as monotherapy in early stages of the disease and in combination with levodopa in advanced PD. Ergot derivatives were the first DAs available, but use of these drugs is currently restricted due to risk of fibrotic valvular heart disease1,2 and they should never be considered for first-line treatment.
Non-ergoline DAs may be administered orally (pramipexole and ropinirole), transdermally (rotigotine) or subcutaneously (apomorphine). Extended-release oral formulations allowing patients to take a single daily dose have recently been introduced. These formulations were developed in order to achieve more stable plasma drug concentrations, thereby minimising motor fluctuations as much as possible. These fluctuations are at least partially derived from oscillations in plasma drug concentrations.2 Another DA, rotigotine, was recently designed for the same purpose; the first non-ergoline, transdermally delivered DA has an effectiveness and safety profile similar to those of other drugs in its class.
While the new DAs are not without severe adverse effects, particularly impulse control disorder (ICD), their effect on quality of life for PD patients has been unquestionably beneficial. They have also considerably widened the range of treatment strategies available to neurologists. In this review, we will use a practical approach to analyse pharmacological factors, adverse effects, and clinical utility of the three most widely used dopaminergic agonists in clinical practice (pramipexole, ropinirole, and rotigotine).
Pharmacokinetics and pharmacodynamicsPramipexolePramipexole's chemical name is (S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole. It is a potent D2 agonist with maximum affinity for the D3 subtype (Table 1).2 Immediate-release (IR) pramipexole is rapidly absorbed and reaches a maximum concentration between 1 and 2hours after administration, or later if administered with food. Its bioavailability is high at around 90%. Pramipexole displays linear pharmacokinetics, low protein-binding (<20%), and a large volume of distribution (400L). Most of the dose (80%) is eliminated unchanged in urine by active tubular secretion. It should not be used in patients with advanced renal failure, and doses must be adjusted in patients with mild or moderate renal insufficiency (see ‘Posology’). No significant drug interactions have been described except for potential decrease in urinary excretion of pramipexole among patients treated concomitantly with cimetidine and amantadine.3
Affinity of dopaminergic agonists for different subtypes of dopamine, serotonin, and alpha-adrenergic receptors, expressed as an inhibitor constant (nmol/L, inversely proportional to the affinity for the receptor).
| Receptor | Pramipexole | Ropinirole | Rotigotine |
| D1 | >10 000 | >10 000 | 83 |
| D2 | 1326.6 | 804.7 | 13.5 |
| D3 | 10.5 | 37.2 | 0.71 |
| D4 | 128.8 | 851.1 | 8.3 |
| D5 | >10 000 | >10 000 | 5.4 |
| 5-HT1A | 691.8 | 288 | 30 |
| 5-HT1B | 8317.6 | >10 000 | N/A |
| 5-HT1D | 1659.6 | 1380.4 | 853 |
| 5-HT2A | >10 000 | >10 000 | N/A |
| 5-HT2B | >10 000 | 3801.9 | 1950 |
| α2B | 631.0 | 758.6 | 27 |
| α2C | 69.2 | 1202.3 | 135 |
In the case of extended-release (ER) pramipexole, the formulation uses a matrix tablet with carbomer which promotes slow and constant release of the agent by diffusion and erosion. Maximum concentration is reached 6hours after drug administration, and fluctuations in drug plasma levels are minimal compared to those reached in patients taking the IR formulation 3 times daily. Plasma concentrations stabilise after 5 days of treatment.4,5
RopiniroleRopinirole is an indole derivative whose chemical name is 4-[2-(dipropylamino)ethyl]-1,3-dihydroindol-2-one. It is highly lipophilic and has a large volume of distribution (6.7L/kg). This potent DA binds to both peripheral and central receptors, and has maximum affinity for the D3 subtype (Table 1). The IR formulation is rapidly absorbed and reaches its maximum concentration in 1 to 2hours (or 1 to 2hours later if taken with food); bioavailability is 50%. It displays low plasma protein binding (10%-39%) which is not clinically relevant. Ropinirole is metabolised in the liver by cytochrome P450 prior to elimination in urine; 10% remains active at elimination. Regarding drug interactions, ciprofloxacin, fluvoxamine, and high-dose oestrogen (as in hormone replacement therapy) increase plasma concentrations of ropinirole, while tobacco use may decrease them. Ropinirole clearance has been calculated as 15% lower in subjects older than 65 years.6,7
