Different types of therapies were proven effective for the medical management of motor and non-motor symptoms in Parkinson’s disease (PD). We aimed to gain consensus on the dopamine agonist (DA) therapy use in different clinical scenarios of Parkinson’s disease (PD) patients.
MethodsThis consensus study was based on the nominal group technique. Initially, a consensus group comprising 12 expert neurologists in the PD field identified the topics to be addressed and elaborated different evidence-based preliminary statements. Next, a panel of 48 Spanish neurologists expressed their opinion on an internet-based systematic voting program. Finally, initial ideas were reviewed and rewritten according to panel contribution and were ranked by the consensus group using a Likert-type scale. The analysis of data was carried out by using a combination of both qualitative and quantitative methods. The consensus was achieved if the statement reached ≥ 3.5 points in the voting process.
ResultsThe consensus group produced 76 real-world recommendations. The topics addressed included 12 statements related to DA therapy in early PD, 20 statements concerning DA treatment strategy in patients with motor complications, 11 statements associated with DA drugs and their side effects, and 33 statements regarding DA therapy in specific clinical scenarios. The consensus group did not reach a consensus on 15 statements.
ConclusionThe findings from this consensus method represent an exploratory step to help clinicians and patients in the appropriate use of DA in different stages and clinical situations of PD.
Se demostró la efectividad de diferentes tipos de terapias para el tratamiento médico de los síntomas motores y no motores en la enfermedad de Parkinson (EP). Nos propusimos lograr un consenso sobre el uso de la terapia con agonistas dopaminérgicos (DA) en diferentes escenarios clínicos de pacientes con enfermedad de Parkinson (EP).
MétodosEste estudio de consenso se basó en la técnica de grupo nominal. Inicialmente, un Grupo de Consenso formado por 12 neurólogos expertos en el campo de la EP identificó los temas a tratar y elaboró diferentes declaraciones preliminares basadas en la evidencia. A continuación, un panel de 48 neurólogos españoles expresó su opinión en un programa de votación sistemática a través de Internet. Finalmente, las ideas iniciales fueron revisadas y reescritas de acuerdo con la contribución del panel y fueron clasificadas por el grupo de Consenso utilizando una escala tipo Likert. El análisis de los datos se llevó a cabo mediante una combinación de métodos cualitativos y cuantitativos. El consenso se alcanzaba si la afirmación alcanzaba ≥3,5 puntos en el proceso de votación.
ResultadosEl Grupo de Consenso elaboró 76 recomendaciones para el mundo real. Los temas abordados incluyeron 12 afirmaciones relacionadas con la terapia con DA en la EP temprana, 20 afirmaciones relativas a la estrategia de tratamiento con DA en pacientes con complicaciones motoras, 11 afirmaciones asociadas con los fármacos DA y sus efectos secundarios, y 33 afirmaciones relativas a la terapia con DA en escenarios clínicos específicos. El Grupo de Consenso no llegó a un consenso en 15 afirmaciones.
ConclusionesLos resultados de este método de consenso representan un paso exploratorio para ayudar a clínicos y pacientes en el uso apropiado de la DA en diferentes estadios y situaciones clínicas de la EP.
Parkinson’s disease (PD) is a chronic, progressive disorder caused by the gradual loss of dopaminergic neurons in the midbrain dopaminergic nucleus.1 Different therapies were proven effective for treating motor symptoms in PD, including dopamine agonists (DA) agents, amantadine, monoamine oxidase type B (MAO B) inhibitors, catechol-O-methyl transferase (COMT) inhibitors, and levodopa. Even though the treatment arsenal for PD has been available for decades, there are still differences in treatment approaches for patients facing similar clinical scenarios and consensus favouring one particular strategy is lacking.2 Although the levodopa treatment still represents the most effective symptomatic treatment for PD, its chronic use might be associated with challenging complications such as dyskinesias, motor fluctuations, or lack of efficacy. Because of this, it is preferable to only use low doses of levodopa therapy during the initial stages of PD.3
Interestingly, different formulations of the approved drugs have been progressively available, which provided a prolonged half-life in some cases, or the possibility to use enteral or parenteral drug delivery in others.4 These additional pharmacological formulations of commonly used drugs in PD facilitated patient adherence and tolerance in most cases but added more heterogeneity in patient care. The DA drugs have a widespread use in daily practice, as they can be prescribed both at disease onset as a symptomatic treatment, or in patients facing motor fluctuations during advanced stages of the disease.5 At present, only the non-ergot DA drugs are being used in PD, which included pramipexole, ropinirole, rotigotine, and apomorphine.6 There is a current variability regarding which DA is prescribed to a specific patient, and this might be related to the drugs’ formulations, potential adverse effects, or miscellaneous. This study aimed to reach an expert consensus on the appropriate use of DA in different clinical scenarios of PD.
