Patients with temporal lobe epilepsy (TLE) perform poorly on semantic verbal fluency (SVF) tasks. Completing these tasks successfully involves multiple cognitive processes simultaneously. Therefore, quantitative analysis of SVF (number of correct words in one minute), conducted in most studies, has been found to be insufficient to identify cognitive dysfunction underlying SVF difficulties in TLE.
ObjectivesTo determine whether a sample of patients with TLE had SVF difficulties compared with a control group (CG), and to identify the cognitive components associated with SVF difficulties using quantitative and qualitative analysis.
MethodsSVF was evaluated in 25 patients with TLE and 24 healthy controls; the semantic verbal fluency test included 5 semantic categories: animals, fruits, occupations, countries, and verbs. All 5 categories were analysed quantitatively (number of correct words per minute and interval of execution: 0-15, 16-30, 31-45, and 46-60seconds); the categories animals and fruits were also analysed qualitatively (clusters, cluster size, switches, perseverations, and intrusions).
ResultsPatients generated fewer words for all categories and intervals and fewer clusters and switches for animals and fruits than the CG (P<.01). Differences between groups were not significant in terms of cluster size and number of intrusions and perseverations (P>.05).
ConclusionsOur results suggest an association between SVF difficulties in TLE and difficulty activating semantic networks, impaired strategic search, and poor cognitive flexibility. Attention, inhibition, and working memory are preserved in these patients.
Los pacientes con epilepsia del lóbulo temporal (ELT) muestran dificultades en tareas de fluidez verbal semántica (FVS). La ejecución exitosa de estas requiere la participación simultánea de múltiples procesos cognitivos; por esto, el análisis cuantitativo (número de palabras emitidas en un minuto) realizado en la mayoría de los estudios no ha permitido identificar la disfunción cognitiva subyacente a las dificultades de FVS en la ELT.
ObjetivosDeterminar si una muestra de pacientes con ELT, contrastada con un grupo de comparación (GC), presentaba alteración en la FVS e identificar los componentes cognitivos relacionados con la alteración mediante análisis cuantitativo y cualitativo.
MétodosSe evaluó la FVS de 25 pacientes con ELT y 24 participantes sanos, mediante 5 categorías: animales, frutas, profesiones, países y verbos. Se analizaron cuantitativamente (número de palabras correctas por minuto e intervalos de ejecución: 0-15, 16-30, 31-45, 46-60s) las 5 categorías y cualitativamente (agrupaciones, tamaño de la agrupación, saltos, perseveraciones e intrusiones) las de animales y frutas.
ResultadosLos pacientes generaron menor número de palabras en todas las categorías e intervalos; y para las de animales y frutas, un menor número de agrupaciones y de saltos que el GC (p < 0,01). No hubo diferencias respecto al tamaño de la agrupación, las intrusiones y las perseveraciones (p > 0,05).
ConclusionesLas dificultades de FVS en la ELT podrían relacionarse con fallos en la activación de las redes semánticas, en la búsqueda estratégica y flexibilidad mental. Los procesos de atención, inhibición y memoria de trabajo están conservados.
Temporal lobe epilepsy (TLE) is a neurological syndrome characterised by epileptic seizures predominantly originating in the hippocampus, amygdala, and parahippocampal gyrus1,2; hippocampal sclerosis is the most frequent pathological finding.3,4
Patients with TLE display language alterations, particularly affecting semantic processing. Approximately 33% of patients report word finding difficulties during spontaneous speech and 40% show naming difficulties.5,6 These alterations become apparent during semantic verbal fluency (SVF) tasks, in which patients perform poorly compared to controls.7–9
Besides linguistic processes, SVF tasks also involve cognitive processes, including processing speed, sustained and selective attention, working memory, semantic processing, and such executive functions as inhibition, strategic search, and cognitive flexibility.10 SVF involves multiple simultaneous processes: accessing the semantic store, strategically searching and retrieving words pertaining to a specific semantic category while inhibiting words from other semantic categories (intrusions), storing previously retrieved words in the working memory to avoid repetitions (perseverations), and sustaining attention on the task.
Words are clustered by subcategory (e.g., domestic animals, marine animals, etc.). When an individual is unable to evoke any more words from a specific subcategory, they change the search criterion and switch subcategories (e.g., from domestic animals to marine animals).11,12 Clusters and cluster size reflect semantic categorisation and depend on cognitive domains associated with the temporal lobe and involving automated semantic network activation. Switching requires the cognitive flexibility to shift between subcategories and involves such processes as strategic search, cognitive flexibility, and executive control; these processes involve frontal lobe function.12–14
A time-dependent decrease has been observed in the number of words generated, due to increasing difficulty15: as the task progresses, it requires greater cognitive control since the words generated in the previous seconds must be related to those already mentioned, which must be recalled to avoid repetitions. It has been suggested that the early phase of the task (first 15seconds) reflects access and automatic activation of semantic networks, whereas the late phase (last 15seconds) reflects such cognitive strategies as systematic search in the semantic store.16
Studies based on quantitative analysis have reported SVF deficits in patients with TLE. However, this type of analysis provides no information on the specific cognitive processes underlying impaired SVF. Qualitative analysis (clusters, switches, perseverations, and intrusions) and an analysis of time-dependent word production may provide a deeper understanding of these processes.
