The use of oral anticoagulants in patients with a history of atrial fibrillation (AF) and intracranial haemorrhage (ICH) is controversial on account of the risk of haemorrhagic stroke recurrence. This study presents our experience regarding the safety and efficacy of percutaneous left atrial appendage closure (LAAC), an alternative to anticoagulation in these patients.
MethodsWe conducted a retrospective, single-centre, observational study. LAAC was performed in patients with a history of ICH and non-valvular AF. Risk of ischaemic and haemorrhagic events was estimated using the CHA2DS2-VASc and HAS-BLED scales. We recorded periprocedural complications, IHC recurrence, cerebral/systemic embolism, mortality and use of antithrombotic drugs following the procedure.
ResultsLAAC was performed in 9 patients (7 men, 2 women) using the AMPLATZER Amulet device in 7 cases and the AMPLATZER Cardiac Plug device in 2. Mean age was 72.7±8.2 years. Time between ICH and LAAC was less than one month in 5 patients and more than one month in 4 patients. Median CHA2DS2-VASc score was 4 (interquartile range of 2.5). Median HAS-BLED score was 3 (interquartile range of 0). No periprocedural complications were recorded. All patients received single anti-platelet therapy (clopidogrel in 5 patients, aspirin in 4) after the procedure; 5 patients received this treatment for 6 months and 4 received it indefinitely. No ischaemic or haemorrhagic events were recorded during follow-up (mean duration of 15 months).
ConclusionsIn our series, LAAC was found to be safe and effective in patients with a history of ICH who required anticoagulation due to AF.
El uso de anticoagulantes orales es controvertido en pacientes con antecedentes de fibrilación auricular (FA) y hemorragia intracraneal (HIC), por riesgo de recurrencia de ictus hemorrágico. Presentamos la experiencia de nuestro centro en relación con la seguridad y la eficacia del cierre percutáneo de orejuela (CPO), una alternativa a la anticoagulación en dicho contexto.
MétodosEstudio observacional, retrospectivo y unicéntrico. El CPO se realizó en pacientes con antecedentes de HIC y FA no valvular. El riesgo de eventos isquémicos y hemorrágicos se estimó usando las escalas CHA2DS2Vasc y HAS-BLED. Se registraron: complicaciones periprocedimiento, recurrencia de HIC, embolismo cerebral/sistémico, mortalidad tras el cierre y al seguimiento y uso de antitrombóticos tras el procedimiento.
ResultadosEl CPO se realizó en 9 pacientes (7 hombres, 2 mujeres). Se utilizó en 7 casos el dispositivo Amplatzer Amulet y en 2 el Amplatzer Cardiac Plug. La media de edad fue 72,7±8,2 años. El tiempo entre la HIC y el CPO fue menor de un mes en 5 pacientes y mayor en 4. La mediana y el rango intercuartil para la escala CHA2DS2Vasc fueron de 4 y 2,5, respectivamente, siendo de 3 y 0 para la escala HAS-BLED. No hubo complicaciones periprocedimiento. Todos recibieron antiagregación simple tras el procedimiento (5 clopidogrel y 4 aspirina); en 5 se mantuvo 6 meses, en 4 indefinidamente. Durante el seguimiento (15 meses de promedio) no se registraron eventos isquémicos ni hemorrágicos.
ConclusionesEn nuestra serie, el CPO supone una alternativa segura y eficaz en pacientes que han presentado HIC y que precisan ser anticoagulados por FA.
One of the most feared complications associated with oral anticoagulant (OAC) treatment is intracranial haemorrhage (ICH). The new OACs seem to decrease haemorrhagic events when compared to classic drugs.1 However, this has not been demonstrated in patients with history of ICH, in whom anticoagulation is especially controversial.2–4 It should be noted that up to 13% of patients with history of ICH meet criteria for anticoagulation.5 Therefore, in the management of patients with atrial fibrillation (AF) and history of ICH, physicians frequently face the dilemma of choosing whether to start anticoagulation (with the associated risk of bleeding), antiplatelet treatment, or no treatment at all (which entails a significant risk of ischaemic events).
