Scrub typhus is a potentially life-threatening but curable disease that can produce multi-organ failure. Neurological manifestations in scrub typhus have gained attention recently, where the entire neural axis except the myoneural junction can be involved. Although the pathogenesis of neurological involvement has not been established, immune-mediated mechanisms are suspected. This article reports the clinicopathological features of scrub typhus cases presenting several rare neurological and neuropsychiatric manifestations.
MethodsThree hundred fifty-four serologically confirmed scrub typhus cases were admitted to the Department of General Medicine of Burdwan Medical College and Hospital (West Bengal, India) between May 2018 and May 2022. There were 50 patients who had predominantly neurological manifestations. Of these 50 cases, ten patients presented with extremely rare neurological manifestations.
ResultsWe report 10 cases of scrub typhus (four men and six women) who presented with complex neurological pictures (posterior reversible encephalopathy syndrome, Opalski syndrome, parkinsonism, cerebellitis, isolated opsoclonus, acute transverse myelitis, myositis, polyradiculoneuropathy with cranial neuropathy, acute transient behavioral changes, and fibromyalgia). Immune-mediated mechanisms might have mediated the pathogenesis of most cases following scrub typhus infection.
ConclusionFrom a clinicopathological point of view, each case was unique in its presentation and treatment response. In any acute onset neurological disorders associated with febrile illness in the tropics or subtropics, scrub typhus infection should be included in the differential diagnosis, despite the absence of eschar and unremarkable neuroimaging findings. This otherwise curable disease may result in multi-organ dysfunction syndrome and death if the diagnosis is delayed.
El tifus de los matorrales es una enfermedad potencialmente mortal pero curable que puede producir fallo multiorgánico. Las manifestaciones neurológicas en el tifus de los matorrales han cobrado fuerza recientemente, donde todo el neuroeje excepto la unión mioneural puede estar involucrado. Aunque no se ha establecido la patogenia de la afectación neurológica, se sospechan mecanismos inmunomediados. En este artículo se describen las características clinicopatológicas de casos de tifus de los matorrales que presentan varias manifestaciones neurológicas y neuropsiquiátricas raras.
MétodosTrescientos cincuenta y cuatro casos de tifus de los matorrales confirmados serológicamente fueron admitidos en el Departamento de Medicina General del Burdwan Medical College and Hospital, Bengala Occidental (India) entre mayo de 2018 y mayo de 2022. Hubo 50 pacientes con manifestaciones neurológicas predominantes. De estos 50, diez presentaron manifestaciones neurológicas extremadamente raras.
ResultadosPresentamos 10 casos de tifus de los matorrales (cuatro hombres y seis mujeres) que presentaron cuadros neurológicos complejos (síndrome de encefalopatía posterior reversible, síndrome de Opalski, parkinsonismo, cerebelitis, opsoclono aislado, mielitis transversa aguda, miositis, polirradiculoneuropatía con neuropatía craneal, cambios transitorios de comportamiento y fibromialgia). Los mecanismos inmunomediados mediaron la patogenia de la mayoría de los casos después del tifus de los matorrales.
ConclusiónDesde el punto de vista clinicopatológico, cada caso fue único en su presentación y respuesta al tratamiento. En cualquier trastorno neurológico de inicio agudo asociado con enfermedad febril en los trópicos o subtrópicos, la infección por tifus de los matorrales debe incluirse en el diagnóstico diferencial, a pesar de la ausencia de escaras y de neuroimágenes normales. Esta enfermedad, por lo demás curable, puede provocar un síndrome de disfunción multiorgánica y la muerte si se retrasa el diagnóstico.
Tsutsugamushi disease, better known as scrub typhus, is a potentially curable life-threatening reemerging zoonosis caused by Orientia tsutsugamushi, an obligate intracellular Gram-negative bacterium.1 In Japanese, ‘tsutsugamushi’ denotes ‘some small and dangerous creature’.2 Geographically, the region extending from eastern Russia and northern Japan towards Pakistan in the west and northern Australia in the south is known as the “tsutsugamushi triangle,” as scrub typhus is endemic in that region.1–4
Scrub typhus commonly infects farmers and outdoor laborers.2 The route of transmission for scrub typhus/tsutsugamushi disease is the bite of the larval ‘chiggers’ of the trombiculid mite, both the reservoir and the vector of the disease.1,2 Rats are the reservoir hosts on whom these larvae usually feed, and when humans contact the ‘mite islands,’ they become accidental hosts.1,2 The “eschar,” considered pathognomonic of the disease, results from the bites of these chiggers, creating a “cigarette burn”-like wound.1,2
Ranging from relatively mild non-specific febrile illness, scrub typhus can produce fatal multi-organ dysfunction syndrome resulting in significant mortality. Many immune mechanisms, including vasculitis, are thought to be underpinning the pathogenesis, although they are yet to be established.4–12 In the last decade, neurological manifestations of scrub typhus, either in isolation or part of multi-organ dysfunction syndrome, have been a topic of concern.6–9 Recent literature suggests that nervous system involvement in scrub typhus can occur in up to 20% of the patients and is often the predominant manifestation that compels the patients/caregivers to seek medical attention, apart from accompanying acute undifferentiated febrile illness.6–9 The entire neuro-axis, from the cerebral cortex to peripheral nerve and muscle, except the myoneural junction, has been affected by tsutsugamushi infection.6–9 Meningitis and meningoencephalitis are the commonest neurological manifestations; rare and difficult to diagnose illnesses like post-infective seizures (even status epilepticus), limbic encephalitis, parkinsonism, isolated cranial neuropathy, opsoclonus-myoclonus-ataxia, diaphragmatic myoclonus, acute transverse myelitis, polyradiculoneuropathy, and myositis/rhabdomyolysis remain lesser described but established clinical entities.4–12
Continuous and updated knowledge on changing trends in this infectious disease is sine-qua-non because 1) despite the potential lethality in undiagnosed and therefore untreated cases, scrub typhus remains amenable to affordable antibiotic therapy — namely doxycycline and azithromycin13; 2) the presentations are heterogeneous, non-specific and the absence of the so-called pathognomonic eschar in as many as 50% of cases, means the disease complex can easily be mistaken for some other tropical neurological syndromes and tubercular/viral or pyogenic meningitis and treated incorrectly; 3) since the advent of the current COVID-19 pandemic, surveillance systems for other infectious diseases have become knot-less for several reasons; however, monitoring for vector-borne infectious tropical diseases should be re-strengthened as these entities will remain with us (interestingly, the cases that the authors present here were admitted and treated successfully during the COVID-19 pandemic when almost any case of fever with headache was being treated as suspected COVID-19); 4) finally, point-of care testing for scrub typhus with a reasonably accurate testing method is essential but remains unavailable even at tertiary-care hospitals in eastern India, making the diagnosis either impossible or unacceptably delayed.