Tröster et al.17 compared SVF performance in a group of patients with TLE and in a control group to determine whether SVF impairment in TLE is due to a disruption of semantic networks, or to inefficient information search and retrieval strategies and impaired cognitive flexibility. Compared to controls, patients produced fewer words and more clusters (e.g., fruits, vegetables, etc.) than subordinate exemplars (e.g., lemon, orange, apple); however, no differences were observed in the number of switches, perseverations, or intrusions. The researchers concluded that SVF impairment in TLE is due to semantic network impairment rather than to inefficient search and retrieval strategies. N’Kaoua et al.18 report findings to the contrary, with patients and controls showing differences in switching and clustering, but a similar mean cluster size. The researchers conclude that patients with TLE show word search and retrieval inefficiency rather than semantic network disruption.
The present study aims to identify the cognitive processes underlying SVF impairment in patients with TLE based on the hypotheses described above.17,18 Combined quantitative and qualitative analysis of SVF may provide valuable information on a wide range of cognitive processes,19,20 guiding neuropsychological rehabilitation strategies. Our objectives were to determine whether a sample of patients with TLE showed poorer SVF than a control group, and to describe SVF impairment using quantitative and qualitative analysis, based on the following hypotheses: (1) if patients display semantic network disruption, they will produce fewer exemplars per category than controls; (2) if patients show impaired semantic network activation, they will produce fewer words during the early phase of the task and fewer clusters than controls; and (3) if patients show inefficient search and retrieval strategies (associated with strategic search, cognitive flexibility, working memory, and inhibition), they will produce fewer words during the late phase of the task and will make fewer switches and show more perseverations and intrusions than controls.
To determine whether SVF varied according to word category, we evaluated the categories “jobs,” “countries,” and “verbs,” in addition to the more widely used categories “animals” and “fruits.”11,16,20-22
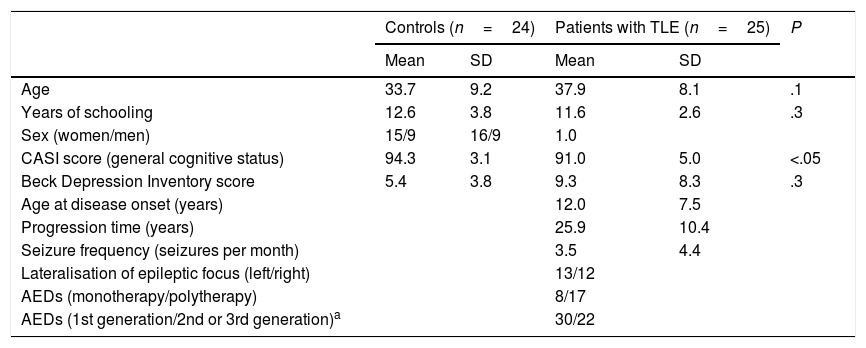
Patients and methodsWe evaluated 25 patients with unilateral TLE from the epilepsy clinic at the National Institute of Neurology and Neurosurgery, in Mexico City. TLE was diagnosed according to the 1989 International League Against Epilepsy criteria,23 based on the predominant ictal and interictal electrical activity, ictal semiology, and presence of unilateral hippocampal sclerosis. Patients had to meet the following inclusion criteria: ages 20 to 50 years, at least 6 years of schooling, normal general cognitive status (Cognitive Abilities Screening Instrument [CASI]24), no symptoms of severe depression (Beck Depression Inventory25), no lesions other than hippocampal sclerosis, absence of secondarily generalised seizures over the past year, and no history of neurosurgery. The study also included 24 healthy age-, sex-, and education-matched controls with no history of neurological or psychiatric disease, no history of alcohol or drug abuse, no cognitive impairment, and no symptoms of severe depression. Table 1 summarises participants’ demographic and clinical characteristics.