The extended-release (ER) formulation makes use of a matrix core with a double-layer coating (one impermeable and the other semi-permeable). The tablet becomes gelatinous in the presence of intestinal juices, which permits slow and uniform release and absorption of the drug over 24hours, regardless of mealtimes. Comparative studies have shown that the ER formulation achieves more stable ropinirole plasma concentrations, with fewer oscillations, than when similar doses of IR formulations are administered 3 times daily. Bioavailability of ER formulations remains near 50%, with linear pharmacokinetics and a latency of 6 to 10hours before the maximum concentration is reached.6,7
RotigotineRotigotine's chemical name is (6S)-6-[propyl(2-thiophen-2-ylethyl)amino]-5,6,7,8-tetrahydronaphthalen-1-ol. This compound has a low molecular weight and it is highly lipid-soluble; these characteristics allow it to diffuse through the stratum corneum of the skin. It is delivered on a silicone adhesive matrix with an aluminised polyester film coating. Drug release is constant and proportional to the surface area of the patch. It has a high affinity for D3 receptors (Table 1), as well as an agonist effect on 5HT1A serotonin receptors and an antagonist effect on α2B and α2C adrenergic receptors.8
While its bioavailability is less than 1% by the oral route, and despite inter- and intra-individual variations, rotigotine displays good transdermal absorption which amounts to an average of 46.1%±10.6% of the total patch content. For this reason, the 2mg patch contains a total of 4.5mg of the drug; the 4mg patch, 9mg; the 6mg patch, 13.5mg; and the 8mg patch, 18mg. Two new formats were recently added which deliver 1 and 3mg. Non-absorbed content remains in the patch. Provided the patch is changed daily, plasma concentrations are stable with no peaks and valleys; differences due to where the patch is placed are not significant. Plasma concentrations stabilise after 2 to 3 days of treatment. If a patch is left unchanged for more than 24hours, concentrations would be less stable and undesirable fluctuations could occur. Rotigotine is metabolised in the liver by cytochrome P450 and glucuronidation. Its metabolites are eliminated in urine (<1% eliminated actively) and haemodialysis does not affect its plasma concentrations. Rotigotine's protein binding rate is over 90%. No relevant drug interactions have been described (not even for drugs metabolised by cytochrome P450), and there are no reports of plasma concentration changes in cases of hypoalbuminaemia or administration of other plasma protein-bound drugs.8
PosologyTable 2 shows the recommended doses for each of these DAs in the up-titrations that are typically performed on a weekly basis. An initial evaluation at approximately 1 month of treatment is recommended in order to evaluate drug tolerability and safety, especially after recognising severe adverse effects which may appear in early stages when high doses of DAs are used (see ‘Adverse effects and specific precautions’). In most cases, an additional increase will be needed at a later date to reach the usual therapeutic dose. At present, the ER formulations of pramipexole and ropinirole demonstrate clear advantages compared to IR formulations. These include simpler posology and dose titration, increased ease of use for the patient, and probably better treatment adherence (see ‘Equivalence, drug switching, and combination treatment’).
Recommended dose titration for pramipexole, ropinirole, and rotigotine.
| Mirapexin IRa 0.18/0.7mg | Mirapexin ERa 0.26/1.05/2.1mg | Requip IR 0.25/0.5/1/2/5mg | Requip prolib 2/4/8mg | Neupro 1/2/3/4/6/8mg | |
| 1 | (½0.18)/8h | 0.26mg | 0.5/8h | 2mg | 2mg (10cm2) |
| 2 | (0.18)/8h | 2×0.26mg | 0.5+½0.5/8h | 4mg | 4mg (20cm2) |
| 3 | (2×0.18)/8h | 1.05mgb | 1/8h | 2+4mg | 6mg (30cm2) |
| 4 | (½0.7)/8hb | 1.05+0.26mg | 1.5-2/8hb | 8mgb | 8mgb (40cm2) |
| 5 | (½0.7+0.18)/8h | 1.05+2×0.26mg | 3-4/8h | 12mg | 8mg+4mgc |
| 6 | (0.7)/8hc | 2.1mgc | 5-6/8hc | 16mgc | 8mg+8mg |
| 7 | (0.7+0.18)/8h | 2.1+2×0.26mg | 7/8 hc | 20mgc | |
| 8 | (0.7+½0.7)/8h | 3.2mg | 8/8h | 24mg |
IR: immediate release; ER: extended release.
Source: compiled by authors.