Although there are several case reports, case series, and reviews from several other parts of India, any such study from eastern India is unavailable. We take this opportunity to put up the real scenario of this reemerging acute undifferentiated febrile illness and present a series of 10 cases of scrub typhus, which presented with several rare neurological syndromes.
MethodsThree hundred fifty-four serologically confirmed scrub typhus cases were admitted to the Department of General Medicine of Burdwan Medical College and Hospital, Burdwan, West Bengal, India, between May 2018 and May 2022. Of these, there were 50 patients who had predominantly neurological manifestations. Of these 50, ten patients presented with extremely rare neurological manifestations.
Written informed consent was obtained from the patients participating in the study (consent for research). The data supporting the findings of this study are available within the article.
ResultsCase 1: posterior reversible encephalopathy syndrome (PRES)A 24-year-old previously healthy woman presented with fever and headache for the last seven days, for which she was taking acetaminophen without any resolution. For the last three days, she has had recurrent episodes of vomiting and vision difficulties. On admission, she was febrile with relative bradycardia; vital parameters, including serial blood pressure measurements, were within normal physiological ranges. General physical examination failed to identify any eschar or any other clue to aid in diagnosis. Neurological examinations were grossly unremarkable except for terminal neck rigidity, Brudzinski’s sign, mild photophobia, and papilledema.
Complete blood cell counts showed leukocytosis (12 900/µL) with normal platelet count and raised erythrocyte sedimentation rate (24 mm in the first hour). Liver and renal function were normal. Brain magnetic resonance imaging (MRI) revealed asymmetric bilateral T2-weighted (T2-WI) and T2-fluid-attenuated inversion recovery (FLAIR) sequences hyperintensities in the parietooccipital and frontal region with patchy diffusion restriction, suggestive of vasogenic edema (PRES) (Fig. 1). 1–70 Hz electroencephalogram was normal and cerebrospinal fluid (CSF) studies revealed lymphocytic pleocytosis (26 cells, all lymphocytes), low glucose (26 mg/dL), and raised protein (85 mg/dL). Detailed investigations for febrile illness were negative for typhoid, malaria, leptospira, dengue, and chikungunya infections. Hepatitis A, B, C, and D status were negative. However, scrub typhus was diagnosed by IgM ELISA and later confirmed by an immunochromatographic card test method. Qualitative-polymerase chain reaction (PCR) in the CSF ruled out relevant neuro infections. Hence, scrub typhus infection associated with PRES was considered. Intravenous doxycycline (200 mg/day) was administered in two divided doses, followed by doxycycline 100 mg twice a day for four weeks. Since the eighth day of antibiotic therapy, she remained afebrile and was discharged asymptomatic after ten days. A follow-up brain MRI was performed four weeks later and showed complete resolution of the brain lesions.
MRI of the brain revealing asymmetric bilateral T2-weighted and T2-fluid-attenuated inversion recovery sequences hyperintensities in the parietooccipital and frontal region with patchy diffusion restriction, suggestive of vasogenic edema (posterior reversible encephalopathy syndrome).
A 35-year-old previously healthy man presented with sudden onset right hemiparesis, reduced sensation involving the left limbs, burning sensation over the right half of the face, unsteadiness of gait, and several episodes of fall, always to his right side, for the last three days. The family members initially thought the falls were due to extreme weakness associated with febrile illness. He also had complained of fever associated with headache, body ache, and a few episodes of vomiting for the last seven days. He had also recently noticed a peculiar change in the quality of his voice (hoarseness). There was no history of craniocervical trauma, nasal regurgitation, addiction, COVID-19, or vaccination in the recent past.
He was febrile (101 °F) and had tachycardia (100 bpm). Other vital parameters were unremarkable. Cognitive functions were normal. Cranial nerve examination revealed dysesthesia over the right half of the face and right-sided lower facial weakness (i.e., right-sided upper motor neuron type of facial palsy). Examination of the motor system revealed right-sided spastic hemiparesis (mMRC grade 4/5) with brisk deep tendon reflexes and a Babinski’s response on the same side. Sensory system examination showed decreased pain and temperature perception over the left half of his trunk and limbs. He also had truncal and gait ataxia. Clinical examination also disclosed the presence of right-sided incomplete/partial Horner’s syndrome (partial ptosis and miosis).
A complete blood cell count was suggestive of neutrophilic leukocytosis (15 080/µL with 81% neutrophils), raised erythrocyte sedimentation rate (58 mm in the first hour), and thrombocytopenia (96 000/µL). Renal, hepatic, and thyroid functions were normal. Serum electrolytes, fasting lipid profile, and blood glucose profile were within normal range, and so were the chest X-ray and echocardiography. Serologies for hepatitis B, C, HIV (1, 2), malaria, typhoid, Japanese encephalitis, and dengue were negative. Serologies for scrub typhus were positive (IgM-ELISA). A brain MRI with contrast revealed an altered intensity lesion hyper on T2-WI and T2-FLAIR sequences without diffusion restriction involving the lateral aspect of the right half of the medulla (Fig. 2). Both computerized tomography and magnetic resonance angiography of the brain did not reveal any abnormalities. CSF study revealed raised protein (60 mg/dL) with lymphocytic pleocytosis (15 cells; all lymphocytes) and low glucose (35 mg/dL). CSF oligoclonal bands were negative and tested for other relevant neuroviruses, anti-aquaporin 4-antibodies, and anti-myelin oligodendrocyte glycoprotein antibodies. Scrub typhus infection was further confirmed by testing for the organism using the PCR method in CSF.
Considering the diagnosis of an infective demyelinating event, he was put on oral doxycycline (200 mg/day) for two weeks. The condition of the patient improved after five days with antibiotics and physiotherapy. Neurological examination at discharge revealed only mild impairment of sensation over the left half of his body and facial pain over the right half of his face, for which he was prescribed pregabalin (75 mg/day) at bedtime. Follow-up three months after discharge revealed persistence of mild sensory abnormalities, but the patient was able to lead a fully independent life.