In such a situation, percutaneous left atrial appendage closure (LAAC) represents a third way. Thus, LAAC would be an alternative in patients with AF and contraindicated for OACs due to previous haemorrhagic stroke; this would enable us to simultaneously prevent ischaemic events and haemorrhagic events secondary to anticoagulation.
While there are clinical trials and meta-analyses comparing LAAC with OACs in patients with AF and ischaemic stroke,6 no clinical trial includes patients with history of ICH. Only 3 observational studies have indicated that LAAC is safe and effective.7–9
Further studies are needed to demonstrate the efficacy and safety of the treatment for this patient profile. In this article, we present results from our centre.
MethodsThis is an observational, retrospective, single-centre study of consecutive cases (with prospective data collection during follow-up) gathered between 2013 and 2016. LAAC has been performed at our centre since 2012, with a total of 66 procedures to date, 9 of which were due to ICH (the remaining 57 were performed in patients presenting non-intracranial [mainly gastrointestinal] haemorrhages while taking OACs).
We prospectively recorded data on demographic variables (age, sex), cardiovascular risk factors (hypertension, diabetes, dyslipidaemia), smoking and alcohol abuse, type of ICH (intraparenchymal, subarachnoid, or subdural), cause of ICH, score on the National Institutes of Health Stroke Scale (NIHSS) before the procedure, score on the modified Rankin Scale (mRS) after the ICH, anticoagulant and antiplatelet treatment before ICH and after LAAC, and ischaemic and haemorrhagic risk based on the CHA2DS2-VASc and HAS-BLED scales. Inclusion criteria included AF with indication of OACs and history of ICH. We excluded those patients with poor functional status (mRS score of 4 or more one year after ICH).
Mean follow-up time was 15 months (range, 3 months to 2 years); all patients signed a standardised informed consent form before the LAAC in which they agreed to the procedure and to inclusion in the study. Both the procedure and the study protocol were approved by the local ethics committee.