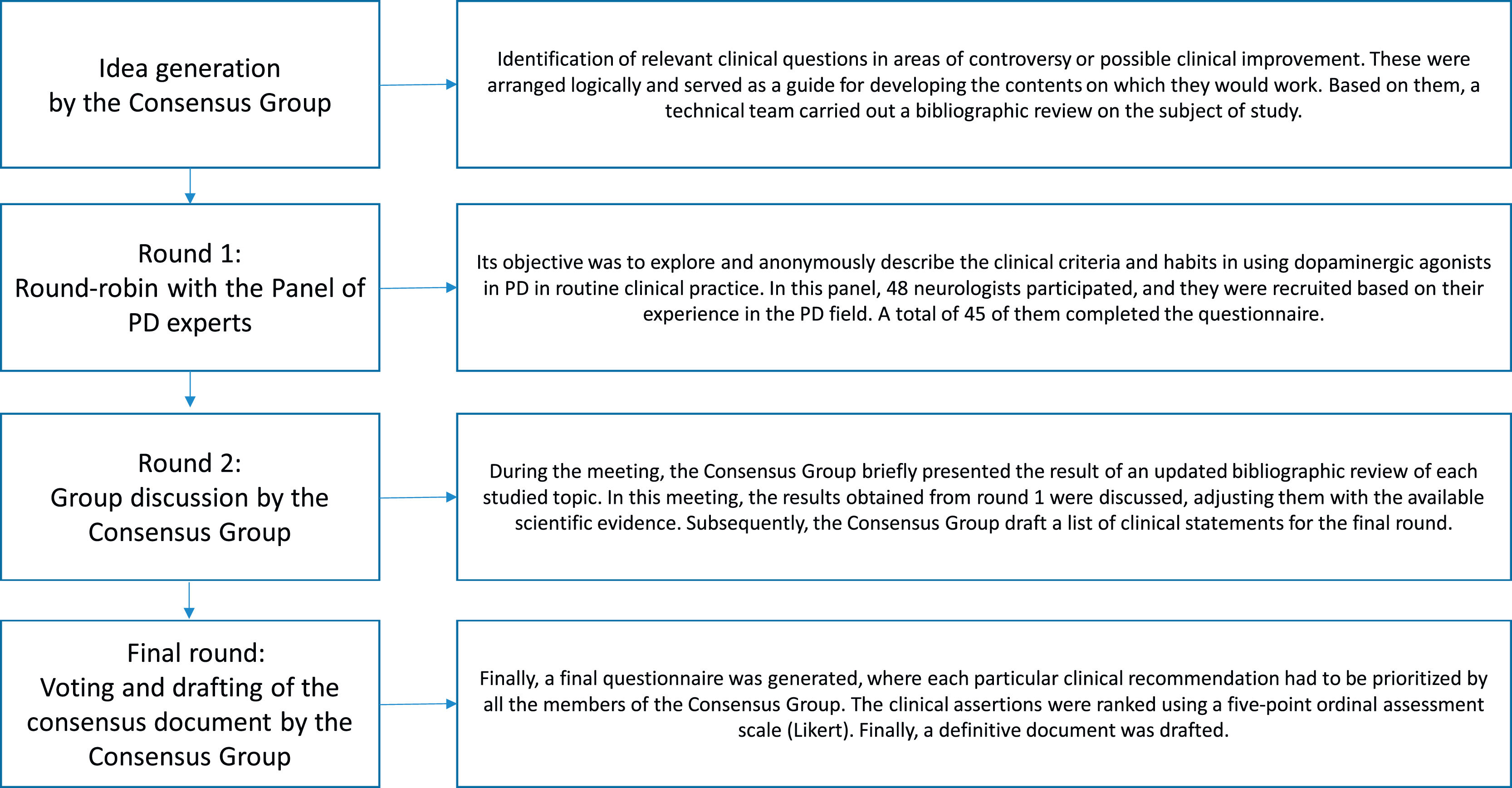
MethodsStudy designA nominal group technique procedure was conducted between June 2020 and May 2021. The consensus rounds were web-based and in-person due to epidemiological concerns related to the COVID-19 pandemic. The workflow of the process used in this study is presented in Fig. 1.
Initially, a consensus group was recruited by using a snowball sampling technique. This group was composed of 12 neurologists, experts in the PD field, who identified the topics to be addressed.
Topic selectionThe document was meant to address consensus on using DA agents in different clinical settings for patients with PD. The main topics to be addressed were done by 3 members of the consensus group (PMR, JPM, DSG) after thoughtful deliberation. The clinical scenarios considered included: 1) DA therapy in early PD; 2) DA therapy in PD patients with motor complications; 3) DA therapy in patients experiencing its potential side effects (impulse control disorders [ICD], pathological gambling, treatment intolerance); and 4) DA therapy in patients facing non-motor symptoms of PD (neuropsychiatric symptoms, autonomic dysfunction, sleep disorders).
Next, a systematic search on PubMed, EMBASE, Índice Médico Español, and LILACS was performed. This task was assigned to a specialised team of documentalists from the Investigation Unit, Francisco de Vitoria School of Medicine, Madrid, who adjusted and drafted the relevant clinical questions using the PICO model (P-problem, I-intervention, C-comparison, O-outcome).
Then, the consensus group underwent a web-based meeting on May 24, 2021, in order to systematically discuss the selected papers, exchange ideas, and start the 2-round nominal group technique voting.7 In the first round, different topics were presented to a panel of 48 neurologists from all over Spain to explore their opinion on using DA in various clinical scenarios. The first survey used an open-ended round in order to facilitate the panellists’ feedback. Because of this, statements could be reviewed or modified by new ideas, according to the panellists’ responses, to facilitate future agreement. In the second round, the consensus group combined the results obtained from round 1 with their systematic literature research. After this, the conclusions were written as clinical recommendations and structured by thematic blocks. In this second round, the consensus group expressed their level of agreement or disagreement on the different statements using a Likert-type scale. For doing so, the panellists had to specify their level of agreement with each statement using a 5-point response key, ranging from 1 (strongly disagree) to 5 (strongly agree). Finally, a consensus document was drafted.
Panel selectionThe expert panel consisted of 48 neurologists from different Spanish regions. The panellists were selected as recognised specialists in the clinical and academic fields of PD. A total of 45 (93.7%) of them completed all the voting rounds.