Pramipexole IR is available in 0.18mg or 0.7mg tablets to be administered 3 times daily. Pramipexole ER is distributed in extended-release tablets dosed at 0.26mg, 1.05mg, and 2.1mg. These tablets should not be broken and they are to be taken once daily in the morning, either with or without food. We recommend planning dose up-titration in 7 to 10 day periods, as shown in Table 2. For patients previously treated with pramipexole IR, it has been shown that overnight switching to the single dose closest to the previous total dose is safe and efficacious (data from same study: IR:ER ratio of 1:1.05). Pramipexole ER should not be used in patients with a creatinine clearance level below 30mL/min. The dose should be titrated in patients whose creatinine clearance level falls between 30 and 50mL/min, beginning with 0.26mg every 48hours in the first week, continuing with 0.26mg daily in the second week and increasing the dose by an additional 0.26mg weekly until reaching a maximum daily dose of 1.57mg (1.05+0.26+0.26mg).3–5
Ropinirole is available in IR tablets dosed at 0.25, 0.5, 1, 2, and 5mg and ER tablets dosed at 2, 4, and 8mg. As with pramipexole ER, ropinirole ER tablets should not be broken and they are administered in a single dose in the morning with or without food. Dose up-titration is also performed on a weekly basis until reaching 8mg daily in the first month; most patients will need their doses increased to 16 to 18mg daily. Ropinirole does not need dose adjustments in cases of mild to moderate renal insufficiency. However, it should not be used in patients with renal failure and creatinine clearance of less than 30mL/min, or in those with severe liver failure.6,7
Rotigotine is available as patches dosed at 1, 2, 3, 4, 6, and 8mg. Rotigotine patches are affixed to clean, dry skin (free from lotions or creams) on the trunk or limbs by applying local pressure for 30-60seconds. The patch is changed every 24hours, rotating the site of patch application so as not to use the same site twice in a 2-week period. It may be necessary to shave or clip hair at the application site to achieve better adhesion. Patches are to be stored refrigerated (2-8°C). Cutting the patch is not recommended; while this will not affect delivery of the drug, it could create problems for patch adhesion. Its aluminium content may set off metal detectors in airports, become hot in saunas or in direct sunlight, and cause burns if the patch remains on when an MRI scan or electric cardioversion is being performed. The initial 2mg dose is titrated in increments of 2mg every week until reaching 8mg at one month. It will then be adjusted according to clinical response until reaching a maximum dose of 16mg. Dose adjustment is not necessary in cases of moderate liver failure or advanced renal failure.8
Evidence of effectivenessAll non-ergoline DAs have demonstrated class I level of evidence as monotherapy for early PD (Table 3) and advanced PD when associated with levodopa (Table 4).9,10 A 2010 meta-analysis of all the controlled trials performed with DAs (ergoline and non-ergoline) found a mean reduction in ‘off’ time of 1.54hours, a mean reduction in levodopa of 116mg, and a decrease of 10 points on the total Unified Parkinson's Disease Rating Scale (UPDRS) score, with an odds ratio of 2.70 for dyskinesias, compared to placebo.11
Comparative efficacy of different dopaminergic agonists in early-stage Parkinson's disease.
| DA | Change in UPDRS score | Motor complications in the long term | Study period |
| Pramipexole12,13,49 | [IR] −3.4 (−7.3 LD) | [IR] 28% dyskinesias (51% LD); RR 0.37 for dyskinesias, RR 0.68 for wearing off | [IR] 5 years |
| [ER] −8.1 (5.1 PL) | [ER] N/A | [ER] 18 weeks | |
| Ropinirole7–19 | [IR] −0.8 (−4.8 LD) | [IR] 5% (35% LD); OR 0.3 for dyskinesias, 0.3 for wearing off | [IR] 5 years |
| [ER] decrease>30% in 77% (66% IR, NS) | [ER] N/A | [ER] 20 weeks | |
| Rotigotine24–26 | −7.2 (−2 PL) | N/A | 13 weeks |
LD: levodopa; IR: immediate release; ER: extended release; N/A: not available; NS: not significant; OR: odds ratio; PL: placebo; RR: relative risk.
Comparative efficacy of different dopaminergic agonists in advanced-stage Parkinson's disease.
| DA | Decrease in ‘off’ time | Change in UPDRS score | Reduction in levodopa dose |
| Pramipexole15,16 | [IR] −12% (+2.1% PL) | Improvement: 37.3% on UPDRS (12% PL) | −27% (+5% PL) |
| Ropinirole2,20,22,23 | [IR] −20% (−11% PL) | [ER] Improvement: 10.2 points (7.9 IR) | [IR] 20% in 7% of the patients |
| [ER]>20% in 66% of the patients (51% IR) | |||
| Rotigotine27,28 | −2.7 (0.9 PL) | Improvement: 8.7 points (3.4 PL) | N/A |
IR: immediate release; ER: extended release; N/A: not available; PL: placebo.
CALM-PD, a double-blind randomised trial, showed that starting PD treatment with pramipexole IR significantly reduced the incidence of dyskinesias and motor fluctuations compared to starting levodopa (28% vs 51%). Despite the fact that control over motor symptoms was better in the levodopa group (−7.3 points vs −3.4 points on UPDRS for pramipexole), 32% of the patients remained on pramipexole monotherapy at the end of the study (5 years).12 Results from the two extension phases of the study have been published. In the first phase (4 years after randomisation), initial treatment with pramipexole was associated with a lower risk of dyskinesias (relative risk [RR] 0.37) and of ‘wearing-off’ phenomena (RR 0.68). However, disabling dyskinesias occurred in similar percentages in both groups and levodopa was associated with a lower risk of freezing of gait (25.3% vs 37.1%, RR 1.7).13 This last finding was not confirmed in the second extension phase (6 years after randomisation): an analysis of 108 subjects in the pramipexole group and 114 in the levodopa group showed a lower risk of freezing of gait, 26.2% vs 34.7% (P=.3). Furthermore, the rate of motor fluctuations (wearing-off and on/off fluctuations) and dyskinesia remained lower in the group beginning treatment with pramipexole (50% vs 68.4%). Both groups had similar values on the UPDRS (daily life and motor subscales) and on quality of life scales.14 The group treated with pramipexole scored significantly higher for drowsiness (11.3 vs 8.6 on the Epworth Sleepiness Scale [ESS]) as well as having higher rates of oedema (27.1 vs 14.4) than the levodopa group. The researchers concluded that both pramipexole and levodopa are effective over a long term as initial treatments for PD, with a lower risk of onset of motor complications with the former drug and a lower risk of drowsiness with the latter.