Complications were defined according to the PROTECT AF study6: ischaemic or haemorrhagic stroke, death, pericarditis, and device embolism were considered major complications. Groin pseudoaneurysm, arteriovenous fistula, haematomas, thrombus formation on the device, and minor bleeding requiring no reintervention were considered minor complications. The statistical methods used were exclusively descriptive, with means, standard deviations, and interquartile ranges (IQR) used in the case of continuous quantitative variables, and medians for ordinal quantitative variables.
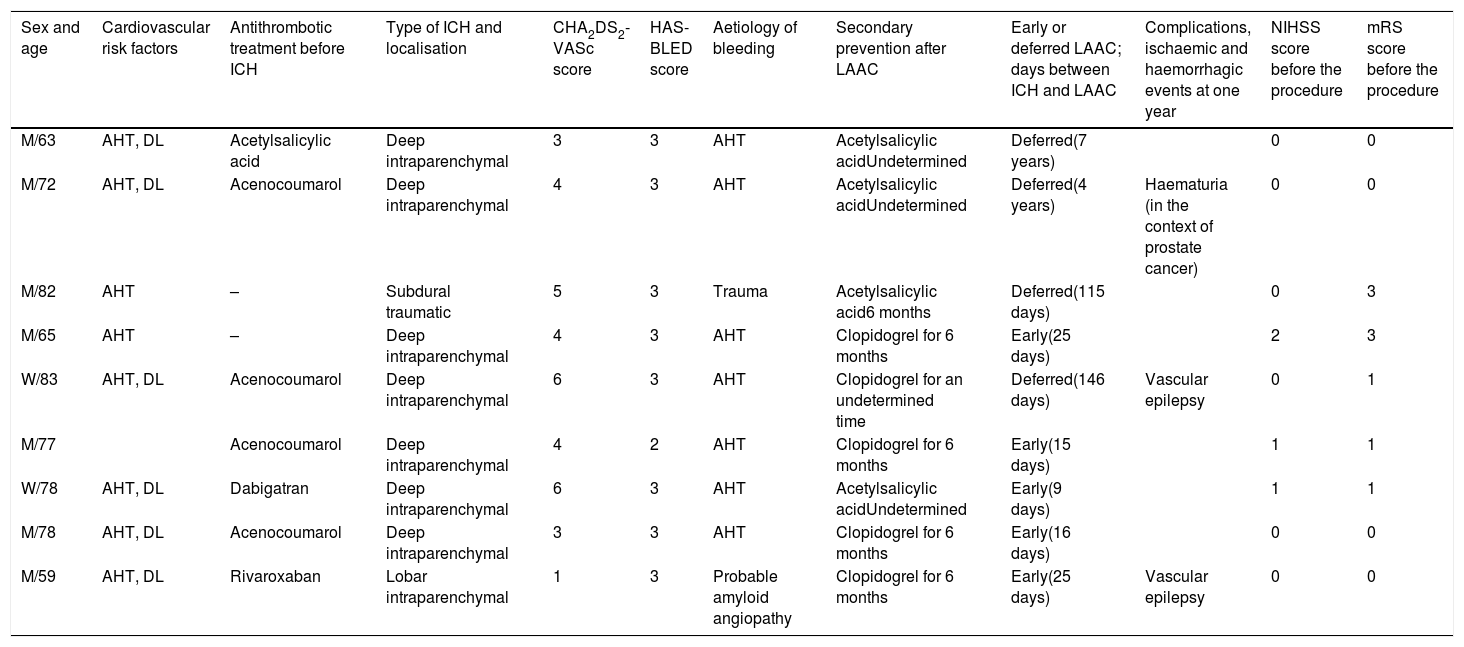
ResultsOf 49 patients presenting ICH and AF between 2013 and 2016, 9 underwent LAAC: 7 men and 2 women. Patients’ characteristics are listed in Table 1.
Summary of clinical data from our 9 patients.
| Sex and age | Cardiovascular risk factors | Antithrombotic treatment before ICH | Type of ICH and localisation | CHA2DS2-VASc score | HAS-BLED score | Aetiology of bleeding | Secondary prevention after LAAC | Early or deferred LAAC; days between ICH and LAAC | Complications, ischaemic and haemorrhagic events at one year | NIHSS score before the procedure | mRS score before the procedure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M/63 | AHT, DL | Acetylsalicylic acid | Deep intraparenchymal | 3 | 3 | AHT | Acetylsalicylic acidUndetermined | Deferred(7 years) | 0 | 0 | |
| M/72 | AHT, DL | Acenocoumarol | Deep intraparenchymal | 4 | 3 | AHT | Acetylsalicylic acidUndetermined | Deferred(4 years) | Haematuria (in the context of prostate cancer) | 0 | 0 |
| M/82 | AHT | – | Subdural traumatic | 5 | 3 | Trauma | Acetylsalicylic acid6 months | Deferred(115 days) | 0 | 3 | |
| M/65 | AHT | – | Deep intraparenchymal | 4 | 3 | AHT | Clopidogrel for 6 months | Early(25 days) | 2 | 3 | |
| W/83 | AHT, DL | Acenocoumarol | Deep intraparenchymal | 6 | 3 | AHT | Clopidogrel for an undetermined time | Deferred(146 days) | Vascular epilepsy | 0 | 1 |
| M/77 | Acenocoumarol | Deep intraparenchymal | 4 | 2 | AHT | Clopidogrel for 6 months | Early(15 days) | 1 | 1 | ||
| W/78 | AHT, DL | Dabigatran | Deep intraparenchymal | 6 | 3 | AHT | Acetylsalicylic acidUndetermined | Early(9 days) | 1 | 1 | |
| M/78 | AHT, DL | Acenocoumarol | Deep intraparenchymal | 3 | 3 | AHT | Clopidogrel for 6 months | Early(16 days) | 0 | 0 | |
| M/59 | AHT, DL | Rivaroxaban | Lobar intraparenchymal | 1 | 3 | Probable amyloid angiopathy | Clopidogrel for 6 months | Early(25 days) | Vascular epilepsy | 0 | 0 |
AHT: arterial hypertension; DL: dyslipidaemia; M: man; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; W: woman.