Case 3: parkinsonismA 62-year-old previously healthy man was admitted to our hospital with a high-grade fever associated with chills, myalgia, headache, and decreased appetite for the last three weeks. On examination, a “cigarette burn” like eschar was found over the right shoulder. Apart from febrile illness and a mild tinge of jaundice, he had developed a new-onset symmetrical resting tremor, axial and appendicular rigidity, and severe slowness of all body movements (bradykinesia to akinesia) and hypophonic speech. His face seemed expressionless (“masked”). His family members also complained of associated behavioral changes suggestive of recent-onset obsessive symptoms. He denied having any of these parkinsonian symptoms before this febrile illness. He also denied any history of long-term intake of drugs/addictive substances that could result in parkinsonian features. There was no associated hearing impairment, seizures, altered consciousness level, or other cognitive deficits. None of his family members had any neurological disease.
Complete blood cell count revealed anemia (hemoglobin 10 g/dL), leukopenia (3700/µL) with relative lymphocytosis, thrombocytopenia (92 000/µL), and raised erythrocyte sedimentation rate (68 mm/first hour). Renal and thyroid function tests were normal. Liver function tests revealed transaminitis (AST 90 U/L and ALT 78 U/L) with mildly raised bilirubin (total bilirubin 3 mg/dL). Serum electrolytes, urinalysis, and blood glucose levels were unremarkable. Repeated serological tests for malaria, dengue, hepatitis A, B, C, E, Japanese encephalitis, leptospirosis, and typhoid fever were negative, but serology for scrub typhus (both IgM-ELISA and Weil-Felix) was positive. Contrast-enhanced MRI of the brain failed to demonstrate any abnormality. CSF study revealed raised protein (68 mg/dL) with lymphocytic pleocytosis (21 cells, all lymphocytes) with low glucose levels (23 mg/dL). Other relevant hepatic and neuro-infectious etiologies were ruled out with qualitative PCR-based methods and other appropriate modalities. He was put on oral doxycycline (200 mg/day) for four weeks. Signs and symptoms of infection resolved completely within two weeks of antibiotic therapy, but the parkinsonism and behavioral problems persisted. Hence, pramipexole (2 mg/day in divided doses), levodopa–carbidopa (500 mg/day in divided doses), sertraline (100 mg/day) and trihexyphenidyl (2 mg/day) were initiated. After three months of follow-up, there were mild parkinsonian features left without any restrictions on activities of daily living. After six months, we gradually lowered the drug doses and stopped all anti-parkinsonian drugs (except sertraline). A review after one year revealed no further relapse or neurological deterioration.
Case 4: cerebellitisA 28-year-old previously healthy woman was admitted to the emergency department with a fever of 10 days of evolution, severe headache, progressive unsteady gait, and falls over the last five days. General physical examination was only remarkable for fever, tachycardia, papilledema, neck rigidity, positive Brudzinski’s sign, and a typical eschar over inter-gluteal cleft. Cognitive function was normal. She had all signs of pan-cerebellopathy (intention tremor, scanning/ataxic speech, cerebellar nystagmus, frequent square wave jerks, hypotonia, impaired coordination, rebound phenomenon, dysdiadokinesia, and pendular knee jerks). She had both truncal and gait ataxia.
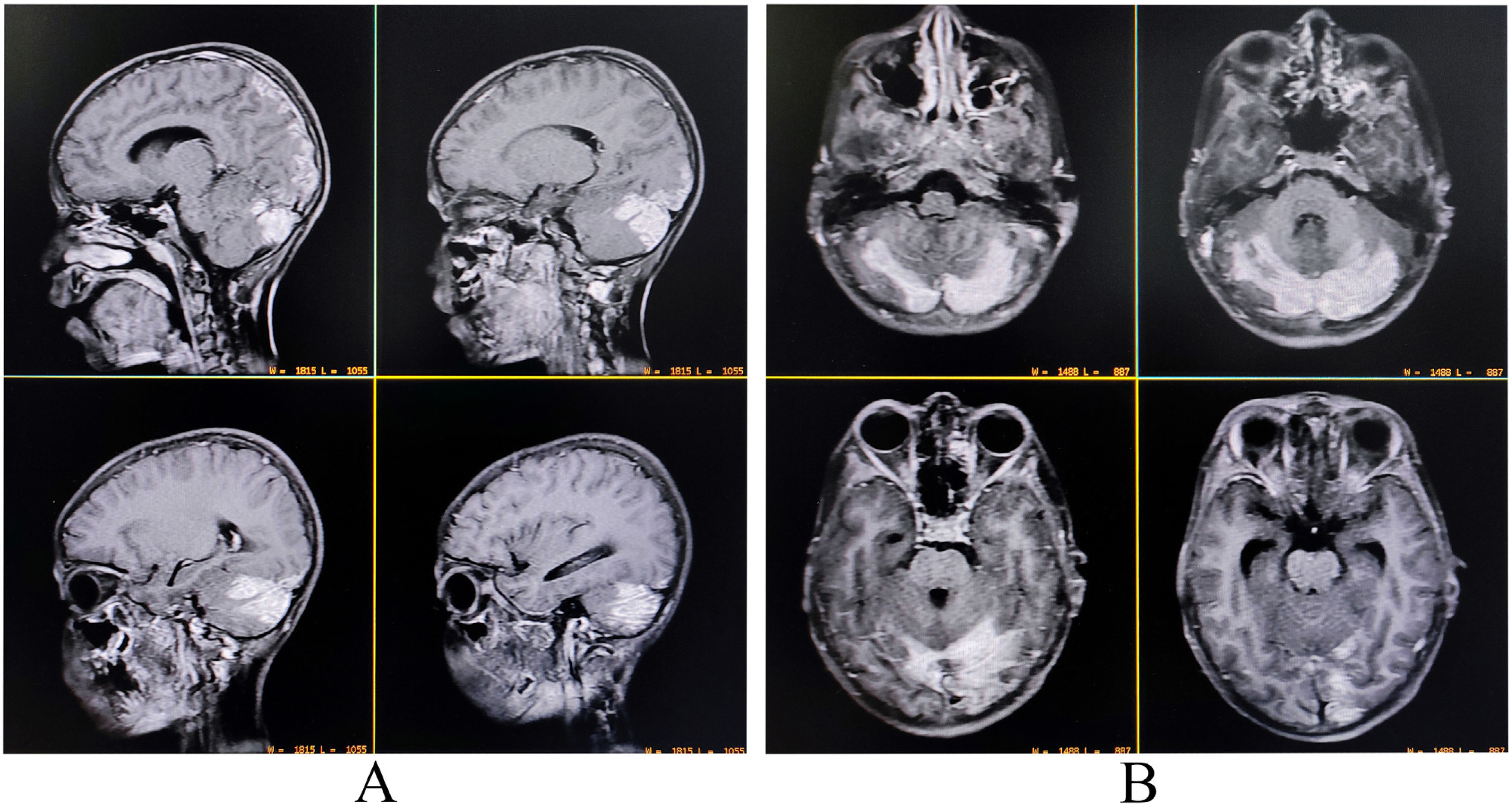
A complete blood cell count revealed neutrophilic leukocytosis (14 670/µL; 78% neutrophils), thrombocytopenia (11 100/µL), and raised erythrocyte sedimentation rate (60 mm in the first hour). Liver function tests revealed transaminitis (AST 132 U/L and ALT 121 U/L) without significant hyperbilirubinemia. Thyroid, blood glucose, kidney, and electrolytes profile were all within normal ranges. Serologies for malaria, dengue, Japanese encephalitis, typhoid fever, hepatitis A, B, C, E, and HIV (1, 2) were negative. Serology for tsutsugamushi infection (IgM-ELISA and Weil-Felix) was positive. Contrast-enhanced brain MRI demonstrated mildly asymmetrical involvement of bilateral cerebellar hemispheres and adjacent meninges with uniform contrast enhancement suggestive of infective cerebellitis (Fig. 3). CSF study revealed low sugar (30 mg/dL), high protein (80 mg/dL) and lymphocytic pleocytosis (45 cells, all lymphocytes). CSF studies were negative for other relevant neuroviruses, neurotropic organisms, and paraneoplastic cerebellitis. Other ancillary investigations like chest X-ray and echocardiography were negative.