In advanced PD, several placebo-controlled studies have shown that pramipexole causes significant decreases in UPDRS scores in both ‘on’ and ‘off’ times (overall decrease of 37.3% vs 12% for placebo in a study of 78 patients over 11 weeks). Pramipexole treatment also resulted in a decrease in ‘off’ time (by 12% vs a 2% increase with placebo in the same study)15 and a decrease in the levodopa dose (27% vs 5% with placebo in another study of 360 patients over 32 weeks).16 Adverse dopaminergic effects were more frequent in the group of patients treated with pramipexole in both studies, although the rate of treatment withdrawal due to poor tolerance was low (9% and 11.6% for pramipexole vs 11% and 10.1% for placebo). A high percentage of randomised patients in both groups completed the study (87% and 80.8%).15,16
RopiniroleThe 056 Study showed that administering ropinirole IR as monotherapy in early PD resulted in a lower dyskinesia rate than with levodopa (5% with ropinirole monotherapy, 35% with levodopa, and 20% with combined treatment), plus a higher probability of remaining dyskinesia-free (OR 2.82). As in pramipexole studies, scores on the UPDRS part II were similar in both patient groups, but patients treated with levodopa experienced a more marked decrease on the motor subscale score than the group treated with ropinirole (−0.8±10.1 for ropinirole and −4.8±8.3 for levodopa). At the end of the study (5 years), the mean dose of ropinirole used was 16.5mg; 30% of the patients assigned to that treatment group remained in monotherapy.17 The open extension of the trial lasted 10 years and included 42 patients from the ropinirole group and 27 from the levodopa group. The study confirmed a lower risk of developing dyskinesia with ropinirole (OR 0.3), as well as a longer time before appearance of dyskinesias and a lower incidence of ‘wearing off’ (OR 0.3).18 The high withdrawal rate constitutes a significant limitation for this study. Among trials in advanced PD, we find a noteworthy study in 149 patients demonstrating potential for reducing the total levodopa dose (decrease of 20% in 27% of the patients) and ‘off’ time (from 20% vs 11%) for ropinirole IR compared to placebo after 6 months of treatment.2
At the same time, the EASE-PD Study 168 (ropinirole IR vs ER at doses of 8 to 12mg) and Study 169 (ER controlled with placebo) demonstrated the safety and efficacy of treatment with ropinirole ER in monotherapy for early PD and ropinirole-levodopa combination treatment for advanced PD. In cases of early PD, ropinirole ER reduced the baseline UPDRS score by 77% in treated patients (vs 66% in treatment with ropinirole IR; difference was not significant).19 In advanced PD (mean of 7hours ‘off’ time at the start of the study), ropinirole ER reduced ‘off’ time by a mean of 2.1hours, compared to 0.3hours for the placebo (mean difference of 1.7hours). It increased ‘on’ time and ‘on’ time without dyskinesias, and resulted in significantly better scores on the depression and quality of life scales compared to placebo.20 Similarly, the PREPARED study demonstrated the non-inferiority and tolerability of ropinirole ER compared to the IR formulation in combination therapy with levodopa for advanced PD. At 24 weeks of treatment with a mean dose of 18.4mg ropinirole ER vs 10.6mg ropinirole IR, the study found that the ER group had a higher percentage of patients with decreases in ‘off’ time exceeding 20% (66% ER vs 51% IR) and a more pronounced mean decrease on UPDRS part III (10.2 ER vs 7.9 IR). Both differences were statistically significant. The incidence of adverse effects was lower in the group treated with ER, although this difference was not significant.21 The 228 Study compared adding ropinirole ER to increasing the levodopa dose in patients treated with levodopa whose symptoms were not optimally controlled. Results showed a lower incidence of dyskinesia at 28 weeks of treatment in the group treated with ropinirole ER compared to the group treated with increased doses of levodopa (3% with a mean ropinirole dose of 10mg vs 17% with a mean additional levodopa dose of 284mg). These researchers did not observe significant differences in UPDRS scores between the two patient groups (−3.7 and −3.4 points on the motor subscale), or in the percentage of patients who developed motor fluctuations (54% and 57%). Regarding side effects, we should point out that the group treated with ropinirole presented a higher level of drowsiness as measured by the ESS than the group treated with levodopa (0.9±3.5 vs −0.2±2.9). This study's limitations included its early termination and its small sample size.22,23
RotigotineThere have been several placebo-controlled trials of rotigotine, both as monotherapy for early PD and as combination treatment with levodopa in advanced PD.24,25 The monotherapy studies in early PD revealed that the score on UPDRS part III was approximately 3.5 points lower in the low-dose rotigotine group (2–6mg) than in the placebo group.24 The SURE-PD study (SP513) compared the efficacy of rotigotine (8mg) to ropinirole IR (up to 24mg). Both treatments were more effective than placebo (at 13 weeks, decreases in UPDRS score of 11 and 7.2 points vs 2 points for placebo). The study was not able to show non-inferiority of 8mg rotigotine to ropinirole. However, analysis of the patient subgroup treated with 12mg ropinirole IR found no significant differences in efficacy compared to rotigotine (decrease in UPDRS score of 7.2 points for ropinirole vs 8.4 for rotigotine).26