Consensus group methodThe nominal group technique is a valuable method used to achieve consensus among participants in different academic fields, and it is convenient for exploring experts’ agreement in healthcare research.8 In this technique, a facilitator asks the participants to contribute with varying points of view about a specific topic in a structured manner in order to generate a list of statements. Then, the group discusses and complements the statements with new ideas as necessary. Each participant usually ranks the assertion by voting on an ordinal scale. Finally, the results are analysed and reviewed in order to determine the overall group agreements.9
Statistical AnalysisThe analysis of data was carried out by using a combination of both qualitative and quantitative methods. Experts’ opinions and quotes recorded during the meetings and the online process were used to help understand individual and group rationale. In some cases, the personal comments from the panellists were used for cross-checking against written information to improve the consensus group’s ideas. The quantitative analysis was obtained and statistically analysed after the consensus group scored and ranked the different statements. Results are shown by using the mean and standard deviation (SD). In our study, consensus was considered to have been reaced for statements scoring ≥ 3.5 points on the consensus group voting.10
ResultsAs a result, the advisory committee produced 76 real-world recommendations on the proposed main topics (Tables 1-4). The consensus group reached agreement on 11 statements related to DA therapy in early PD, 14 statements concerning DA treatment strategy in patients with motor complications, 6 statements associated with DA drugs and their side effects, and 27 statements regarding DA therapy in specific clinical scenarios. The consensus group did not reach a consensus on 15 statements.
Dopamine agonist therapy in early Parkinson’s Disease.
| Mean (SD) | ||
|---|---|---|
| 1 | Non-ergot DA (pramipexole, ropinirole, and rotigotine) are effective in early PD as monotherapy (level of evidence A). | 4.91 (0.29) |
| 2 | The early use of DA reduces the incidence of developing motor fluctuations compared to levodopa. On the contrary, DA has less therapeutic efficacy and more significant side effects. | 4.27 (0.86) |
| 3 | The compared efficacy studies between different DA agonists showed no statistical differences. | 4.18 (0.57) |
| 4 | The efficacy of extended-release formulations of pramipexole and ropinirole is not inferior to immediate-release tablets during the early stages of PD. | 4.64 (0.48) |
| 5 | In elderly patients (over 70 years of age) without dementia, treatment with DA can be initiated if the patient has mild or moderate symptoms. | 3.82 /1.03) |
| 6 | In elderly patients (over 70 years of age), renal function should be monitored if pramipexole is initiated. | 4.55 (0.50) |
| 7 | Extended-release formulations of pramipexole and ropinirole favor treatment compliance due to their easy posology compared to immediate-release tablets. | 4.64 (0.48) |
| 8 | There is no evidence supporting the idea that pramipexole is more efficient in reducing tremor in PD patients compared to other DA agonists. | 4.09 (0.79) |
| 9 | Rotigotine improves sleep quality, pain perception, and morning akinesia. | 4.45 (0.50) |
| 10 | Pramipexole has a mild antidepressant action. | 3.91 (0.29) |
| 11 | DA treatments, including pramipexole, ropinirole, and rotigotine, are associated with impulse control disorder. It is necessary to discuss this potential side effect with patients, especially younger ones. | 5.00 (0) |
| 12 | Further clinical studies are needed to target the best initial treatment strategy for early PD. | 4.36 (0.64) |
DA: dopamine agonist; PD: Parkinson’s disease.
Dopamine agonist therapy in advanced Parkinson’s disease: treatment strategy in patients with motor complications.
| Mean (SD) | ||
|---|---|---|
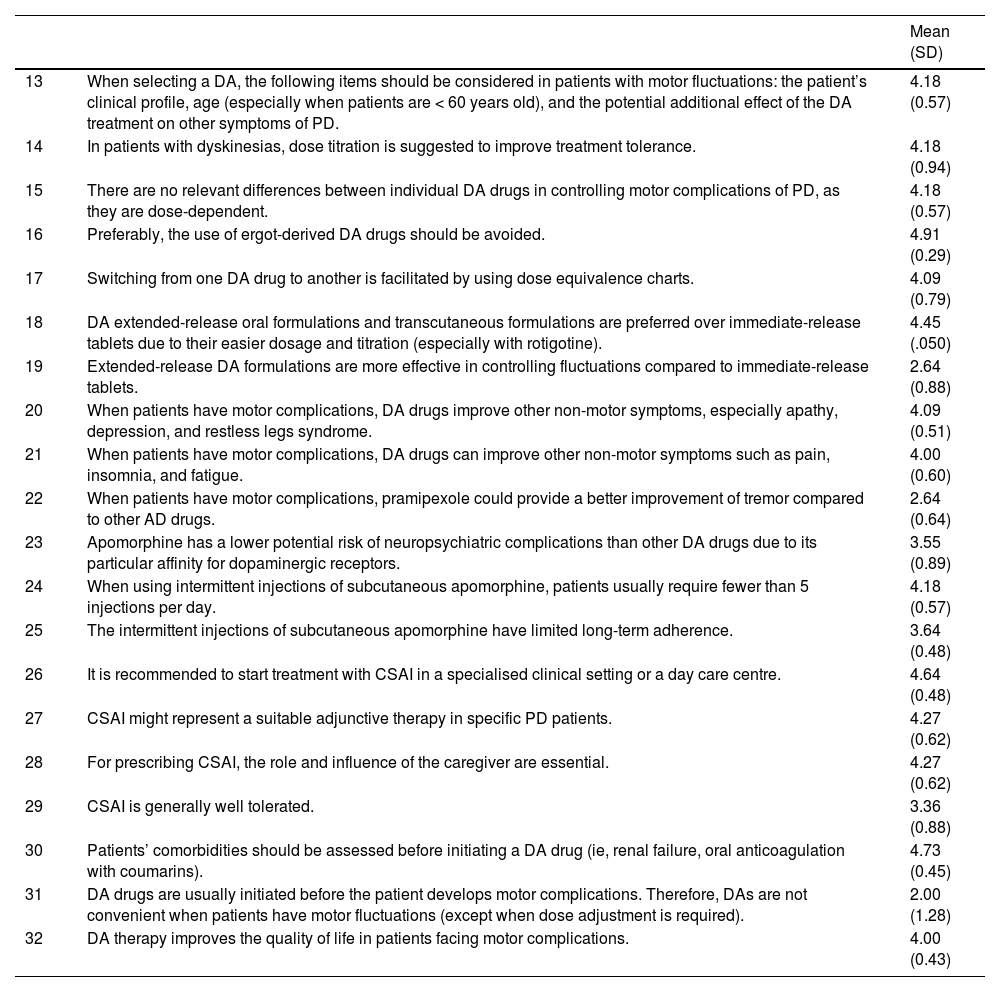
| 13 | When selecting a DA, the following items should be considered in patients with motor fluctuations: the patient’s clinical profile, age (especially when patients are < 60 years old), and the potential additional effect of the DA treatment on other symptoms of PD. | 4.18 (0.57) |