Contrast-enhanced magnetic resonance imaging of the brain revealing mildly asymmetrical involvement of bilateral cerebellar hemispheres and adjacent meninges with uniform contrast enhancement suggestive of infective cerebellitis (A, sagittal T1-weighted imaging; B, T1-weighted imaging).
She was put on oral doxycycline (200 mg/day for four weeks) and intravenous dexamethasone (12 mg/day for 10 days). She was afebrile since the 6th day of hospital stay, and cerebellar features of cerebellopathy significantly abated after the 10th day. She could sit in bed without assistance on the 10th day and walk with assistance on the 13th day of therapy. She was discharged after four weeks, and she was able to walk without support; however, there was still subtle cerebellar dysfunction which improved on her follow-up visit after eight weeks.
Case 5: isolated opsoclonus (without myoclonus or ataxia)A 40-year-old previously healthy woman was brought with complaints of fever and headache for the last ten days, for which a local physician treated her without any improvement. She presented to us with sudden onset oscillopsia, abnormal involuntary to-and-from movements of eyeballs, and difficulties in the fixation of visual objects. General examination was unremarkable except for the fever and relative bradycardia. There was no eschar noticed despite an adequate search. Neurological examination failed to reveal any significant deficit. Neuro-ophthalmological examination revealed involuntary rapid, arrhythmic, chaotic multidirectional conjugate saccades with horizontal, vertical, and torsional components, which were continuous in fashion and present during fixation, pursuit, convergence, and even during eyelid closure and sleep suggestive of opsoclonus. Due to opsoclonus, visual acuity, color vision, fundoscopic examination, and swinging flashlight test could not be performed. There was no myoclonus or ataxia, and meningeal signs were absent. The systemic examination was otherwise normal. Complete blood cell count revealed anemia (hemoglobin 9.8 g/dL), lymphocytic leukocytosis (13 400/µL; lymphocytes 40%), mild thrombocytopenia (120 000/µL), and raised erythrocyte sedimentation rate (40 mm in the first hour). Plasma glucose, thyroid function, and kidney function tests were normal. Electrolytes were normal except for mild asymptomatic hyponatremia (130 mEq/L). Hepatic function tests revealed raised transaminases (AST 110 U/L and ALT 78 U/L) and low albumin (3.3 g/dL). Serologies for malaria, dengue, Japanese encephalitis, typhoid fever, chikungunya, hepatitis A, B, C, E, and HIV (1, 2) were negative. However, serology for tsutsugamushi infection was positive (IgM-ELISA followed by immunochromatographic card test). Contrast-enhanced brain MRI was normal. CSF testing revealed no pleocytosis (3 cells, all lymphocytes), raised protein (88 mg/dL), and low glucose (29 mg/dL) and showed normal IgG index and no oligoclonal bands. CSF qualitative PCR for relevant neuroinfectious agents was negative. Autoimmune and paraneoplastic workup was negative too. For three weeks, the patient was put on oral doxycycline (200 mg/day). Her abnormal eye movements improved after four days of antibiotic therapy. At the sixth month of follow-up, there was neither any sign/symptoms of relapse/recurrence of similar episodes.
Case 6: acute longitudinally extensive transverse myelitisA 36-year-old previously healthy man presented to the emergency department with acute onset paresthesias below mid-chest level, urinary retention, and weakness of lower limbs for the last two days. Urinary retention was relieved by Foley catheterization. He complained of intermittent lancinating/sharp-shooting pain involving his lower limbs and altered perception of hot and cold below the mid chest level. He also complained of a high-grade fever associated with headache and body ache for the last 12 days, which did not subside with some local remedies from an indigenous medical practitioner. On examination, he was febrile with relative bradycardia. Neurological examination was compatible with dorsal transverse myelopathy.
Complete blood cell count was noteworthy for anemia (hemoglobin 10.9 g/dL), neutrophilic leukocytosis (16 300/µL; 76% neutrophils), and raised erythrocyte sedimentation rate (62 mm in the first hour). Serologies for malaria, dengue, Japanese encephalitis, borreliosis, typhoid fever, hepatitis A, B, C, E, and HIV (1, 2) were negative. Contrast-enhanced MRI of the spinal cord revealed a high signal intensity lesion and swelling extending from the D4–D5 level to the distal cord region (Fig. 4). CSF examination showed high protein (97 mg/dL), normal glucose (45 mg/dL) and lymphocytic pleocytosis (19 cells; all lymphocytes). Serology for tsutsugamushi infection (IgM-ELISA and Immunofluorescence antibody) was positive. Anti-aquaporin 4-antibodies, anti-myelin oligodendrocyte glycoprotein antibodies, serologies for neuroviruses, neurotuberculosis, and neurosarcoidosis were tested negative. Based on the MRI findings and Orientia tsutsugamushi titers, acute longitudinally extensive transverse myelitis following scrub typhus was the most tentative diagnosis. Intravenous doxycycline (200 mg/day) for 14 days and high-dose pulse intravenous methylprednisolone therapy (1 g/day) for five days was administered. Following steroidal therapy, leg weakness improved gradually to an mMRC grade 4+ within the next two weeks, and urinary control improved. He was discharged after four weeks from the hospital with minimal neurological deficits. Follow-up MRI at 12 months was normal. At one-year follow-up, the patient could walk without assistance.