In advanced PD (PREFER study, SP650), the 8mg and 12mg doses of rotigotine caused significant decreases in the UPDRS part III score at 24 weeks of treatment (6.8 and 8.7 points, respectively, vs 3.4 points for placebo). Researchers also observed a significant decrease in ‘off’ time for the 8mg dose (2.7hours vs 2.1hours for the 12mg dose and 0.9hours for placebo).27 The CLEOPATRA study (SP515) studied the efficacy of rotigotine for reducing ‘off’ time in patients with advanced PD and treated with levodopa. It compared adding pramipexole (mean dose 3.1mg), rotigotine (mean dose 12.95mg) or placebo to levodopa treatment. Researchers found similar reductions in ‘off’ time for both agonists (1.94hours for pramipexole and 1.58hours for rotigotine), in addition to similar responder rates (67% and 59.7%, respectively, as defined by decreases in the UPDRS score of more than 30%).28 Lastly, the RECOVER study analysed the effect of rotigotine treatment on sleep in both de novo patients and those already treated with levodopa. In both groups, rotigotine significantly improved sleep quality (decrease of 5.9 points on the PD sleep scale [PDSS] vs 1.9 points for placebo) and the morning score on UPDRS part III (7 points vs 3.9 for placebo).29
NeuroprotectionIt has been suggested that DAs may have a neuroprotective effect due to their antioxidant properties (pramipexole functions as a scavenger of free radicals, inhibits apoptosis, and acts directly on the mitochondrial membrane). In addition, all DAs decrease the release of dopamine (mediated by presynaptic autoreceptors), thus decreasing the formation of free radicals derived from dopamine metabolism as well.30 Although in vivo studies and animal models have shown that many ergoline and non-ergoline DAs have a neuroprotective effect, there is no evidence in PD patients that DAs would exert a clinically relevant disease-modifying effect. Some neuroimaging studies with a fluorodopa PET scan (for ropinirole) or DAT scan (pramipexole) indicated that treatment with DA resulted in less pronounced loss of nigrostriatal projections than levodopa treatment. Nevertheless, later studies have shown that DAs and levodopa modify the activity of central DOPA decarboxylase and dopamine transporters, which invalidates the results obtained in other neuroimaging studies that pointed to a neuroprotective effect.31
Clinical utility and recommendations for useIn initial stages of PD, DAs elicit significant improvement on the patient's disability scales, delay the appearance of dyskinesia, and allow doctors to push back levodopa treatment. In advanced PD, they reduce ‘off’ time, improve UPDRS score in ‘on’ and ‘off’ time, and enable us to decrease the total levodopa dose.2,9,10 Additionally, the three non-ergoline DAs elicit significant improvements, compared to placebo, on health-related quality of life scales (pramipexole in advanced PD and PD with depression, ropinirole in patients with fluctuations, and rotigotine in patients experiencing wearing-off).32
In studies with small sample sizes, pramipexole was shown to significantly reduce scores on tremor scales and logs (by 48% to 65% compared to placebo).33,34 Nevertheless, we lack well-designed studies that directly compare the two drugs and conclude that pramipexole may be superior to levodopa (which has an estimated anti-tremor effect of 30%-50%).34 The antidepressant effect of pramipexole, both as monotherapy and associated with levodopa, has been highlighted by various controlled studies. Efficacy results show pramipexole to be superior to placebo (63%-64% vs 43%-45%) and even comparable to sertraline for treating symptoms of depression in PD. These results are independent from improvements in motor symptoms, which would indicate that the drug has a direct antidepressant effect. Nevertheless, most of these studies include a heterogeneous population and varied measurement instruments, which limits the extent to which its conclusions can be generalised.35,36 Dose titration for ropinirole ER is simple, and the drug provides motor benefits with a low risk of dyskinesia. In patients on levodopa who display poor motor control, especially those already presenting troublesome dyskinesia, associating ropinirole ER is an effective alternative to increasing the levodopa dose.23 Rotigotine may be especially useful in patients with nocturnal and morning symptoms.29 It also provides the additional advantage of not needing to be suspended before surgery.37
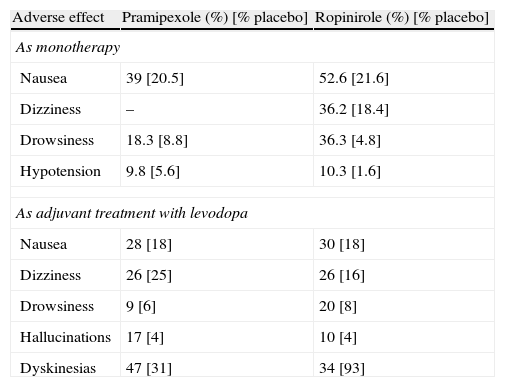
Adverse effects and special precautionsCentral dopaminergic adverse effects (hallucinations, psychosis, dyskinesias, dizziness, drowsiness) and peripheral effects (nausea, orthostatic hypotension, oedema) have been reported in various percentages of the population for the three non-ergoline DAs. They may also appear in levodopa treatment (Table 5).2,31 All clinical trials have shown a higher incidence rate of hallucinations and drowsiness with DAs than with levodopa (DA overall number needed to harm: 15 for hallucinations vs only 3 for drowsiness).38
Adverse dopaminergic effects of pramipexole (immediate release) and ropinirole (immediate release) vs placebo.