In the remaining 40 patients in whom LAAC was ruled out as therapeutic alternative, we found the following exclusion criteria: deceased (20 patients), mRS score ≥ 4 (18), or patient's refusal to undergo the procedure (2).
Mean age was 72.7±8.2 years. Aetiology was determined according to the H-ATOMIC system.10 Seven of these haemorrhages were deep and hypertensive, one was a subdural haematoma, and the other a lobar haemorrhage with probable amyloid aetiology.
Median CHA2DS2-VASc and HAS-BLED scores were 4 (IQR, 2.5) and 3 (IQR, 0), respectively.
Prior to ICH, 4 patients were taking anticoagulation with acenocoumarol, one with dabigatran, and one with rivaroxaban; another patient was taking antiplatelet treatment with acetylsalicylic acid; and 2 were receiving no treatment.
Antithrombotic treatment after LAAC consisted of acetylsalicylic acid or clopidogrel for 6 months in 5 cases and was not specified in the remaining patients; treatment depended on the patient's circumstances (stent carrier, diabetic, etc.). The cardiology department was responsible for deciding on this treatment.
No major or minor complication was recorded either during the periprocedural period or during follow-up. One patient presented haematuria in the context of prostate cancer, and a further 2 patients presented seizures of vascular aetiology.
Time from haemorrhage to LAAC varied greatly, with a mean of 361±479 days. Patients were divided into 2 groups: those who underwent the procedure early (5 patients who underwent LAAC less than a month after the ICH; mean, 18 days), and those who underwent LAAC later on an outpatient basis (4 patients; mean, 2 years and 2 months).
Patients undergoing LAAC presented a good functional status before the procedure (mRS score≤3) and an NIHSS score < 3.
DiscussionAutopsy and echocardiography findings show that the left atrial appendage is the origin of 90% of thrombi in patients with non-valvular AF.5 Therefore, one way of preventing ischaemic stroke is to isolate the site of thrombus generation and embolisation, which gives rise to the need for anticoagulation.7,11,12 At our centre, 9 LAAC were performed in patients with a history of AF and ICH between 2013 and 2016, with no major or minor complications recorded during follow-up.
Copresence of AF meeting criteria for anticoagulation with ICH is a frequent finding in clinical practice. In fact, up to 13% of patients presenting ICH are eligible for anticoagulation.5 However, continuing anticoagulation after an ICH significantly increases the risk of haemorrhage recurrence.13 Although new anticoagulants have shown an initially lower risk of ICH than classic anticoagulants,1 it is not known whether they are associated with lower risk of recurrence in those patients who have previously suffered a haemorrhagic event.
To date, few studies have analysed how best to approach patients with ICH who need to continue anticoagulation or the right time to start anticoagulation.2,3,14 Some of these studies report that it would only be recommendable to start anticoagulation treatment in those patients with a CHA2DS2-VASc score ≥ 6, whereas antiplatelet treatment is not recommended as it increases the risk of bleeding without protecting against ischaemic events.15
The Spanish Society of Neurology's guidelines contraindicate anticoagulation in the case of lobar haemorrhages, given the significant theoretical risk of haemorrhagic recurrence. In the case of deep haemorrhages of hypertensive aetiology, the use of OACs is permitted only in cases of optimal blood pressure management.4
With regard to treatment selection, the new OACs are associated with lower risk of stroke, systemic embolism, and haemorrhagic stroke when compared to classic anticoagulants. However, prognosis has not been shown to be different in patients with ICH. These drugs have also been observed to increase the risk of gastrointestinal haemorrhage.1
Although some clinical trials do compare LAAC with OACs, no such study has been conducted in patients with ICH.