Case 7: polyradiculoneuropathy with cranial neuropathyA 28-year-old previously healthy woman presented to the emergency department with rapidly evolving tetraparesis and lancinating pain over the nape of the neck and lower back, radiating respectively to upper and lower limbs over the last three days. The weakness progressed quickly from distal to proximal lower limbs, followed by a similar pattern of evolving weakness involving the upper limbs and neck. She also complained of fever, body-ache, upper abdominal pain, nausea, deviation of her angle of the mouth toward the left side, grossly decreased taste sensation, and difficulties in closing her eyes, blowing, and whistling for the last ten days. There was no history of associated headaches, loss of consciousness, seizures, stiffness, changes in bladder/bowel habits, respiratory distress, joint pains, or recent travel and vaccination history.
General examination was remarkable for fever, tachycardia, abdominal right-upper quadrant tenderness, mild jaundice, and an eschar inside her umbilicus. Her cognitive functions were intact, and she had a “masked” facial expression. Cranial nerve examination revealed asymmetric (right > left) bilateral lower motor neuron–type facial weakness (House-Brackmann Grade II) with reduced taste sensation on both sides of the anterior tongue and hyperacusis (tearing was preserved) and weakness of neck flexors (mMRC grade 3+/5). Motor system examination revealed flaccid areflexic quadriparesis (muscle power estimation by mMRC showed 3/5 in proximal upper and lower limbs and 2+/5 in distal upper and lower limbs). Babinski’s response was flexor bilaterally. Sensory examination revealed reduced pinprick sensations up to both knees and decreased joint position and vibration senses. Autonomic functions were intact; cerebellar functions and gait could not be tested. Examination of bony cranium and spine were normal. There were no signs of meningeal irritation or papilledema.
A complete blood cell count revealed anemia (hemoglobin 9.8 g/dL), neutrophilic leukocytosis with relative lymphopenia (16 800/µL; neutrophils 88%; lymphocytes 10%), thrombocytopenia (105 000/µL) and raised erythrocyte sedimentation rate (78 mm in the first hour). Renal and thyroid function tests were unremarkable. Hepatic function tests revealed elevation of transaminases and bilirubin. Glycemic indices were within normal limits, and so were electrolytes, arterial blood gases, and muscle enzymes (creatine kinase, aldolase, and lactate dehydrogenase). Serologies for malaria, typhoid, and SARS-CoV-2 were negative, but scrub typhus IgM (ELISA) was positive. Contrast-enhanced MRI of the brain and spinal cord failed to reveal any abnormality. A nerve conduction study, coupled with needle electromyography, on day ten after the onset of limb weakness, revealed a sensorimotor (motor > sensory) primary axonal and secondary demyelinating poly-(radiculo)-neuropathy. CSF study revealed lymphocytic pleocytosis, with raised protein levels and low glucose levels. Anti-nuclear antibodies panel, serum angiotensin-converting enzyme, and vasculitis panel were unremarkable. Urine for porphobilinogen and total porphyrins and toxins were negative. Relevant tests for SARS-CoV-2, hepatitis A, B, C, E, HIV (1, 2), cytomegalovirus, Epstein–Barr virus, Japanese encephalitis, dengue, Campylobacter jejuni, Hemophilus influenzae, Borrelia sp., and Tropheryma whipplei were negative. Serum protein electrophoresis with immunofixation failed to reveal any paraproteinemia. An 18-FDG-PET-CT scan of the whole body could not find any neoplastic lesion.
She was put on intravenous doxycycline (200 mg/day), oral azithromycin (600 mg/day) for 14 days, and intravenous immunoglobulins. Pregabalin (150 mg/day) and nortriptyline (25 mg/day) were also prescribed to relieve radicular pain. After the seventh day of antibiotic and immune therapy, she became afebrile, and the radicular pain abated significantly. With regular physiotherapy, her deficits improved significantly, and she could walk unaided after two months of discharge from the hospital. However, it took almost six months to resolve her facial weakness. At the sixth month of follow-up, she had no demonstrable neurological deficit.
Case 8: myositisA 30-year-old previously healthy woman presented to the emergency department with a history of high-grade fever, chills, and generalized body aches for ten days. For five days, she noticed increasing pain involving her thigh and calf muscles of bilateral lower limbs, making it impossible for her to get up from a sitting position and ambulate even a step forward. For the last two days, she noticed pain and weakness involving the upper limb muscles and found it difficult to raise her arms above shoulder level and feed herself using her own hands. There was no history of severe exertion, skin disease, joint pains, oral ulcers, photosensitivity, Raynaud’s phenomena, shortness of breath, decreased urinary output, cough, jaundice, or history suggestive of urinary tract infection. On examination, vitals were unremarkable except for relative bradycardia and fever. There was an eschar seen in the right sub-mammary region. The other systemic examinations were within the normal limits. Neurological examination revealed painful muscle swelling especially involving the proximal muscles of both lower and upper limbs, and motor weakness (mMRC grade 2/5 in proximal muscles and 3+/5 in distal muscles) without the involvement of neck muscles. Deep tendon reflexes were unaltered, and Babinski’s responses were bilaterally flexor. No other clinically demonstrable neurological deficit was found.
Complete blood cell count revealed neutrophilic leukocytosis (18 765/µL; 72% neutrophils) with relative lymphopenia and thrombocytopenia (87 000/µL) and raised erythrocyte sedimentation rate. Renal, hepatic, and thyroid functions were within normal physiological limits. Serologies for malaria, dengue, Japanese encephalitis, typhoid fever, Borrelia, Chikungunya, SARS-CoV-2, hepatitis A, B, C, and E were negative. However, serology for scrub typhus was positive. Serum muscle enzymes (CPK, aldolase, LDH) and CRP were raised. Autoimmune and connective tissue profiles were negative, including myositis-specific autoantibodies. Needle electromyography revealed a myopathic pattern. She was put on intravenous doxycycline (200 mg/day) for two weeks. Fever and muscle pain abated after starting antibiotic therapy ten days after, but the weakness persisted. Hence, she was put on intravenous methylprednisolone (1 g/day) for five days, to which her muscle weakness dramatically improved, and she was discharged after four weeks of hospital stay with no neurological deficits. A follow-up three months after discharge revealed a normal examination.