| Adverse effect | Pramipexole (%) [% placebo] | Ropinirole (%) [% placebo] |
| As monotherapy | ||
| Nausea | 39 [20.5] | 52.6 [21.6] |
| Dizziness | – | 36.2 [18.4] |
| Drowsiness | 18.3 [8.8] | 36.3 [4.8] |
| Hypotension | 9.8 [5.6] | 10.3 [1.6] |
| As adjuvant treatment with levodopa | ||
| Nausea | 28 [18] | 30 [18] |
| Dizziness | 26 [25] | 26 [16] |
| Drowsiness | 9 [6] | 20 [8] |
| Hallucinations | 17 [4] | 10 [4] |
| Dyskinesias | 47 [31] | 34 [93] |
A recent meta-analysis compared the adverse effects of several ergoline and non-ergoline DAs to establish any differences. The RR for nausea was similar between ropinirole and rotigotine (RR 2.25 and 2.08) and superior in both cases to pramipexole in the indirect comparison (RR 1.48) The RR for vomiting was considerably higher for rotigotine than for placebo (5.31); the difference was also significant for ropinirole (2.84), but not for pramipexole (0.82). In the comparison between ropinirole and pramipexole, the RRs for dizziness (1.87 vs 1.20), somnolence (2.45 vs 1.68) and dyskinesias (2.71 vs 2.27) were moderately higher for ropinirole. Comparison of ropinirole and rotigotine revealed no significant differences regarding somnolence (RR 1.15 in direct comparison, ropinirole vs placebo 2.45, and rotigotine vs placebo 1.35). All DAs had higher RRs than placebo for hallucinations; RRs for rotigotine (4.02) and pramipexole (3.36) were higher than those for ropinirole (2.84). The RR for confusional state was significantly higher for pramipexole than placebo (RR 2.64), with no significant differences in the cases of ropinirole and rotigotine vs placebo.39 In general terms, intolerance to one of the three non-ergoline DAs does not predict cross-intolerance to others. If intolerance persists despite reducing the DA in question to the minimum effective dose, trying one of the other two drugs is recommended. An exception would be the appearance of psychosis or ICD, conditions which would require non-DA treatment options.
The global odds ratio for drowsiness with AD treatment is estimated at 2.9 vs 1.9 with levodopa. Identified risk factors include the DA dose, concomitant levodopa dose, age, clinical severity of PD, and being male.38 In an open study of patients with sleep disorders and treated with ropinirole IR, researchers observed significant improvement on the ESS and PDSS in various parameters after switching to ropinirole ER.40
Sleep attacks are sudden episodes of sleep that occur unexpectedly with no prodromes. They can be truly dangerous in certain situations, such as driving a vehicle. The first description of these attacks in patients treated with DAs (pramipexole and ropinirole) appeared in 1999.41 A multi-centre study of 6620 patients with PD treated with DAs revealed a rate of excessive daytime drowsiness of 42%, and 10% of these patients experienced sleep attacks (4% of the total). Both ergoline and non-ergoline DAs have been linked to sleep attacks, with onset being earlier for the non-ergoline class.33 Rotigotine has also been associated with this adverse effect. A study comparing the drug to ropinirole IR found six cases in the rotigotine group (2.8%) and four for rotigotine (1.8%).26 Age younger than 70 years and a history of PD of less than 7 years may also constitute risk factors.33
Impulse control disorder (ICD) associated with PD is gaining in recognition and it is a frequent phenomenon (prevalence between 6% and 15%). Many factors contribute to this entity, but DAs play an undeniable role in its pathogenesis.42 The most frequent manifestations are pathological gambling, hypersexuality, compulsive shopping, compulsive eating, and excessive internet use. It typically affects younger males with early-onset PD. A personal or family history of ICD or substance abuse, or certain premorbid personality traits (impulsiveness, thrill-seeking behaviour) constitute clear risk factors.42,43 Polymorphisms of dopamine receptors have been associated with substance abuse and ICT in individuals with and without PD. In addition, analogous changes have been described in functional neuroimaging studies (increased dopamine release in the ventral striatum, lower density of D2/D3 receptors, and hypofunction of dopaminergic structures in the reward system) in both population groups.42,44 Although these data point towards a probable neurobiological vulnerability, the crucial element in the clinical onset of ICD is treatment with DA. Although the traditional view holds that pramipexole is more active in ICD than the other DAs (the fact that pramipexole is the most commonly prescribed drug in this risk group may constitute a bias), ropinirole has a similar risk potential, and cases with rotigotine have also been published.42,45,46 The absolute increase in risk has been calculated at 13% for all DAs.38 DOMINION, a recent cross-sectional study of more than 3000 patients, calculated an OR of 2.72 (2.08-3.54) for all DAs and found no significant differences between pramipexole (17.7%) and ropinirole (15.5%).47 Polytherapy and high doses of DA are the factors most frequently associated with developing ICT, although cases have also been described in patients treated for restless legs syndrome with low doses of DAs.48 Concern for this situation has led researchers to employ different ICT screening tests (modified Minnesota Impulse Disorders Interview [mMIDI], Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease [QUIP]) as a safety measure in DA clinical trials. Two randomised trials comparing pramipexole ER to pramipexole IR, placebo (259 patients), or to pramipexole IR only (156 patients), found 6 cases of ICD (3 with the IR formulation and 3 with the ER formulation). This accounted for approximately 1% of the patients actively being treated in short time periods (18 and 9 weeks, respectively).49,50 ICT should be monitored closely because of its frequency and severity, and also due to potential medical and legal repercussions. Patients and family members should be informed before this treatment is started, and doctors must be alert to any symptoms that might indicate a pathological increase in impulsive behaviour. Treatment for ICT is multidisciplinary and includes use of psychotherapy and support groups. The first phase entails reducing or suspending the DA drug. It is often necessary to start levodopa treatment, or else increase the dose.43 While treatment with amantadine is controversial, controlled trials have demonstrated its utility in treating ICD.42,51 Other drugs have also demonstrated their benefits in open trials (clozapine, quetiapine, topiramate, bupropion, zonisamide, naloxone, and valproate).42,43 Results from deep stimulation therapy are not very encouraging. In published series, symptoms in half of the patients improve and the other half may experience exacerbations or even onset of a de novo ICT.52 The overall prognosis for ICT in PD is unfavourable. The condition may persist despite suspending DA treatment, and withdrawing the drug may also result in cases of apathy or depression which will not necessarily be reversible. Consequences in the patient's family and social environments may be difficult to overcome.