Sample characteristics.
| Controls (n=24) | Patients with TLE (n=25) | P | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 33.7 | 9.2 | 37.9 | 8.1 | .1 |
| Years of schooling | 12.6 | 3.8 | 11.6 | 2.6 | .3 |
| Sex (women/men) | 15/9 | 16/9 | 1.0 | ||
| CASI score (general cognitive status) | 94.3 | 3.1 | 91.0 | 5.0 | <.05 |
| Beck Depression Inventory score | 5.4 | 3.8 | 9.3 | 8.3 | .3 |
| Age at disease onset (years) | 12.0 | 7.5 | |||
| Progression time (years) | 25.9 | 10.4 | |||
| Seizure frequency (seizures per month) | 3.5 | 4.4 | |||
| Lateralisation of epileptic focus (left/right) | 13/12 | ||||
| AEDs (monotherapy/polytherapy) | 8/17 | ||||
| AEDs (1st generation/2nd or 3rd generation)a | 30/22 | ||||
First-generation AEDs: carbamazepine, clonazepam, phenytoin, valproic acid.
Second- and third-generation AEDs: clobazam, lamotrigine, levetiracetam, vigabatrin.
AED: antiepileptic drug; CASI: Cognitive Abilities Screening Instrument; SD: standard deviation; TLE: temporal lobe epilepsy.
This study was approved by the Ethics Committee at the National Institute of Neurology and Neurosurgery; all participants signed informed consent forms as stipulated by the 1975 Declaration of Helsinki.
InstrumentsWe used the CASI24 to select patients with normal cognitive function (scores 82 and above) and the Beck Depression Inventory25 to exclude participants with severe depressive symptoms (scores over 30).
SVF was evaluated patient by patient using a protocol specifically designed for this study. In 60seconds, participants had to name as many words as possible from each of the following categories: animals, fruits, countries, jobs, and verbs. We used a voice recorder during the task and transcribed all the words produced.
The quantitative analysis evaluated (1) number of words per category and (2) total number of words for all categories.
The qualitative analysis evaluated performance in the SVF animals and fruits tasks; as these are the most widely used categories in research and clinical assessment, the literature includes criteria for this type of analysis. Furthermore, as explained by Goñi et al.,26 the category “animals” is universal across languages and cultures, with only minor differences between countries, education systems, and generations. For this type of analysis, we recorded the number of clusters, mean cluster size, number of switches, number of perseverations, and number of intrusions in each category. Table 2 lists the criteria of the qualitative analysis; these are based on previous studies14,22,27–29 but include clearer and more specific word classification instructions. Table 3 presents the criteria for clusters and the exemplars considered within clusters.
Qualitative analysis criteria.
| 1. Correct words: words pertaining to the category requested (animals or fruits) and included in the dictionary of the Royal Academy of the Spanish Language |
| The following words were regarded as correct: (1) words for animals that change depending on sex (e.g., hen, cow, mule) or age (e.g., kid, chicken, foal), and (2) derivatives and synonyms (e.g., horse-pony, sex-related changes [perro/perra: in Spanish, male/female dog], plátano/banana [Spanish synonyms for banana]) if the alternative form had not been mentioned previously. The total number of correct words was obtained by adding together all correct words except for the following: words from supraordinate subcategories (e.g., fish) when naming exemplars from a specific subcategory (e.g., tuna, salmon) and subordinate exemplars (e.g., dog breeds) |
| 2. Clusters: clusters had to include at least 2 consecutive words pertaining to the same semantic subcategory, including perseverations, derivative words, and synonyms, as these provide information about the strategy followed by the participant regardless of whether they are correct. Subcategories were established prior to testing based on previous studies and responses given by controls |
| Table 3 lists the semantic subcategories considered in this study, with the corresponding exemplars |
| We applied the following classification rules: (1) words pertaining to 2 different subcategories were assigned to both (for example, in the list “dog, cat, tiger, lion,” the first 2 words were regarded as domestic animals and the last 3 words as felines; “cat” was therefore included in both subcategories), and (2) when small clusters were incorporated into larger clusters, or when 2 subcategories overlapped but all elements may be assigned to a single subcategory, we only considered the largest common category |
| 3. Mean cluster size: mean number of words within each cluster. It was calculated by summing all words within a cluster and subtracting one (e.g., a cluster containing 3 words has a size of 2). We also included perseverations, derivative words, and synonyms, as long as they were not consecutive or did not appear in the same cluster |
| 4. Switches: shifts between words were regarded as switches in the following cases: (a) shifting between isolated words, that is, words nor pertaining to a cluster (e.g., the list “dog, viper, butterfly” contains 2 switches, one from dog to viper and the second from viper to butterfly); (b) shifting from an isolated word to a cluster, or vice versa (e.g., the list “cat, puma, parrot” contains one switch, from the cluster “felines” to the isolated word “parrot”), or (c) shifting between clusters (e.g., the list “cat, puma, parrot, canary” contains one switch, from the cluster “felines” to the cluster “birds”). Perseverations, derivative words, and synonyms were also considered in switching |
| 5. Perseverations: repetition of previously generated words |
| 6. Intrusions: words with a different semantic category to the one being tested |
| 7. Performance/time: the analysis of performance by time interval included all 5 semantic categories evaluated: animals, fruits, jobs, countries, and verbs. We counted the number of words per category in each time interval (0-15, 16-30, 31-45, and 46-60seconds) |
Clusters and exemplars.