Case 9: acute transient behavioral changesA 23-year-old man was brought to the general medicine department with complaints of extreme fears of contamination and excessive cleaning habits. The concerned wife complained that for the last two weeks following hospital admission for scrub typhus infection, there had been a remarkable change in personality and behavior of her husband as he was spending up to 3 hours/day “cleaning” their household and was invariably taking a bath every time he returned from outside. The patient himself complained that for the last 3–4 days, he heard voices making him do things in a way; otherwise, those voices were threatening him with some impending harm and using derogatory comments. He was also alone most of the time inside his room and was not talking to even familiar people except his family members. His wife also reported that he washed his hands about 50–100 times per day and cleaned the washroom several times after using it.
According to his family members, he never had any psychiatric symptoms previously, and neither had any of his ancestors ever had any history suggestive of harboring neuropsychiatric ailments. During the interview, he admitted that he was feeling stressed and irritable. He described lightheadedness, unsteadiness, lack of stamina, palpitations, chest discomfort, muscle pain, band-like headache, shortness of breath, nightmares, and decreased sleep and appetite. He also admitted that his behavior was not rational and was excessive. But at the same time, he was unable to control it and felt compelled to do things he was doing. He said he had tried multiple times to resist these irrational thoughts and related behaviors. Interestingly, all these symptoms started following an event of hospital admission for documented scrub typhus infection three weeks ago. He had been treated for scrub typhus meningitis with antibiotic therapy and was discharged in a hemodynamically stable condition.
A complete metabolic panel, blood cell count, hepatic, thyroid, and renal function tests were non-contributory. Contrast-enhanced MRI of the brain and CSF study failed to reveal any abnormality. Autoimmune and encephalitis profile and relevant neuroviruses panel were negative too. He was prescribed sertraline (100 mg/day), clobazam (10 mg/day), and cognitive behavioral therapy. After four months of follow-up, his symptoms abated significantly.
Case 10: fibromyalgia following tsutsugamushi diseaseA 40-year-old previously healthy woman was diagnosed and successfully treated for scrub typhus infection in May 2021. Since the advent of December 2022, she came with a new-onset perception of excessive anxiety, unexplained fear, band-like headache, generalized paresthesia especially involving both upper limbs, perception of pain to non-painful stimuli, lack of adequate balance sleep, and severe lack of concentration.
General, neurological and rheumatological examination revealed no major physical abnormalities except 18/18 tender points for fibromyalgia. Other systemic examinations were normal. The patient’s global pain assessment on the Visual Analogue Scale (VAS) scale was 100.
Complete blood cell count, thyroid, liver, kidney, electrolytes, and blood glucose profiles were within normal physiological ranges. C-reactive protein was mildly raised. Anti-CCP antibodies, rheumatoid factor, anti-nuclear antibodies panel, vasculitis profile, ferritin, HLA-B27, HLA-B5, angiotensin-converting enzyme, CPK, aldolase, and LDH levels were normal. Vitamin D3, calcium, phosphate, and parathyroid hormone levels were also within normal ranges. There were negative serologies for hepatitis B, C, HIV (1, 2), tuberculosis, chikungunya, and dengue. According to the 2010 ACR preliminary diagnostic criteria, post-scrub typhus fibromyalgia was the most tenable diagnosis. She was put on pregabalin (75 mg/day), nortriptyline (50 mg/day), and vitamin C daily. This regimen improved the overall well-being of the patient by approximately 70%–80% by the fourth month of follow-up. The VAS score had reduced significantly to 30. On clinical examination, there were fewer tender points (7/18).
DiscussionDespite the increasing number of clinical studies mentioning neurological complications of tsutsugamushi disease, remarkably little research has been dedicated to divulging the undermined mechanisms of neuroinvasion and neuroinflammation.14 Before marching on case-based discussion, we would like to summarize scrub typhus’s plausible pathogenesis.
Orientia tsutsugamushi has a tropism for endothelial cells and invades dendritic cells, monocytes, and tissue macrophages.15 The endothelial invasion causes vascular injury accompanied by perivascular mononuclear infiltration in the viscera, leading to complications.15 A mice model study revealed cerebral T-cell infiltration and vascular damage on the backdrop of endothelial dysfunction, increased proinflammatory and type 1 cytokine and chemokines, increased CXCR3 ligands, and an altered complement pathway.14 On the other hand, microglial expansion and macrophage infiltration aid in the disease progression.14 Orientia tsutsugamushi also induces endothelial expression of cytokines and chemokines, as observed in HDMEC and HUVEC cells.15 Endothelial dysfunction in the viscera, modulated by angiopoietins (Ang1 and Ang2), underlies the scrub typhus pathology.15 Moreover, monocytic infiltration caused by Orientia tsutsugamushi results in high cytokine expression and secretion, possibly leading to systemic inflammation.15
The bacterial dissemination from the periphery to the central nervous system occurs through hematogenous spread.6,16,17 Several mechanisms have been postulated behind the cellular invasion-induced damage: a) nonprofessional phagocytic binding and cell surface interaction between bacterial lectins and heparan sulfate proteoglycans16; b) activation of the nuclear factor-κB signaling pathway in macrophages and endothelium, and AP-1 in endothelial cells, inducing the expression of MIP-1α/β, MIP-2, (macrophages), IL-8, RANTES (endothelium), and MCP-1 (both)16; c) induction of apoptosis in endothelial cells16; and d) inhibition of apoptosis in monocytes (as observed THP-1 cells), actively suppressing the cytokine production by infected macrophages.16
Case-based discussionsCase 1: PRES, a potentially reversible clinical-radiological syndrome caused by a breach in the blood-brain barrier, leading to capillary leakage into the surrounding interstitium,18 is extremely uncommon in scrub typhus.19,20 During the COVID-19 pandemic, discussions on infection-associated PRES have gained some momentum.21 From existing knowledge of infection-associated PRES cases, it can be presumed that PRES in scrub typhus arises as a result of a) infection-related septic shock and dysregulation of the cerebral blood flow, or b) direct endothelial damage and vasogenic edema secondary to the infection-induced systemic inflammatory state and associated cytokinemia.18–21 The second mechanism is likely to have occurred in our case as there was no change in blood pressure levels during the hospital stay.