| 14 | In patients with dyskinesias, dose titration is suggested to improve treatment tolerance. | 4.18 (0.94) |
| 15 | There are no relevant differences between individual DA drugs in controlling motor complications of PD, as they are dose-dependent. | 4.18 (0.57) |
| 16 | Preferably, the use of ergot-derived DA drugs should be avoided. | 4.91 (0.29) |
| 17 | Switching from one DA drug to another is facilitated by using dose equivalence charts. | 4.09 (0.79) |
| 18 | DA extended-release oral formulations and transcutaneous formulations are preferred over immediate-release tablets due to their easier dosage and titration (especially with rotigotine). | 4.45 (.050) |
| 19 | Extended-release DA formulations are more effective in controlling fluctuations compared to immediate-release tablets. | 2.64 (0.88) |
| 20 | When patients have motor complications, DA drugs improve other non-motor symptoms, especially apathy, depression, and restless legs syndrome. | 4.09 (0.51) |
| 21 | When patients have motor complications, DA drugs can improve other non-motor symptoms such as pain, insomnia, and fatigue. | 4.00 (0.60) |
| 22 | When patients have motor complications, pramipexole could provide a better improvement of tremor compared to other AD drugs. | 2.64 (0.64) |
| 23 | Apomorphine has a lower potential risk of neuropsychiatric complications than other DA drugs due to its particular affinity for dopaminergic receptors. | 3.55 (0.89) |
| 24 | When using intermittent injections of subcutaneous apomorphine, patients usually require fewer than 5 injections per day. | 4.18 (0.57) |
| 25 | The intermittent injections of subcutaneous apomorphine have limited long-term adherence. | 3.64 (0.48) |
| 26 | It is recommended to start treatment with CSAI in a specialised clinical setting or a day care centre. | 4.64 (0.48) |
| 27 | CSAI might represent a suitable adjunctive therapy in specific PD patients. | 4.27 (0.62) |
| 28 | For prescribing CSAI, the role and influence of the caregiver are essential. | 4.27 (0.62) |
| 29 | CSAI is generally well tolerated. | 3.36 (0.88) |
| 30 | Patients’ comorbidities should be assessed before initiating a DA drug (ie, renal failure, oral anticoagulation with coumarins). | 4.73 (0.45) |
| 31 | DA drugs are usually initiated before the patient develops motor complications. Therefore, DAs are not convenient when patients have motor fluctuations (except when dose adjustment is required). | 2.00 (1.28) |
| 32 | DA therapy improves the quality of life in patients facing motor complications. | 4.00 (0.43) |
CSAI: continuous subcutaneous apomorphine infusion; DA: dopamine agonist; PD: Parkinson’s disease.
Dopamine agonists and side effects.
| Mean (SD) | ||
|---|---|---|
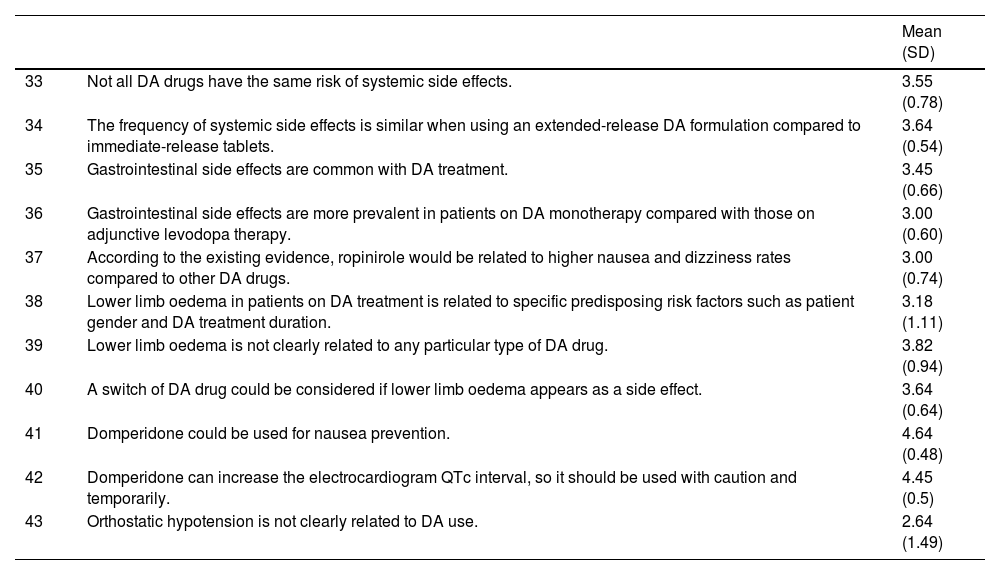
| 33 | Not all DA drugs have the same risk of systemic side effects. | 3.55 (0.78) |
| 34 | The frequency of systemic side effects is similar when using an extended-release DA formulation compared to immediate-release tablets. | 3.64 (0.54) |
| 35 | Gastrointestinal side effects are common with DA treatment. | 3.45 (0.66) |
| 36 | Gastrointestinal side effects are more prevalent in patients on DA monotherapy compared with those on adjunctive levodopa therapy. | 3.00 (0.60) |
| 37 | According to the existing evidence, ropinirole would be related to higher nausea and dizziness rates compared to other DA drugs. | 3.00 (0.74) |
| 38 | Lower limb oedema in patients on DA treatment is related to specific predisposing risk factors such as patient gender and DA treatment duration. | 3.18 (1.11) |
| 39 | Lower limb oedema is not clearly related to any particular type of DA drug. | 3.82 (0.94) |
| 40 | A switch of DA drug could be considered if lower limb oedema appears as a side effect. | 3.64 (0.64) |
| 41 | Domperidone could be used for nausea prevention. | 4.64 (0.48) |
| 42 | Domperidone can increase the electrocardiogram QTc interval, so it should be used with caution and temporarily. | 4.45 (0.5) |
| 43 | Orthostatic hypotension is not clearly related to DA use. | 2.64 (1.49) |
DA: dopamine agonist; PD: Parkinson’s disease.