The meta-analysis of the PROTECT AF and PREVAIL studies (which included more than 2400 patients with AF) revealed comparable efficacy between warfarin and the WATCHMAN device for the overall prevention of neurovascular events (without differentiating between ischaemic and haemorrhagic events), and lower rates of ICH and mortality in the group undergoing LAAC; however, this group also showed a higher rate of ischaemic stroke.6 It should be noted that whereas OACs can lead to long-term complications, complications associated with LAAC are short-term and arise mainly during the procedure (device embolism, periprocedural stroke, death, and pericardial effusion).1 Such complications were observed at a rate of 7.4% in the PROTECT AF study and 4.2% in the PREVAIL study.
The limited medical literature on the subject only compares anticoagulant treatment, antiplatelet treatment, and no treatment,15,16 and does not address LAAC; anticoagulant treatment is recommended in patients presenting high risk of ischaemic events. Thus, the aim of our study is to address the difficult decision of how to prevent ischaemic events in patients with history of non-valvular AF and ICH.
Only 3 descriptive studies analyse the safety and effectiveness of LAAC in patients with history of ICH,7–9 and none compares the procedure with the remaining therapeutic options. With regard to the 3 studies including patients with ICH, in the first (published in 2014), LAAC was performed in 20 patients with history of ICH, with no periprocedural complications or ischaemic or haemorrhagic events after a mean follow-up time of 13.8 months. Only 4 minor complications were detected.7 In the second study (published in 2016), LAAC was performed in 24 patients with history of ICH and 2 with history of intraocular haemorrhage; 3 complications were observed after a mean follow-up time of 11±9 months: a transient ischaemic attack of unknown cause, a death unrelated to the procedure, and a case of device thrombus, which was resolved percutaneously.8 In the third study (published in 2016), LAAC was performed in 46 patients with history of ICH; a periprocedural complication rate of 6% and 3 deaths were observed after a 12-month follow-up period (one death due to ICH, one due to multiorgan failure, and another of unknown cause).9
We observed no major or minor periprocedural complications in our 9 patients, nor ischaemic or haemorrhagic events during follow-up. We should highlight that, based on the CHA2DS2-VASc and HAS-BLED scales, the yearly risk of stroke and haemorrhagic complications was calculated at 4%-6.7% and 1.8%-3.7%, respectively.
In our series, the percentage of brain ischaemic and haemorrhagic events was 0%. As a novel observation with regard to studies on LAAC, we found no difference between patients undergoing LAAC during admission due to ICH and those undergoing LAAC on an outpatient basis (as can be observed in Table 1, there are no differences regarding complications, subsequent treatment, or subsequent ischaemic or haemorrhagic events). Therefore, the procedure enables us to reduce the time during which the patient is not protected against ischaemic events.
However, robust conclusions cannot be drawn, given the study design (retrospective and single-centre), the small number of patients included, the short follow-up period, and the heterogeneity of patients. Our findings may be confirmed by further multi-centre observational studies with larger samples, and clinical trials comparing the safety and effectiveness of LAAC against antiplatelet or anticoagulant treatment. While we await conclusive data, our findings suggest that LAAC may be a good alternative to OACs in patients with ICH with a view to preventing vascular ischaemic and haemorrhagic events.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fayos-Vidal F, Arzamendi-Aizpurua D, Millán-Álvarez X, Guisado-Alonso D, Camps-Renom P, Prats-Sánchez L, et al. Cierre de orejuela en pacientes con hemorragia intracraneal y fibrilación auricular. Neurología. 2020;35:10–15.
This study has not been presented at any of the SEN's Annual Meetings or at any other meetings or congresses