| The following clusters are based on results from our control group, as well as on clusters reported in previous studies.22,27–29 |
| Clusters for animals |
| Paired words (pairs of words strongly associated to one another due to the influence of popular culture, stories, and fables): elephant-mouse, elephant-horse-camel, cat-mouse/rat, hare-tortoise, wolf-pig |
| Domestic/farm animals: bull, calf, canary, cat, chicken, cow, dog, donkey (ass), duck, ferret, Guinea pig, goat, hamster, hen, horse, kid, lamb, parakeet, parrot, pig (hog, boar, swine), rabbit, rooster, sheep, etc. |
| Woodland/mountain animals: bear, beaver, coyote, deer, eagle, elk, falcon, fox, lynx, mole, otter, owl, porcupine, puma, rabbit, raccoon, raven, squirrel, vulture, wild boar, wolf, etc. |
| Jungle/rainforest animals: antelope, armadillo, boa constrictor, cheetah, chimpanzee, cobra, crocodile, deer, elephant, falcon, giraffe, gorilla, hedgehog, hippopotamus, hyena, jaguar, kangaroo, koala, lemur, leopard, lion, mandrill, monkey, ocelot, orangutan, otter, panda, panther, porcupine, puma, python, rhinoceros, tiger, viper, wild boar, zebra, etc. |
| Desert animals: antelope, black vulture, camel, chameleon, coati, coyote, dingo, dromedary, elephant, fennec fox, lizard, lynx, meerkat, mongoose, scorpion, snake, tarantula, toad, tortoise, etc. |
| Flying animals or birds: bat, bee, butterfly, canary, chicken, duck, eagle, falcon, flamingo, goose, hummingbird, ostrich, owl, parakeet, parrot, peacock, pelican, penguin, pigeon, quetzal, raven, robin, rooster, swallow, toucan, etc. |
| Aquatic animals: cod, crab, crocodile, dolphin, fish (jellyfish, swordfish, blowfish, clownfish), hammerhead shark, iguana, lobster, manatee, octopus, oyster, piranha, prawn, salamander, seahorse, seal, shark, turtle, walrus, whale, etc. |
| Reptiles or amphibians: chameleon, crocodile, frog, gecko, iguana, Komodo dragon, toad, turtle, all types of snakes, etc. |
| Insects: ant, bedbug, beetle, butterfly, cockroach, dragonfly, earthworm, flea, fly, ladybird, louse, mantis, mosquito, scorpion, snail, spider, tick, etc. |
| Canines: coyote, dingo, dog, fox, hyena, jackal, wolf, etc. |
| Felines: cat, cheetah, jaguar, lion, leopard, lynx, ocelot, panther, puma, tiger, etc. |
| Ruminants: camel, deer, dromedary, gazelle, giraffe, goat, lamb, llama, ox, reindeer, etc. |
| Primates: baboon, chimpanzee, gorilla, lemur, mandrill, monkey, orangutan, etc. |
| Rodents: beaver, chinchilla, Guinea pig, ferret, hamster, hedgehog, marmot, mouse, otter, porcupine, rat, squirrel, etc. |
| Clusters for fruits |
| Sweet fruits: apple, apricot, banana, cherimoya, cherry, date, dragon fruit, fig, guava, guarana, grape, loquat, lychee, mammee, melon, nectarine, orange, papaya, peach, pear, plum, pomegranate, prickly pear, redcurrant, sapote, soursop, watermelon, etc. |
| Acid fruits: apple, grape, grapefruit, guava, kiwi, lime, lemon, orange, pineapple, quince, etc. |
| Sub-acid fruits: apricot, blackberry, guava, lime, mandarin, mango, nectarine, passionfruit, peach, raspberry, strawberry, etc. |
| Neutral fruits/nuts: almond, avocado, chestnut, cocoa bean, coconut, hazelnut, peanut, walnut, etc. |
| Citrus fruits: apple, cherry, grape, grapefruit, lemon, lime, mandarin (tangerine), orange, pear, persimmon, quince, etc. |
| Tropical fruits: avocado, banana, cherry, coconut, cranberry, dragonfruit, fig, grape, grapefruit, guava, kiwi, loquat, lychee, mammee, mandarin, mango, melon, orange, papaya, passionfruit, peach, pineapple, plum, pomegranate, sapote, soursop, strawberry, watermelon, etc. |
| Forest fruits: blackberry, cherry, cranberry, raspberry, redcurrant, strawberry, etc. |
| Dried fruit and nuts: almond, chestnut, date, fig, hazelnut, peanut, pistachio, prune, tamarind, tejocote, walnut, etc. |
We also analysed performance by time interval, that is the total number of correct words produced for each of the 5 categories in 4 time intervals (0-15, 16-30, 31-45, and 46-60seconds).
