Posthypoxic leukoencephalopathy is an infrequent disease, and is especially underdiagnosed in paediatric patients. It is classified as acute or delayed, according to the time interval between the ischaemic insult and the onset of clinical symptoms. The delayed form was first described by Shillito1 in 1936 and later in 1962 by Plum et al.2 It is characterised by clinical worsening after a period of stability, between 2 and 40 days after the initial hypoxic event.3 This form of the disease exclusively affects the white matter, sparing the U fibres. To date, only 2 cases of paediatric patients have been reported, with ours being the youngest.4 The acute form, known as anoxic-ischaemic encephalopathy, occurs with no significant delay and may affect the U fibres and the grey matter.5 In both cases, it is mainly the white matter that is affected, which is contrary to expectations in an ischaemic context, since the grey matter and basal ganglia are more vulnerable to anoxia. Recent studies suggest that the white matter is more sensitive to ischaemia than previously believed.6
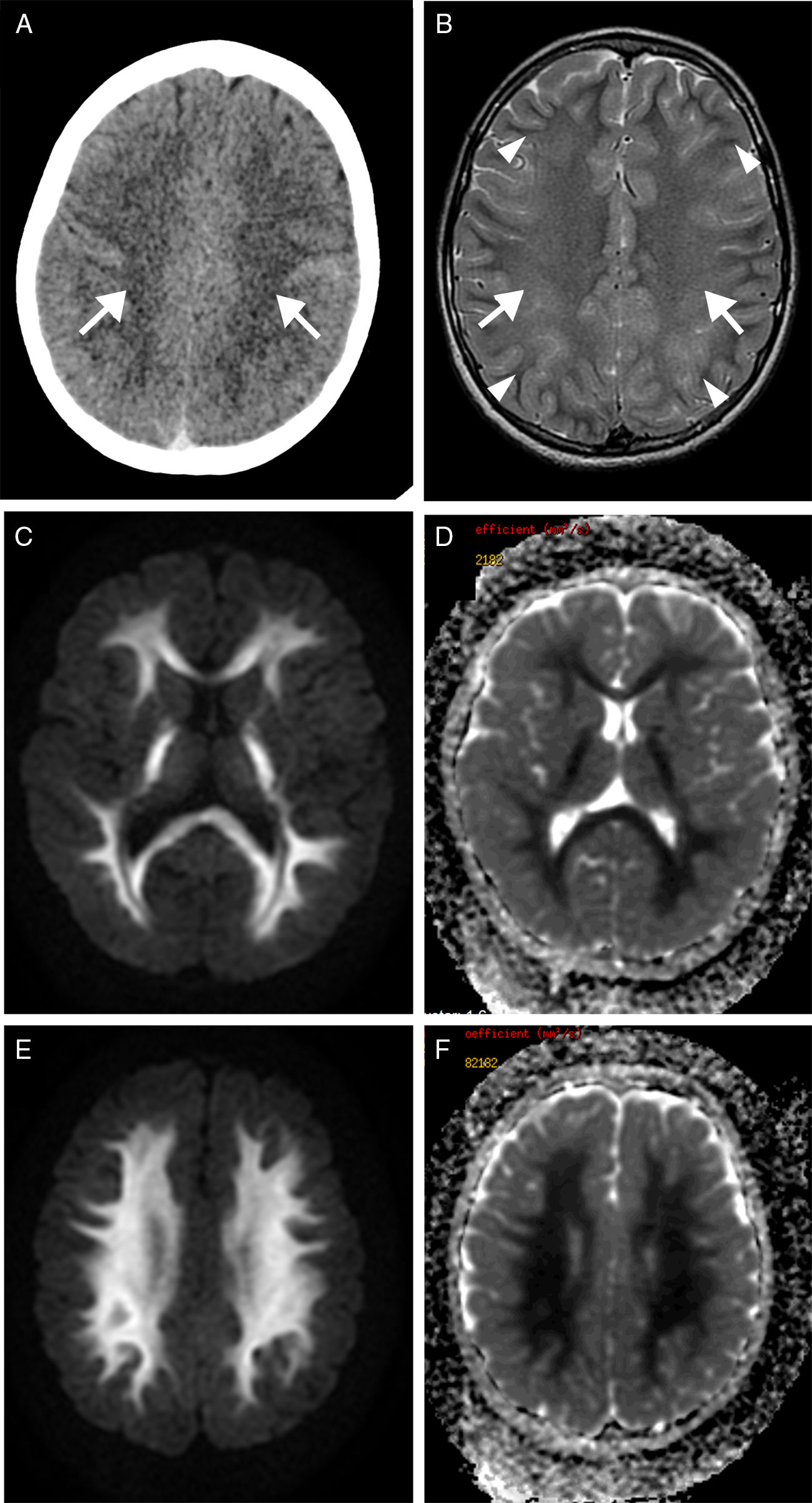
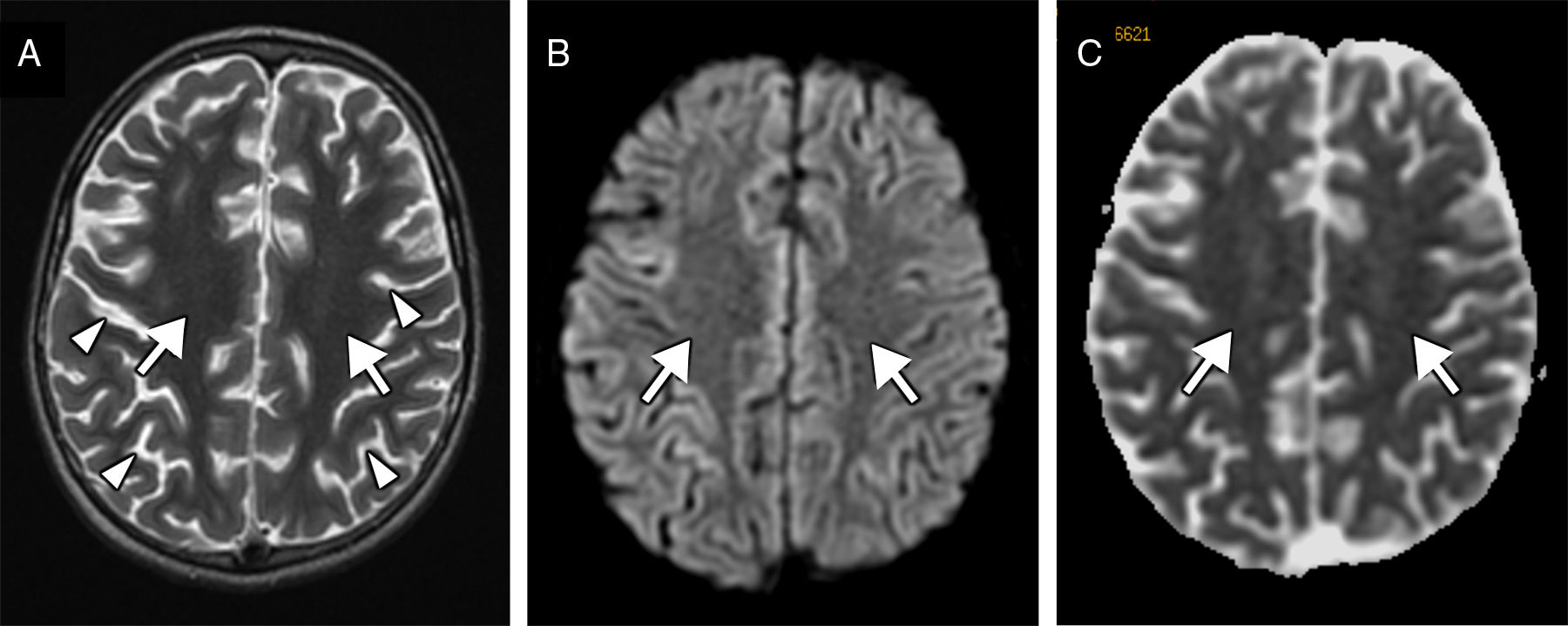
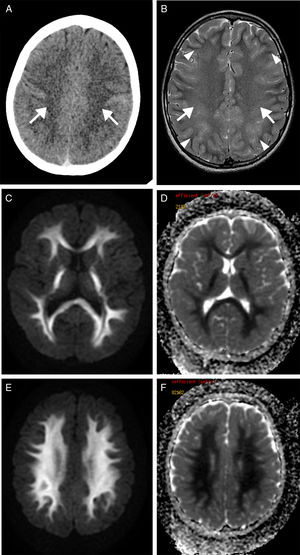
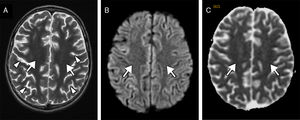
We present the case of a 9-year-old boy with a history of severe obstructive hypertrophic cardiomyopathy who was attended at the emergency department following a cardiorespiratory arrest lasting 5minutes. Seven days after admission, he presented a new episode of haemodynamic instability due to ventricular fibrillation, with recovery after 4minutes of resuscitation. Nine days after the initial event, the patient's level of consciousness decreased; a CT scan revealed a faint generalised hypodensity in the supratentorial white matter (Fig. 1A). An MRI study performed