Dropped head syndrome refers to exaggerated anterior neck flexion resulting from weak extensors or dystonic neck flexors. It has considerable impact on the patient's speech, swallowing, and quality of life.53 It is thought to be uncommon in PD (<5%), but the condition has been linked in some of these cases to treatment with DAs, especially pramipexole and cabergoline. Presentation is subacute, typically occurring a few weeks or months after treatment onset. Reducing or suspending DA treatment and starting levodopa or increasing the levodopa dose may lessen symptoms.53,54
One characteristic and frequent adverse effect of rotigotine patches are skin reactions, which affect up to 46% of the patients included in clinical trials.25 These reactions are normally mild to moderate and rarely result in suspension of treatment (in less than 5% of all cases). These mild rashes should be distinguished from the allergic reactions that do require suspension of treatment and which may present in patients with sulphite allergies (the patch contains metabisulphite). Many patients report that the patch comes loose easily.8 Although prolonged pressure (one minute) or shaving the site may improve patch adhesion, this complication may be a very real obstacle to treatment compliance.
Special precautions when suspending treatmentAbruptly suspending any DA may trigger a dopamine withdrawal syndrome, suspension should always be gradual. For pramipexole, a daily decrease of 0.52mg (0.26mg×2) is recommended; once the dose has reached 0.52mg, it is reduced by 0.26mg daily until treatment has been suspended completely. Ropinirole can be suspended by reducing the dose by 4mg daily to reach 8mg, followed by daily decreases of 2mg until treatment is completely suspended. For rotigotine, decreases of 2mg are applied every 24hours until treatment has been suspended completely.3,6,8
Suspending DA treatment, after deep stimulation surgery for example, has been associated with developing apathy. This is especially true of pramipexole. This complication is difficult to treat and it may entail a poorer outlook. For this reason, suspending treatment is not a priority if adverse effects are not present.
Equivalence, drug switching, and combination treatmentSwitching from one DA to another is common in clinical practice, whether due to lack of effectiveness or the development of adverse effects. Table 6 lists approximate dose equivalents for the different DA drugs.
Approximate dose equivalents for pramipexole, ropinirole, and rotigotine.
| Dopaminergic agonists | Dose equivalents | Levodopa equivalent dose |
| Pramipexole IRa: rotigotine IR | 1mg: 4mg | 1mg: 100mg |
| Pramipexole IRa: ropinirole ER | 1mg: 4mg | |
| Ropinirole IR: rotigotine | 1-1.5mg: 1mg | 3mg ropinirole: 100mg |
| 3mg ropinirole: 100mg | ||
| Ropinirole IR: pramipexole IR | 1.5mg: 1mg | |
| Pramipexole IRa: pramipexole ERa | 1mg: 1.5mg | |
| Ropinirole IR: ropinirole ER | Approximately 1:1; round total dose of IR to 2, 4, 6, 8, 12, 16, 20, and 24mg |
IR: immediate release; ER: extended release.
Source: compiled by authors. References in the text: 3–5, 19, 21,22, 40, 49,50, 55–57.
Regarding the schedule for the switching process, a clinical trial showed good tolerability for an overnight switch from ropinirole IR or pramipexole IR to rotigotine.55 A drug overlap period of 2 days in the first case, and 3 days in the second, was calculated by multiplying mean drug clearance by 5 (see Table 7). Adverse effects were more frequent in this period of the study so patients should be warned about this possibility. There have been no studies that evaluate the switch from the extended-release formulations of these drugs to rotigotine. However, the overlap period would probably be longer in this case.