Data analysisStatistical analysis was performed using the IBM SPSS software, version 19. We used the non-parametric Mann–Whitney U and chi-square tests to analyse the variables not showing normal distribution or homoscedasticity. Repeated measures ANOVA was used to analyse homoscedastic and normally distributed variables in order to compare the number of words produced by each group for each of the 5 categories. The Bonferroni correction was applied for post hoc analysis of statistically significant interactions (paired comparisons).
For the animals and fruits categories, non-parametric tests were used to analyse the number of clusters, mean cluster size, the number of switches, perseverations, and intrusions, and performance by time interval.
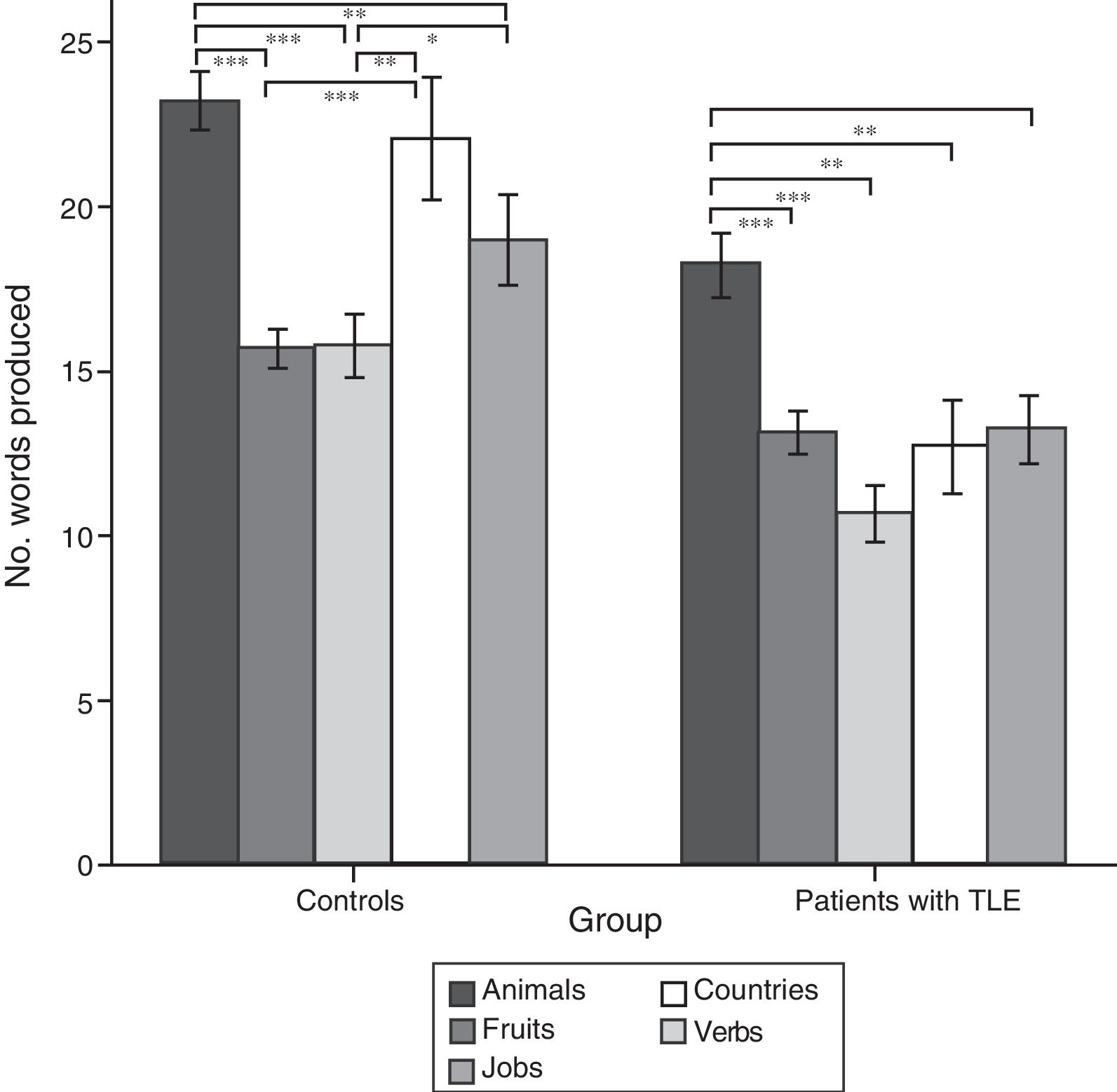
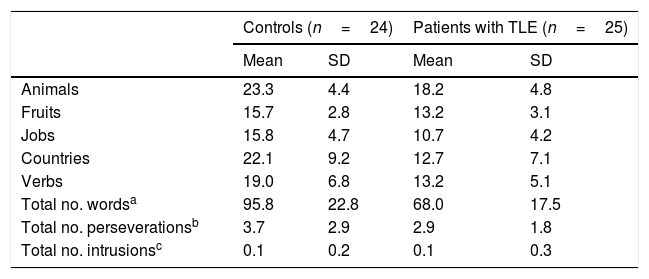
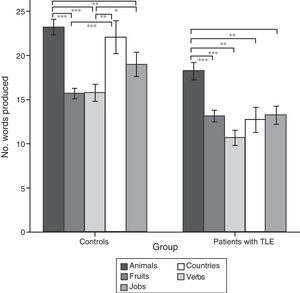
ResultsQuantitative analysis of semantic verbal fluency tasksWe observed a statistically significant interaction between the factors group×category (F[4,188]=4.2; P=.003), indicating significant differences in the number of words produced by each group. According to the intergroup post hoc analysis (Table 4), patients with TLE generated fewer words per category than controls (animals, P<.001; fruits, P=.004; jobs, P<.001; countries, P<.001; verbs, P=.002). The post hoc intragroup analysis (Fig. 1) showed that patients produced more words for the animals category than for the remaining categories, whereas controls generated more words for the animals category, followed by the countries and verbs categories, and fewer words for fruits and jobs.