Dopamine agonists in specific clinical scenarios.
| Mean (SD) | ||
|---|---|---|
| A) ICD | ||
| 44 | The diagnosis of ICD could be underestimated in cases of unawareness from relatives. | 4.91 (0.29) |
| 45 | The diagnosis of ICD may differ depending on the clinimetric properties of the different available diagnosis scales. | 4.91 (0.29) |
| 46 | The presence of depression acts as a premorbid risk factor for suffering an ICD with the use of DA. | 4.45 (0.78) |
| 47 | In patients with premorbid risk factors for an ICD, the use of DA drugs should be avoided. | 4.27 (0.75) |
| 48 | The dose of DA treatment is associated with the risk of an ICD. | 4.45 (0.5) |
| 49 | Levodopa treatment and dose influence the genesis of ICD. | 3.82 (0.83) |
| 50 | Not all DA drugs have the same risk of being associated with an ICD. | 3.73 (0.86) |
| 51 | AD treatments present a different risk of inducing an ICD depending on their release formula. | 3.00 (0.95) |
| 52 | Rotigotine treatment could be associated with less risk of developing an ICD. | 3.82 (0.57) |
| 53 | Apomorphine has a lower risk of being associated with an ICD compared to oral or transdermal DA drugs. | 4.00 (0.60) |
| 54 | The dose of the DA should be reduced in the presence of a mild ICD. | 3.73 (0.75) |
| 55 | A switch of DA could be considered if the ICD is mild to moderate. | 2.82 (0.94) |
| 56 | The dose of the DA should be reduced in the event of a moderate-severe ICD. | 4.00 (1.18) |
| 57 | The DA treatment should be withdrawn if patients develop a mild ICD. | 2.91 (0.79) |
| 58 | The DA should be withdrawn in the event of a moderate-severe ICD. | 4.55 (0.66) |
| 59 | In cases of moderate-severe ICD, the dose of levodopa should also be reduced. | 2.45 (0.5) |
| 60 | Scientific evidence is lacking to recommend the use of other additional drugs for the treatment of ICD. | 4.27 (0.62) |
| 61 | In patients with a history of ICD using DA treatment, the use of these drugs should be restricted in the future. | 4.45 (0.66) |
| 62 | In patients with a history of ICD with oral or transdermal DA, who are candidates for second-line therapies, the use of continuous infusion with subcutaneous apomorphine should be avoided. | 3.09 (0.79) |
| 63 | Dopaminergic dysregulation syndrome is mainly associated with the use of levodopa. | 4.18 (0.83) |
| B) DAWS | ||
| 64 | There are premorbid factors that predict the appearance of a DAWS. | 4.09 (0.51) |
| 65 | In patients in whom DA has been withdrawn due to side effects, such as an ICD, its reintroduction could be considered if they have suffered a DAWS. | 3.82 (0.57) |
| C) Sleep Disorders | ||
| 66 | DA drugs are effective in improving sleep disorders in PD patients (nocturnal akinesia, fragmented sleep, RLS) | 4.45 (0.50) |
| 67 | Although rotigotine has more favorable scientific evidence, other DA therapies may be equally effective in improving sleep disorders in patients with PD. | 4.00 (0.60) |
| 68 | DA drugs have shown to be not effective in treating REM sleep behaviour disorder in patients with PD. | 4.09 (0.67) |
| 69 | In case of drowsiness associated with a DA treatment, it may be helpful to switch the DA drug, preferably to rotigotine. | 3.45 (0.66) |
| D) Cognition and behaviour | ||
| 70 | In a patient with PD and cognitive impairment, it is recommended to avoid the use of DA treatments. | 3.82 (0.72) |
| 71 | It is unnecessary for a patient on DA treatment who develops cognitive impairment without psychotic symptoms to reduce DA dose unless neuropsychiatric complications appear. | 3.73 (0.86) |
| 72 | In patients with PD older than 80 years, without cognitive impairment, it is preferable to avoid the use of DA. | 4.00 (0.74) |
| E) Miscellanous | ||
| 73 | The combination of 2 DA is not recommended, except for the additive use of apomorphine in pen or infusion. | 4.55 (0.66) |
| 74 | In patients using a diurnal apomorphine infusion pump, the concomitant use of a nocturnal DA extended-release treatment may be helpful. | 4.36 (0.48) |
| 75 | In patients with PD on DA therapies who undergo deep brain stimulation therapy, it is recommended not to reduce the DA dose by more than 70% in the early post-surgical stages in order to avoid apathy as a side effect. | 4.18 (0.39) |
| 76 | In patients with PD who are on Duodopa, DA drugs may help treat non-motor manifestations, such as sleep disorders. | 4.45 (0.50) |
DA: dopamine agonist; DAWS: dopamine agonist withdrawal syndrome: ICD: impulse control disorders; PD: Parkinson’s disease; REM: rapid eye movement; RLS: restless legs syndrome.