Researchers studied switching from pramipexole IR to ropinirole ER in an open clinical trial. The most common adverse effects were exacerbation of PD symptoms, drowsiness, nausea, and dizziness; most cases resolved with dose adjustment. Thirteen out of 60 patients stopped treatment with ropinirole ER. All had advanced PD and their pramipexole dose was approximately 4mg/day. Conversion of pramipexole IR to ropinirole ER at a ratio of 1:4 is associated with better adherence to and a preference for ropinirole ER and less need for dose adjustment. The switch was performed overnight in a clinic and patients were monitored for orthostatic hypotension (no significant changes were detected). Telephone check-ups were conducted over the first 5 days as a safety measure.56
An open DA conversion study investigated a switch to pramipexole IR in 34 patients previously treated with ropinirole (mean dose 5.4mg).57 Mean levodopa dose in these patients was 595mg. The final mean pramipexole IR dose was 3.4mg/day with no significant changes to the levodopa dose. Estimated equivalence for a conversion from ropinirole IR to pramipexole IR was 1.5:1. This study recorded an 8.5-point improvement in the UPDRS score at 12 weeks. There were also better results for motor fluctuations (with no significant dyskinesia increases) and on depression scales. Results should be interpreted cautiously as the study lacked a control group.
Lastly, many studies have demonstrated the tolerability and effectiveness of switching from pramipexole IR or ropinirole IR to the ER formulation.4,5,19,21,22,49,50 Tolerability results for pramipexole ER were similar to those for the IR formulation in studies performed in patients with early PD, in both de novo treatment and cases of switching from IR to ER. Additionally, in another study carried out in patients with fluctuations, adverse effect incidence for pramipexole ER was lower than for placebo and the IR formulation (54.9 vs 55.6 and 64%).5 Two studies conducted in 259 and 539 patients with early PD, and one study in 517 patients with advanced PD and on levodopa treatment, showed pramipexole ER to be non-inferior to the IR formulation; another study in 156 patients with early PD could not confirm this result.5 Five studies conducted in more than 800 patients have confirmed that extended-release ropinirole was also non-inferior.22 Furthermore, the PREPARED study, carried out in patients with advanced PD and treated with levodopa, found significantly larger decreases in the UPDRS motor subscale scores (−10.2 points vs −7.9 with ropinirole IR) and the proportion of patients with a sustained reduction in ‘off’ time was more than 20% compared to baseline (66% vs 51%).21 Ropinirole ER seems to display better tolerability than the IR drug at equal doses, especially regarding daytime drowsiness and sleep quality, as indicated above.22 The overall clinical impression is that ropinirole ER is somewhat more effective and better tolerated than the IR formulation, whereas differences between pramipexole ER and IR are probably less noticeable. On the other hand, although treatment adherence in patients with PD is generally considered to be good, some recent studies question that view (and state that up to 61% of 3119 patients displayed poor adherence).58 In a multi-centre study of more than 29 000 patients in the USA (46% of whom exhibited less than 80% compliance), adherence to DA treatment (pramipexole and ropinirole) was poorer than adherence to rasagiline and levodopa. The causes of poor compliance were not studied.59 In contrast, a European multi-centre study measured far lower rates of treatment compliance (12.5% of 112 patients). In this study, adherence was significantly better in patients taking low-dose treatment (both levodopa and DAs) and single daily doses.60 Although adherence may be negatively impacted by drug tolerability and costs (especially in healthcare systems lacking universal coverage), it seems logical that simplifying dosage would improve treatment compliance. However, no controlled studies support this view.
Regarding the chronology in the switch between DAs, equally favourable results have been published for slow titration, rapid titration, and overnight switching.61 Overnight switching seems to be the best option for simplifying treatment, provided that the patient is warned about possible dopaminergic adverse effects during the overlap period.
Few studies in the literature contemplate combination therapy with multiple DAs. Only one trial with design limitations (a short observation period) in patients with motor fluctuations on combined levodopa and DA treatment (pramipexole or ropinirole) detected clinical improvement when associating cabergoline with no increase in adverse dopaminergic effects.61 Due to its different pharmacokinetic and pharmacodynamic properties, associating an extended-release DA (pramipexole or ropinirole) with transdermal rotigotine may be advantageous compared to monotherapy in some situations (for example, poorly controlled night-time or morning symptoms). Clinical trials will be needed to confirm this.
DA combination therapy is off-label in Europe. An exception would be for patients receiving subcutaneous apomorphine injections. Although DAs are typically discontinued in patients treated with continuous infusion of apomorphine, doctors must be alert to the development of apathy and depression, as stated in our earlier mention of surgical treatment.
ConclusionIn conclusion, the DAs pramipexole, ropinirole, and rotigotine fall within the extended options for treating PD in its different phases. Despite the good safety profile for this drug class, doctors should be aware of their associated adverse effects and pathoplastic potential throughout the course of treatment. Maintaining patients and families informed and providing close clinical monitoring, especially during the early phases of titration, promotes early detection of possible cases of toxicity or adverse drug effects and may improve treatment adherence.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Alonso Cánovas A, Luquin Piudo R, García Ruiz-Espiga P, Burguera JA, Campos Arillo V, Castro A, et al. Agonistas dopaminérgicos en la enfermedad de Parkinson. Neurología. 2014;29:230–241.