at 24hours showed altered signal intensity in the supratentorial white matter, sparing the U fibres; the hyperintensity was symmetrical, extensive, confluent, and homogeneous on the T2-weighted sequence (Fig. 1B), displaying diffusion restriction (Fig. 1C and E) and no contrast enhancement. A follow-up MRI performed at 3 weeks revealed normal signal intensity (Fig. 2A) and no diffusion restriction (Fig. 2B and C) in the white matter, with a moderately reduced volume and more prominent sulci (Fig. 2A, arrowheads).
Transverse CT sequence (A) showing a faint hypodensity in the supratentorial white matter (arrows). Transverse T2-weighted MRI sequence (B) showing homogeneous hyperintensity of the supratentorial white matter, confluent with bilateral and symmetrical distribution (arrows), sparing the U fibres (arrowheads). Diffusion restriction is observed on the diffusion sequence (C and E) and corresponding apparent diffusion coefficient maps (D and F).
Follow-up MRI scan performed at 3 weeks. The transverse T2-weighted sequence (A) reveals normalised signal intensity in the white matter (arrows) and moderate loss of volume with more prominent sulci (arrowheads). Diffusion restriction (arrows) is no longer observed on the diffusion sequence (B) and corresponding apparent diffusion coefficient maps (C).
In this clinical context, the imaging findings are characteristic of delayed posthypoxic leukoencephalopathy. On the MRI study, the white matter lesion is supratentorial, sparing the short association fibres; the cortex and basal ganglia are unaffected. Diffusion is typically restricted due to intramyelinic oedema, and the lesion presents no contrast uptake. Differential diagnosis using MRI is very narrow due to the limited number of diseases exclusively affecting the white matter with