Mean number of words per category and total mean number of words, perseverations, and intrusions during semantic verbal fluency tasks.
Intragroup comparison of the number of words produced for each category.
Data are expressed as means and standard deviation (error bars). Statistically significant differences between categories are also indicated.
Post hoc analysis with Bonferroni correction: *P<.05; **P<.01; ***P<.001.
No significant intergroup differences were observed in the number of perseverations (U=269; P=.53) or intrusions (U=276.5; P=.32).
Qualitative analysis of semantic verbal fluency tasksThe qualitative analysis included the animals and fruits categories only.
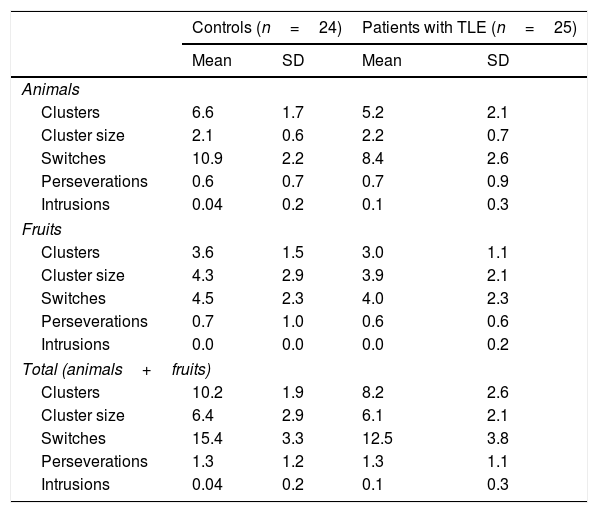
Patients generated significantly fewer clusters (U=167; P=.007) and switches (U=177; P=.013) than controls; no differences were observed in mean cluster size (U=294.5; P=.912) or number of perseverations (U=295.5; P=.925) or intrusions (U=276.5; P=.322) (Table 5).
Mean cluster size and mean number of clusters, switches, perseverations, and intrusions for the animals and fruits categories.
| Controls (n=24) | Patients with TLE (n=25) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Animals | ||||
| Clusters | 6.6 | 1.7 | 5.2 | 2.1 |
| Cluster size | 2.1 | 0.6 | 2.2 | 0.7 |
| Switches | 10.9 | 2.2 | 8.4 | 2.6 |
| Perseverations | 0.6 | 0.7 | 0.7 | 0.9 |
| Intrusions | 0.04 | 0.2 | 0.1 | 0.3 |
| Fruits | ||||