Case 2: the classical presentation of lateral medullary syndrome consists of crossed sensory deficits, specifically loss of pain and temperature sensation affecting trunk and extremities contralateral to the lesion and ipsilateral facial numbness.22 Other features of the syndrome include vertigo, nystagmus, hoarseness, dysphagia, ipsilateral cerebellar signs, and complete Horner’s syndrome.22 However, the clinical presentation is widely varied, depending on the sensorimotor, anatomical, and radiological involvement pattern, with classical features rarely observed.23,24 Our patient’s presentation differed from the classical description of Wallenberg in several aspects. Atypical features in our case, which questioned the diagnosis of the classical lateral medullary syndrome, were as follows: a) ipsilateral hemiparesis (Opalski syndrome); b) incomplete/partial Horner’s syndrome; and c) ipsilateral upper motor neuron type of facial palsy.
The eponym, Opalski syndrome, has been reserved only for those cases with ipsilateral hemiparesis with Babinski positivity and contralateral numbness.25 The cause of hemiparesis had been attributed to either caudal extension of the ischemic lesion to involve corticospinal fibers after the decussation or involvement of medullary penetrating arteries, which supply the post-decussation pyramidal fibers.25 These arteries are a branch of the vertebral artery, the most commonly involved artery in Wallenberg’s syndrome.26
The presence of incomplete or partial Horner’s syndrome (e.g., only partial ptosis and no miosis, anhidrosis) is due to the involvement of deeply located parts of the descending sympathetic tracts, which may be spared in a more dorsal medullary lesion leading to this differential involvement.22,27
The ipsilateral upper motor neuron type of facial palsy may be attributed to hypothetical looping supranuclear corticobulbar fibers, which descend in the contralateral ventromedial medulla and decussate at the level of the upper medulla and then ascend in the dorsolateral medulla to reach the facial nerve nucleus.27 Remarkably, though most frequently, this syndrome has been described following a vascular event, primary demyelinating events can also play the role of a culprit,28 and in this novel case, scrub typhus induced secondary demyelinating lesion over the lateral half of right medulla potentially gave rise to this syndrome.29 Clinicians should be aware of these atypical presentations of tsutsugamushi infections mimicking brainstem strokes.
Case 3: the recent COVID-19 pandemic has improved our knowledge regarding infection-associated movement disorders.30–34 Parkinsonism has been another rarely described manifestation following scrub typhus infection,35–38 and the exact pathogenesis is still unrevealed but presumably shares some of them with SARS-CoV-2 infection-related parkinsonism. Acute onset, symmetrical/asymmetrical extrapyramidal rigidity, tremulousness, expressionless face, and bradykinesia in a febrile patient with or without an eschar must instigate the clinicians in an endemic region to suspect scrub typhus-related parkinsonism. Unmasking of underlying panhypopituitarism, underlying or co-existing hypothyroidism, scrub typhus-induced bilateral upper motor neuron type of facial palsy, post-scrub psychiatric syndrome, and syndrome of inappropriate antidiuretic hormone secretion or cerebral salt wasting-related hyponatremia may mimic some features of this parkinsonian syndrome and have to be meticulously ruled out before stamping the final diagnosis. We also recommend that in appropriate clinical settings, even in the absence of definitive laboratory data, antibiotic therapy for scrub typhus should be started empirically as this can save lives.
Case 4: isolated cerebellitis as the manifestations of scrub typhus infection are rare in adults, unlike children.8,39–41 Acute cerebellitis is a potentially reversible inflammatory syndrome resulting in an acute cerebellar dysfunction following neuro-infections or systemic infections.42 It can be para/syn-infectious, post-infectious, immune-mediated, or post-vaccinal disorder.42 Acute cerebellitis is associated with a good long-term prognosis with early initiation of antimicrobial therapy, as in this case.8,39–41 Brain MRI may illustrate various patterns of cerebellar involvement in addition to bilateral diffuse hemispheric abnormalities and cortical swelling, but it can also be normal and hence, not pathognomonic.8,39–41 Although there are no consensus guidelines on treating acute cerebellitis, antimicrobial therapy should be considered.8,39–41 However, there are no consensus regarding steroids, and therefore treatment needs to be individualized.
Case 5: opsoclonus is a rare but recognized para-infectious immune-mediated neurological manifestation following tsutsugamushi infection.43 It can be associated with myoclonus and/or cerebellar ataxia, extrapyramidal syndromes, or isolation.43 Associated albuminocytological dissociation in CSF study and appearance after a few days of febrile illness might suggest a post-infective immune-modulation as the basis of this phenomenon.44 Neuroimaging is usually normal in most cases.43,44 It is imperative to recognize tsutsugamushi infection as a cause of this acute dramatic neurological condition, particularly in endemic areas with a proper clinical backdrop or with a history of acute undifferentiated febrile illness.
Case 6: only five cases with acute transverse myelitis have been reported.45–49 Longitudinally extensive transverse myelitis (LETM) is defined as a spinal cord lesion that extends over three or more vertebral segments on MRI.50–52 Affecting more than two-thirds of spinal cord thickness from the center, LETM typically involves a dramatic presentation consisting of paraparesis or quadriparesis, sensory disturbances, and bladder, bowel and/or sexual dysfunction.50–52 Although it is a characteristic of neuromyelitis optica, similar spinal lesions can also occur in various diseases involving the central nervous system, e.g., sarcoidosis, Sjögren syndrome, multiple sclerosis, neoplasms, or traumatic spinal cord injury, among others.50–52 Borrelia, Chlamydia, cytomegalovirus, mumps virus, Coxsackie virus, Mycobacterium tuberculosis, Mycoplasma, enterovirus 71, hepatitis C virus, Brucella, Ascaris, Toxocara, and Schistosoma are also known to be associated with LETM.50–52 In our patient, after excluding all the common etiologies of LETM, we were left with para-infectious LETM following tsutsugamushi infection. In the previously reported similar cases of scrub typhus-induced para-infectious LETM, the onset of symptoms ranged from four to 14 days after the onset of fever.45–49 MRI variably showed predominantly grey-matter myelitis involving cervical, dorsal and lumbar cord enhancement and swelling.45–49 All patients were managed with steroids in conjunction with doxycycline, which favors an immunological basis underlying this presentation in scrub typhus.45–49 Scrub typhus should be kept as a differential diagnosis in patients with acute undifferentiated fever and LETM in endemic areas.