The best time to initiate symptomatic treatment varies significantly between patients, according with different factors. The best treatment to administer initially in PD patients is based on the patient’s characteristics, impact of disease on daily activities, and the pharmacodynamics of the selected drug. There is no general consensus to advise clinicians with the selection of the initial pharmacological treatment for PD. The most important factors that might influence the treatment selection are the patient’s age, severity of symptoms, evolution in years, the antiparkinsonian power of the selected drug, and the drug-related side-effects. Based on this, clinicians and patients must discuss the best specific pharmacologic option tolerance.5,11Table 1 shows the sxperts’ agreement on DA use as initial therapy in PD.
2) Dopamine agonist therapy in Parkinson’s disease patients with motor complicationsThe best treatment strategy in PD patients facing motor complications must be balanced between adequate control of the symptoms produced by the selected drug and the appearance of adverse pharmacological effects. Typically, levodopa is the more effective treatment for reducing the motor symptoms of PD, although it is more frequently associated with development of dyskinesia compared to DA, especially in the long-term treatment. Despite the lower risk of inducing motor complications, DA drugs are associated with an increased risk of developing specific adverse events, including somnolence, hallucinations, and behavior disorders. Furthermore, DA are not well tolerated in elderly patients, especially those with previous cognitive impairment. The advantages and disadvantages of the different therapeutic strategies in patients with motor complications must be weighed carefully by both clinicians and patients, prioritising the patient’s specific needs and context. Table 2 shows the experts’ agreement on DA use in PD patients with motor complications.
3-Dopamine agonist therapy in patients experiencing its potential adverse effects (impulse control disorders, treatment intolerance)Some side effects caused by DA agents and levodopa might be similar and may range from mild to severe intensity. They include nausea, vomiting, sleepiness, orthostatic hypotension, confusion, and hallucinations. Nevertheless, patients on DA therapies are more likely to develop peripheral oedema, somnolence, constipation, dizziness, hallucinations, and nausea compared, and DA-treated patients are more likely to discontinue treatment due to adverse events.6 Severity of ICD can range from mild symptoms associated with compulsive behavior (solitaire-playing, compulsive cleaning, etc) to more severe and destructive conduct, such as pathological gambling or hypersexuality. The ICD can be developed at any stage of PD and in patients on any dopaminergic therapy, but it is more frequently observed in PD patients taking DA drugs.5 ICD might improve with DA discontinuation, but some patients might maintain compulsive symptoms after DA cessation.12Table 3 shows the experts’ agreement on DA therapy use in PD patients facing its potential side effects.
4-Dopamine agonist therapy in PD patients with non-motor symptomsPD patients may experience different types of non-motor symptoms, which can be related to the disease proper or the pharmacologic drugs administered. The non-motor symptoms include a different range of neuropsychiatric symptoms that goes from anxiety, apathy, and mood disorder to cognitive dysfunction, hallucinations, and other psychotic symptoms.13 Also, PD patients might have sleep disorders, including insomnia, parasomnias, and restless legs syndrome. Autonomic symptoms can also be present, including orthostatic hypotension, sexual dysfunction, and constipation.13 On the other hand, patients on DA treatment might develop a withdrawal syndrome, especially if the drug is rapidly removed.14 The DA treatment withdrawal usually includes psychological, autonomic, and motor manifestations such as anxiety, panic attacks, depression, sweating, vomiting, weakness, instability, and DA drug craving. These symptoms are typically refractory to other antiparkinsonian treatments, including levodopa, and might only be controlled by replacing the specific DA withdrawn drug.15Table 4 shows the experts’ agreement on DA use in patients with non-motor symptoms of PD.
DiscussionThe current range of treatment options available for idiopathic PD is broad, where treatment strategy can be divided into pharmacological, non-pharmacological, and surgical therapy. Our study used a nominal group technique to help reach an expert consensus regarding the advantages and disadvantages of DA use in different clinical situations. Tailoring a treatment strategy for individual patients requires careful consideration of different factors, that include the patient’s symptoms, age, disease stage, functional disability, and lifestyle.
Unlike levodopa, the pharmacological effects of the DA drugs are exerted by their direct interaction with the dopamine receptors and therefore mimicking the endogenous dopamine. Interestingly, DA treatments can be used both at the early stages of the disease and in more advanced stages when patients exhibit motor and non-motor complications. Furthermore, DA drugs have all been shown of value as monotherapy, typically in patients with early disease course (statement 1). As a result, it is commonly recommended in daily practice to use a minimum dose of levodopa or to delay its initiation until patients have symptoms that interfere with daily functioning or impair their quality of life (statement 2). There is limited information regarding head-to-head comparisons for the efficacy of different DAs, showing no significant difference between each other or only slight superiority of one drug over another (statement 3). As a consequence, the DA drug selected to treat a particular patient is usually based on concerns related to its formulation, dosing frequency, and cost.16,17 This can explain the broad clinical situations where DA therapies are being prescribed, based mainly on the patients’ and doctors’ preferences. When planning a tailored treatment strategy, clinicians should consider cognitive status and renal function in elderly patients (statements 5-6), and drug formulations (statement 7) in order to improve tolerance and adherence. Also, different comorbidities of the patients can help the DA election at disease onset (statements 9-10). Finally, all the non-ergot DA drugs can be associated with ICD, and it is recommended to discuss this potential side effect when prescribing these drugs (statement 11).