| Clusters | 3.6 | 1.5 | 3.0 | 1.1 |
| Cluster size | 4.3 | 2.9 | 3.9 | 2.1 |
| Switches | 4.5 | 2.3 | 4.0 | 2.3 |
| Perseverations | 0.7 | 1.0 | 0.6 | 0.6 |
| Intrusions | 0.0 | 0.0 | 0.0 | 0.2 |
| Total (animals+fruits) | ||||
| Clusters | 10.2 | 1.9 | 8.2 | 2.6 |
| Cluster size | 6.4 | 2.9 | 6.1 | 2.1 |
| Switches | 15.4 | 3.3 | 12.5 | 3.8 |
| Perseverations | 1.3 | 1.2 | 1.3 | 1.1 |
| Intrusions | 0.04 | 0.2 | 0.1 | 0.3 |
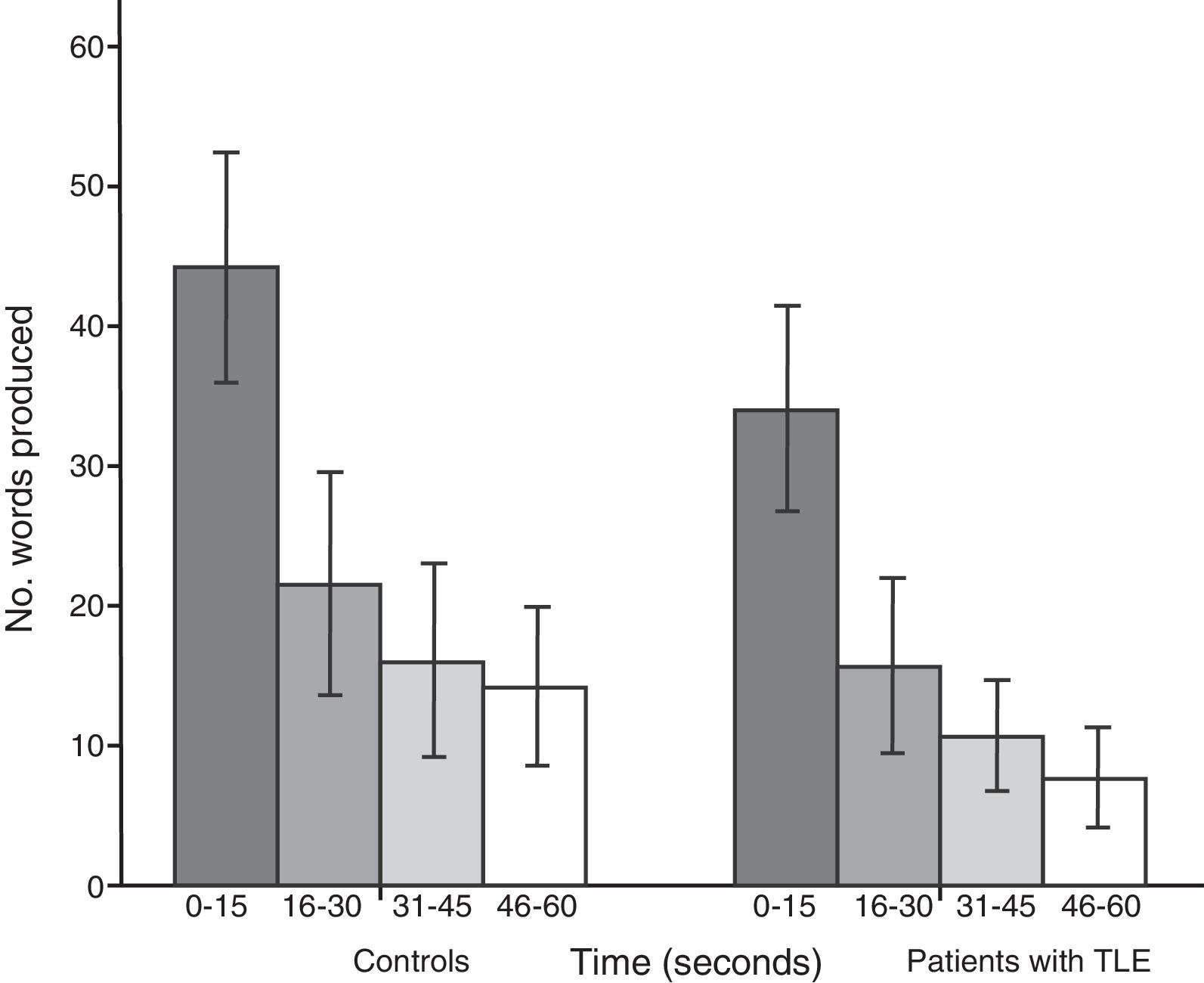
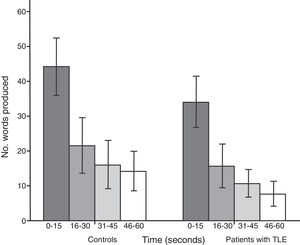
Both groups showed a linear decrease in word production by time interval. Patients with TLE produced significantly fewer words than controls in each time interval: 0-15seconds (U=112.5; P<.001), 16-30seconds (U=171.5; P=.01), 31-45seconds (U=161; P=.005), and 46-60seconds (U=104; P<.001) (Fig. 2).
DiscussionPatients with TLE showed considerable SVF impairment for all semantic categories, generating fewer words than controls. Our results are consistent with those of previous studies, and confirm the presence of SVF impairment in TLE.7–9
The fact that patients with TLE generated fewer clusters for animals and fruits than controls is suggestive of impaired semantic network activation, but does not indicate semantic network disruption as both groups showed similar mean cluster size. A potential explanation for this is that data within semantic networks are preserved, but activation is impaired, hindering retrieval.