Case 7: isolated or multiple cranial neuropathies have been a well-recognized neurological manifestation of scrub typhus infection.44 Pathomechanisms include immune-mediated inflammation, demyelination of individual nerves and infective cavernous sinuses, and leptomeningeal inflammation, microvasculitis of individual nerves, and raised intracranial pressure-mediated neuropathy.44 Though virtually any cranial nerve can be involved in the process, nerves responsible for extra-ocular movements, facial nerve, vestibulocochlear nerve, and optic nerve have some uncanny predilection for involvement.44 In our patient, bilateral lower motor neuron–type facial weakness was likely to be either peri-vasculitis mediated or immune-mediated, which responded to conservative medical management without immunotherapy.
Several case reports of polyradiculoneuropathy following tsutsugamushi infection have been described,53–61 including one with Miller Fisher syndrome.61 At times, there are co-infections with leptospira, malaria, and borrelia, making it difficult to attribute the etiological origin to anyone specifically.53–61 The mean duration from onset of fever to development of weakness is 3–16 days.53–61 Nerve conduction studies reveal both demyelinating and axonal patterns.53–61 All previously reported patients showed improvement to near-complete resolution of weakness and were treated with intravenous immunoglobulins.53–61 The pathogenesis of this presentation appears to be immune-mediated.53–61
Case 8: muscle involvement in scrub typhus occurs in almost half of the cases and is evidenced by weakness, myalgia, raised serum CPK, and myopathic pattern on electromyogram, and in a few cases, muscle histopathology is suggestive of vasculitis.62 The exact mechanism of muscle dysfunction in scrub typhus is not known. It may be due to an immune phenomenon or vasculitis or may arise from myotoxin and inflammatory mediators released in this infectious illness. However, all signs of muscle dysfunction, such as weakness and serum CPK, normalize after treatment with doxycycline.62 A polymyositis-like illness in a 32-year-old woman with severe muscle weakness, markedly elevated CPK, a myopathic pattern on electromyogram, and infiltration of macrophages on muscle biopsy following tsutsugamushi infection has been reported.63 Her muscle weakness and CPK levels normalized within one month following treatment with high dose steroid.63 Further studies are, however, needed to characterize the muscle dysfunction in scrub typhus and probe into its underlying mechanisms.62–65
Case 9 and 10: neuropsychiatric manifestations in scrub typhus are rare.8,16,66–69 Though the exact pathogenesis remains elusive, oxidative stress, endotheliopathy, and increased vascular permeability may be implicated.68 It has been shown in a recent nationwide analysis that patients with tsutsugamushi infection have a 1.56-fold higher risk of depression than the general population.68 Mahajan et al.66 reported a 63-year-old woman presenting with fever, diffuse headache, and body aches who suffered two episodes of visual hallucinations of acute onset, perceiving animals and dead relatives. These hallucinatory symptoms improved over the next 12 hours and were not suggestive of acute delirium. Ripley67 has also described the presence of neuropsychiatric disorders in scrub typhus, similarly to Wisseman.69 Additional studies should be conducted to clarify the cause-and-effect relationship between scrub infection and neuropsychiatric manifestations. Case 9 and 10 in this series discuss two novel presentations following scrub infection, i.e., post-infectious obsessive-compulsive disorder (OCD) and post-scrub fibromyalgia syndrome, respectively. OCD is an extremely distressful neuropsychiatric illness, categorized into primary (more common) and organic/secondary (rarer) forms. The discovery of Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal infection (PANDAS) and Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) introduced its association with secondary autoimmune triggers.70 Sometimes, patients with autoimmune brain diseases (i.e., autoimmune encephalitis and multiple sclerosis, among others) and systemic autoimmune diseases present with symptoms of OCD.70 A subset of OCD patients has “red-flag” features like sub-acute to acute onset, unusual age of onset, atypical presentation of obsessive-compulsive symptoms with associated neuropsychiatric features (e.g., excessive cognitive deficits) or accompanying neurological symptoms (e.g., movement disorders), autonomic dysfunction, treatment resistance, associations of symptoms onset with infections such as group A streptococcus, comorbid autoimmune diseases or malignancies.70 This subgroup has been classified as “autoimmune OCD” subtype.70 These patients show elevated proinflammatory cytokines and autoantibodies against targets that include the basal ganglia or dopamine receptors.70 Our case number 9 likely belongs to this newly described spectrum of autoimmune OCD following scrub typhus infection.
Fibromyalgia syndrome (case number 10), a commonly encountered disorder in the last decade, is now recognized as one of many “central” pain syndromes characterized by chronic widespread musculoskeletal pain and related symptoms and multiple painful tender points.71,72 Genetic and environmental factors may also play a role in the etiopathology of fibromyalgia syndrome and other related syndromes.71–73 Although it cannot be considered an autoimmune disease, fibromyalgia syndrome has been associated with infectious agents (viruses in particular) and vaccination.71–75 Infection has been a well-recognized risk factor for the development of fibromyalgia, as evidenced from recent studies.72,73 Multiple infectious agents have been associated with the development of either full-blown fibromyalgia (e.g., hepatitis C) or with extensively overlapping symptom complexes (e.g., chronic Lyme disease).72,73 Our patient is probably the first reported case of post-scrub typhus fibromyalgia syndrome.
ConclusionFrom a clinicopathological point of view, each case was unique in its presentation and treatment response. Immune-mediated mechanisms mediated the pathogenesis of most of our cases following scrub typhus.
In any acute onset neurological disorder associated with febrile illness in the tropics or subtropics, scrub typhus infection should be included in the differential diagnosis, despite the absence of eschar and unremarkable neuroimaging. This otherwise curable disease may result in multi-organ dysfunction syndrome and death if the diagnosis is delayed.
Author’s contributionDr. Ritwik Ghosh collaborated in 1) the conception, organization, and execution of the research project and 2) the writing of the first draft and the review and critique of the manuscript.
Dr. Arpan Mandal collaborated in 1) the conception and organization of the research project and 2) the review and critique of the manuscript.
Dr. Moises León-Ruiz collaborated in 1) the conception and organization of the research project and 2) the writing of the first draft and the review and critique of the manuscript.
Dr. Dipayan Roy collaborated in 1) the conception and organization of the research project and 2) the writing of the first draft and the review and critique of the manuscript.
Dr. Shambaditya Das collaborated in 1) the conception and organization of the research project and 2) the review and critique of the manuscript.
Dr. Souvik Dubey collaborated in 1) the conception and organization of the research project and 2) the review and critique of the manuscript.
Dr. Benito-León (jbenitol67@gmail.com) collaborated in 1) the conception, organization, and execution of the research project and 2) the writing of the first draft and the review and critique of the manuscript.