The development of motor fluctuations over time is variable and mostly depends on the progressive degeneration of nigrostriatal dopamine terminal, rather than the initial treatment strategy.18 Furthermore, there is increasing evidence that the choice and timing of initial therapy for PD, whether levodopa, DA or MAO B inhibitor might have a minimum impact on the long-term prevalence of motor fluctuations and dyskinesia.19 Nevertheless, a substantial proportion of patients with PD might develop levodopa-related motor complications years after starting levodopa.20 These complications include motor fluctuations and different types of complex oscillations in motor function. In patients experiencing dyskinesias, adding a non-ergot DA might help to reduce challenging symptoms.5,21 In those cases, such drugs as ropirinole, rotigotine or pramipexole can be all equally considered, preferably using an extended-release formulations (statements 15-17). Further, when used as an add-on, DA should be started with a lowest dose and titrated cautiously and starting with the lowest dose possible (statements 14, 18). Additionally, DA drugs can help to improve non-motor symptoms of PD (statements 20-21).
In patients facing advanced stages of PD, apomorphine can also have a key role in their clinical management. Apomorphine is a non-ergoline DA with a high affinity to dopamine D 1-2 receptors.22 It has a similar efficacy to levodopa in controlling motor symptoms of PD, and it is prescribed for the control of motor fluctuations. It is used subcutaneously both as an intermittent injection or as continuous infusion (statements 24-27). Although apomorphine has a rapid and short onset of action, a lower risk of neuropsychiatric complications (statement 23), and a good tolerability when it is used as a continuous infusion, patients and caregiver education might be essential for its correct use (statement 28).
Even though DA therapy has an important role in patients with advanced PD as a treatment for levodopa-induced motor complications, its use might be associated with specific complications (statements 33-34). The spectrum of side effects associated with DA might be wide, and can range from gastrointestinal discomfort and lower limb oedema to significant neuropsychiatric complications (statements 39-41). For instance, DA drugs are most commonly associated with the development of ICDs such as pathological gambling, compulsive sexual behaviour, or compulsive shopping in up to 50% of patients with long-term use.23 Risk factors for its development include male sex, younger age, a higher dose of DA, and previous history of ICD (statements 44-48). Furthermore, depression could act as a premorbid risk factor for suffering an ICD with the use of DA (statement 46).24 Regarding the DA type, rotigotine and apomorphine could be associated with less risk of developing an ICD (statements 50, 52-53). When an ICD is present, it is recommended to taper the DA gradually in order to improve the symptomatology (statements 54, 56, 58, 60).25 Higher doses of levodopa may be associated with a small increase in risk of ICD (statement 49).26
Moreover, although levodopa is considered the most potent trigger for dopamine dysregulation syndrome in PD (statement 63), subcutaneous apomorphine and oral DA may also be responsible.27 It usually involves male patients with early-onset PD who take increasing quantities of dopaminergic drugs, despite having severe drug-related dyskinesia.28 Finally, DA should be avoided in elderly adults due to increased risk cognitive impairment or in patients with a history of ICD, dementia, hypersomnia, or hallucinations (statements 70-72).
The limitations of this study are related to the fact that it is based on expert opinion and authors were challenged to reach consensus on topics who might have a limited research background. To address that issue, the consensus group used the large backup panel of neurologists to gain consistency and homogeneity. Also, it should be noted that authors and participants based their opinion on local regulations, and changes in safety recommendations may not apply in different countries. Finally, regarding the topics that not reached expert consensus, authors believes that it is related the clinical heterogeneity that clinicians must usually face in their daily practice, and consequently those topics should be addressed in a personalised approach.
ConclusionDA represents an effective option to treat PD and allow the use of lower doses of levodopa therapy, postponing their potential side effects. In addition, DA might also provide benefits in more advanced disease stages by reducing motor fluctuations associated with the long-term levodopa therapy. Our work provides insights into where DA eventually fit into the treatment schemes for PD in daily practice. The conclusions derived from it represent an exploratory step from which the outcomes should be used to guide further qualitative and quantitative research designs.29 In any case, DA as a group, has been considered the most potent ancillary antiparkinsonian medication available.5
Conflict of interestSantos García D. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, Teva, Archímedes, Esteve, Stada, Merz, and grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017-2020 por el proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”).
Pagonabarraga Mora J. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, Zambon, Bial, and Esteve.
Escamilla Sevilla F. has received honoraria for educational presentations and/or advice service by Abbvie, Bial, Boston Scientific, Esteve, Medtronic, Stada, UCB Pharma and Zambon.
García Ruiz P.J. has received personal compensation as a consultant/scientific advisory board from Italfarmaco, Britannia, Bial, Stada and Zambon and speaking honoraria from Italfarmaco, Bial, Zambon, Merz, Dysport and Abbvie.
Infante Ceberio J. has received honoraria for educational presentations and advice service from Abbvie, Zambon and Bial.
Jaime Kulisevsky Bojarski J. has no conflicts of interest.
Linazasoro Cristóbal G. CEO, founder and shareholder of VIVE biotech SL. He has received honoraria for educational presentations, advisory service and participation in research projects and clinical trials by Novartis, UCB, Lundbeck, Bial, Abbvie, Asta Médica, Teva, Krka, Medtronic, Boehringer, Schering, Pharmacia, Lilly, Dupont, Italfármaco, Allergan, Ipsen, Merz, Zambón, Biogen, Roche, Genentech and Oryzon.
Luquín Piudo M.R. has received honoraria for educational presentations and advice services by Abbvie, UCB Pharma, Zambon, Bial, Esteve, and grants from the Spanish Health Institute Carlos III and European Commission.
Martínez Castrillo J.C. has received grants/research support from Allergan, AbbVie, Bial, Ipsen, Italfarmaco, Merz, and Zambon ; honoraria or consultation fees from Allergan, AbbVie, Bial, Exeltis, Ipsen, Italfarmaco, Merz, Ipsen, Orion, TEVA, UCB, and Zambon ; and company sponsored speaker’s bureau from Allergan, AbbVie, Bial, Krka, Ipsen, Italfarmaco, Merz, TEVA, UCB, and Zambon.
Jesús S. has received honoraria from Abbvie, Zambon, Bial, Italfarmaco, Merz. She holds the grant “Acción B Clínicos-Investigadores (B-0007-2019)” from the “Consejería de Salud y Familias” and she received the grant PI21/01901 founded by the “Instituto de Salud Carlos III”.