In strategic and executive processes, patients were found to make fewer switches than controls in the animals and fruits categories, although no differences were observed between groups in the number of intrusions and perseverations. This suggests that patients retrieve elements pertaining to the correct semantic categories, inhibit irrelevant or unrelated information, and store previously generated words in the working memory to avoid repetitions. Therefore, fewer switches point to inefficient search strategies and impaired cognitive flexibility: patients with TLE did not shift between clusters once the exemplars from the previous cluster were exhausted. Despite published evidence of executive dysfunction in patients with TLE,30–32 we did not use complementary neuropsychological tests to evaluate executive function and confirm executive dysfunction.
Similarly to the results reported by N’Kaoua et al.,18 we found intergroup differences in the number of switches but similar mean cluster sizes. This suggests that the mechanisms underlying SVF impairment in patients with TLE involve alterations in strategic search and cognitive flexibility. However, unlike other authors, we found evidence that these patients also present impaired semantic network activation, as they produced fewer clusters in the animals and fruits categories.
The results of the analysis of performance by time interval are also consistent with impaired semantic network activation, impaired strategic search, and poor cognitive flexibility: patients showed reduced capacity to retrieve information from the early phase to the late phase of SVF tasks for all 5 categories analysed.
In conclusion, the executive profile of our patients with TLE shows: (1) decreased verbal fluency, characterised by production of fewer words in different semantic categories; (2) fewer clusters than in the control group, but no differences in mean cluster size (qualitative analysis); this combined with production of fewer words during the early phase of the task may indicate impaired semantic network activation; (3) fewer switches and decreased production of words during the late phase of the task, which may reflect inefficient search and retrieval strategies, associated with strategic search and mental flexibility; and (4) similar numbers of perseverations and intrusions to those made by controls, which suggests that attention, inhibition, and working memory are preserved in these patients.
The results of the quantitative and qualitative analysis of SVF show that semantic impairment in TLE may involve both impaired semantic network activation and semantic information retrieval inefficiency in strategic and executive processes.
An alternative hypothesis suggests generalised slowing due to adverse effects of antiepileptic drugs (AED). Reduced alertness and processing speed are the most common adverse effects of first-generation AEDs (e.g., valproic acid, carbamazepine, phenytoin, and phenobarbital).33 Polytherapy has a greater negative impact on cognition than monotherapy.34 Most of the patients included in our sample were receiving polytherapy with first-generation AEDs (Table 1). This treatment may affect information processing speed, slowing strategic search and the access, activation, and dissemination of semantic networks.
In the evaluation of SVF with different semantic categories, patients perform better in the animals category than in the remaining categories, which for the first time shows the type of information these patients recall more easily. This may have important implications for the neuropsychological rehabilitation of memory, linguistic, and semantic processes in these patients, as it provides valuable data on which type of specific information and strategies for information organisation and retrieval are most helpful in improving memory function. Our study also establishes clearer and more specific criteria for classifying some of the words most frequently produced in animals and fruits categories during SVF tasks; these criteria are useful for qualitative analysis and have not been established in previous studies. We also describe in detail the criteria for classifying clusters, mean cluster size, and switches for these 2 categories.
Our study also has a number of limitations. We did not use additional neuropsychological tests, which would provide complementary data in the analysis of such cognitive processes as attention, cognitive flexibility, and working memory. Our sample size constitutes another limitation as it prevents extrapolation of our results. While the sample was small, patients were carefully selected (presence of unilateral hippocampal sclerosis and other clinical variables) to avoid confounding (Table 1). Patients in the previously cited studies using neuropsychological tests vary greatly in terms of disease duration and age at onset, and aetiologies are often different or unspecified.7,17,18 These variables may be regarded as confounders, introducing biases.
The fact that the qualitative analysis included only the animals and fruits categories may prevent us from extrapolating this explanation of the cognitive deficits underlying poor performance in SVF tasks. The qualitative analysis did not include the remaining categories for 2 reasons. Firstly, as these categories have rarely been used in research, no specific criteria have been established for defining clusters. Secondly, the animals and fruits categories are the most frequently used in clinical practice, and our study aimed to provide useful and practical information for the clinical context. Future studies should perform qualitative analyses of different semantic categories, from specific and functional categories to more abstract ones, to determine whether semantic processing difficulties in patients with TLE are generalised or specific to particular categories.
Conflicts of interestThe authors have no conflicts of interest to disclose.
The authors wish to thank CONACYT for its support (project no. 240856).
Please cite this article as: Jaimes-Bautista AG, Rodríguez-Camacho M, Martínez-Juárez IE, Rodríguez-Agudelo Y. Análisis cuantitativo y cualitativo de la fluidez verbal semántica en pacientes con epilepsia del lóbulo temporal. Neurología. 2020;35:1–9.