diffusion restriction; these include: toxic leukoencephalopathy (due to such drugs of abuse as heroin or such medications as vigabatrin, methotrexate, and carmofur), metabolic leukoencephalopathy (phenylketonuria and extrapontine myelinolysis), inflammatory diseases (multiple sclerosis and acute disseminated encephalomyelitis), status epilepticus, post-traumatic diffuse axonal injury, and fat embolism. The clinical symptoms and characteristic distribution pattern of the injury help establish diagnosis in most cases.
The precise pathophysiological mechanism remains unknown, although 2 hypotheses have been proposed; the first is related to the replacement of some myelin-related proteins halting after ischaemia, which would explain the delay in disease onset but not the low incidence3; the second hypothesis is related to a decrease in arylsulphatase A levels,7 although patients with normal levels have been reported.8 In terms of histopathology, the lesion consists of diffuse demyelination with axonal integrity and presence of active macrophages and astrocytes. U fibres and the cortex are typically preserved.2,7
Symptoms tend to remit spontaneously. However, the natural progression remains unknown due to the condition's low incidence and the lack of follow-up in most of the published cases.9
In the appropriate clinical scenario, exclusive involvement of the supratentorial white matter, symmetrically and bilaterally with diffusion restriction, is highly suggestive of delayed posthypoxic leukoencephalopathy. Recognising its characteristic clinical progression and imaging pattern helps in diagnosis and enables ideal management.
Please cite this article as: Mazón M, Montoya-Filardi A. Descripción de un caso de leucoencefalopatía hipóxica tardía: una imagen característica. Neurología. 2020;35:213–216.