Vela Desojo L. has no conflicts of interest.
Campos Lucas F.J. has no conflicts of interest.
Caballero Martínez F. has no conflicts of interest.
Pablo Mir P. has received support for attending meetings and/or travel or honorarium for lecturing from Abbott, Allergan, Abbvie, Bial, Britannia, Italfarmaco, Merz, UCB, Teva, Stada and Zambon.
FundingThis study was funded by the Chair of Epilepsy and Movement Disorders UFV-UCB.
We thank the neurologists who participated in phase 1 for their disinterested collaboration. We thank the Epilepsy and Movement Disorders UFV-UCB Chair for supporting this initiative. We thank Irene Santamaría of the Francisco de Vitoria University for the technical coordination work of this study.
Araceli Alonso Cánovas1; José Matías Arbelo González2; Begoña Ares Pensado3; Asunción Ávila Rivera4; Nuria Caballol Pons5; Mª Teresa Cáceres Redondo6; Víctor Manuel Campos Arillo7; Mar Carmona Abellán8; Fátima Carrillo García9; Mª José Catalán Alonso10; Esther Cubo Delgado11; Beatriz De la Casa Fages12; Eduardo de Pablo-Fernández13; Sevilla14; Mª del Carmen Fernández Moreno15; Juan García Caldentey16; Rocío Garcia-Ramos17; Juan Carlos Gómez Esteban18; Jéssica González Ardura19; Javier Gutiérrez García20; Antonio Koukoulis Fernández21; Monica Kurtis Urra22; Inés Legarda Ramírez23; Luis Javier López del Val24; Lydia López Manzanares25; Eva López Valdes26; Nuria López-Ariztegui27; José Luis López-Sendón Moreno28; Raúl Martínez Fernández29; Irene Martínez Torres30; Marina Mata Álvarez-Santullano31; Adolfo Mínguez Castellanos32; Mariana Hernández González-Monje33; Jesús Olivares Romero34; Juan Manuel Oropesa Ruiz35; Isabel Pareés Moreno36; Berta Pascual Sedano37; José Manuel Paz González38; Jesús Pérez Pérez39; Ana Belén Perona Moratalla40; Víctor Puente Périz41; María Cruz Rodríguez Oroz42; Javier Ruiz Martínez43; Antonio Salvador Aliaga44; Mª Pilar Sanz Cartagena45; Ángel Sesar Ignacio46; Berta Solano Vila47; María Esther Suárez San Martín.48
1-Hospital Universitario Ramón y Cajal. Madrid; 2-Hospital Universitario San Roque. Las Palmas de Gran Canaria; 3-Complejo Hospitalario Universitario de Santiago de Compostela. La Coruña; 4-Complejo Hospitalario Moisès Broggi, Hospital Sant Joan Despí. Barcelona; 5-Complejo Hospitalario Moisès Broggi, Hospital Sant Joan Despí. Barcelona; 6-Hospital Universitario Reina Sofía. Córdoba; 7-Hospital Vithas Xanit Internacional de Benalmádena. Málaga; 8-Hospital Universitario de Basurto. Vizcaya; 9-Hospital Universitario Virgen del Rocío. Seville; 10-Hospital Clínico San Carlos. Madrid; 11-Hospital Universitario de Burgos. Burgos; 12-Hospital General Universitario Gregorio Marañón. Madrid; 13-UCL Queen Square Institute of Neurology. London (United Kingdom); 14-Hospital Universitario Virgen de las Nieves. Granada; 15-Hospital Universitario Virgen de Valme. Seville; 16-Hospital Quirónsalud Palmaplanas. Balearic Islands; 17-Hospital Clínico San Carlos. Madrid; 18-Hospital Universitario de Cruces. Vizcaya; 19-Hospital de Cabuñes. Asturias; 20-Hospital Clínico Universitario San Cecilio. Granada; 21-Hospital Álvaro Cunqueiro de Vigo. Pontevedra; 22-Hospital Ruber Internacional. Madrid; 23-Hospital Universitario Son Espases. Balearic Islands; 24-Hospital HLA Montpellier. Zaragoza; 25-Hospital Universitario de La Princesa. Madrid; 26-Hospital Clínico San Carlos. Madrid; 27-Hospital Universitario de Toledo. Toledo; 28-Hospital Universitario Ramón y Cajal. Madrid; 29-HM CINAC, Hospital HM Puerta del Sur. Madrid; 30-Hospital Universitario y Politécnico La Fe. Valencia; 31-Hospital Universitario Infanta Sofia. Hospital Ruber Juan Bravo. Madrid; 32-Hospital Universitario Virgen de las Nieves. Granada; 33-HM CINAC, Hospital HM Puerta del Sur. Madrid; 34-Hospital Universitario Torrecárdenas. Almería; 35-Hospital Universitario Juan Ramón Jiménez. Huelva; 36-Hospital Universitario Ramón y Cajal. Hospital Ruber Internacional. Madrid; 37-Hospital de la Santa Creu i Sant Pau. Barcelona; 38-Complejo Hospitalario Universitario A Coruña. La Coruña; 39-Hospital de la Santa Creu i Sant Pau. Barcelona; 40-Hospital General Universitario de Albacete. Albacete; 41-Hospital del Mar. Barcelona; 42-Clínica Universidad de Navarra. Navarra; 43-Hospital Universitario Donostia. Gipuzkoa; 44-Hospital Clínico Universitario de Valencia. Valencia; 45-Hospital de Mataró. Barcelona; 46-Hospital Clínico Universitario de Santiago de Compostela. La Coruña; 47-Hospital Universitario Doctor Josep Trueta. Gerona; 48-Hospital Universitario Central de Asturias. Asturias.