Motor function is impaired in multiple neurological diseases associated with corticospinal tract degeneration. Motor impairment has been linked to plastic changes at both the presynaptic and postsynaptic levels. However, there is no evidence of changes in information transmission from the cortex to spinal motor neurons.
MethodsWe used kainic acid to induce stereotactic lesions to the primary motor cortex of female adult rats. Fifteen days later, we evaluated motor function with the Basso, Beattie, Bresnahan (BBB) scale and the rotarod and determined the density of thin, stubby, and mushroom spines of motor neurons from a thoracolumbar segment of the spinal cord. Spinophilin, synaptophysin, and β III tubulin expression was also measured.
ResultsPharmacological lesions resulted in poor motor performance. Spine density and the proportion of thin and stubby spines were greater. We also observed increased expression of the 3 proteins analysed.
ConclusionThe clinical symptoms of neurological damage secondary to Wallerian degeneration of the corticospinal tract are associated with spontaneous, compensatory plastic changes at the synaptic level. Based on these findings, spontaneous plasticity is a factor to consider when designing more efficient strategies in the early phase of rehabilitation.
Diversas enfermedades neuropatologías asociadas a la degeneración del tracto corticoespinal muestran deterioro de las funciones motoras. Tales alteraciones neurológicas se asocian a diversos fenómenos plásticos subsecuentes, a nivel tanto presináptico como postsináptico. Sin embargo, no existe evidencia que indique la existencia de modificaciones en la transmisión de información del tracto corticoespinal a las motoneuronas espinales.
MétodosSe indujo una lesión por vía estereotáxica en la corteza motora primaria de ratas hembra adultas con ácido kaínico y, 15 días después, se evaluó el desempeño motor mediante la escala BBB y en un dispositivo Rota-Rod. Paralelamente, se cuantificó la densidad numérica y proporcional de las espinas delgadas, en hongo y gordas, en motoneuronas de un segmento torácico-lumbar de la médula espinal. Así mismo, se registró la expresión de las proteínas espinofilina, sinaptofisina β III-tubulina.
ResultadosLa lesión farmacológica provocó un desempeño motor deficiente. Así mismo, tanto la densidad de espinas como la proporción de espinas delgadas y gordas fue mayor, al igual que la expresión de las 3 proteínas estudiadas.
ConclusiónLa aparición de los síntomas clínicos de daño neurológico provocado por la degeneración walleriana del tracto corticoespinal se acompaña de respuestas plásticas espontáneas de tipo compensador, a nivel sináptico. Lo anterior indica que durante la rehabilitación temprana de este tipo de pacientes, la plasticidad espontánea constituye un factor que se debe considerar para el diseño de estrategias de intervención más eficientes.
The organisation of voluntary movement depends on the coordinated function of different brain areas.1,2 Pyramidal cells in the 5th layer of the primary motor cortex (M1) integrate information related to voluntary movement,3 whose execution is associated with the direct connections between these cortical neurons and the spinal cord through the corticospinal pyramidal tract.1
Several movement alterations are associated with M1 lesions, including paralysis, paraesthesias,4,5 and hyperreflexia.6 The neuropathologies underlying these alterations are the main cause of disability, and clinical evidence reveals that recovery is usually limited to partial functional recovery, both sensory and motor.7
The main histopathological damage associated with paralysis or paraesthesia is wallerian degeneration, which occurs after a cortical lesion. Wallerian degeneration is characterised by axonal degeneration after a lesion distal to the neuronal soma. In cortical lesions, this type of degeneration causes loss of communication between cortical neurons and spinal cord motor neurons.8,9
There is clinical10,11 and experimental7,12 evidence of partial functional recovery after lesions to the motor cortex. The eventual incipient recovery is reported to be partially responsible for such plastic processes as cortical reorganisation, axonal regrowth of viable afferent pathways to spinal cord motor neurons, and dendritic elongation or generation in spinal cord motor neurons.13–15
Recent studies show epileptogenesis in spinal cord motor neurons, caused by an experimental spinal cord injury. However, there is no experimental evidence on plastic changes associated with synaptic activity mediated by dendritic spines in spinal cord motor neurons resulting from a degenerative lesion to the corticospinal pyramidal tract.
Material and methodsAnimalsWe used 26 female Sprague-Dawley rats (200–250g) kept under standard vivarium conditions (25°C, 12-hour light/dark cycles) with ad libitum access to water and small rodent chow.
SurgeryAnimals were assigned to one of 2 study groups. In a stereotactic procedure, the experimental group (n=13) was administered a single dose of 5nM of kainic acid diluted in 0.3μL of physiological saline solution at 2 different points of the M1 bilaterally to induce a lesion. The coordinates used were: (1) anterior-posterior to Bregma=2.7; dorso-ventral=2.4, lateral=±3.0; and (2) antero-posterior to Bregma=−1.8, dorso-ventral=1.6, lateral=±1.4.17 A second group of rats were used as controls (n=13). Using the same stereotactic coordinates as in the experimental group, we administered 0.3μL of physiological saline solution. Prior to stereotactic surgery, animals were anaesthetised with intramuscular injections of 13mg/kg of xylazine followed by 80mg/kg of ketamine.
Behavioural studyAll the animals underwent a behavioural assessment 15 days after lesion induction. For the assessment of changes in motor performance caused by the injury, we used the BBB18 functional scale for assessing locomotor performance and a balance and motor coordination test on a rotarod.
The BBB scale is scored from 0 to 21, where 0 represents complete absence of spontaneous movement and 21 indicates normal gait. The test was performed in a delimited circular area; movements of the hip, knee, and ankle were recorded, as well as the capacity to stand on the hind legs, plantar position, steps, tail position, and coordination. Rats were assessed during a single session for 4minutes. Thirty minutes after finishing assessment with the BBB functional scale, animals were assessed on the rotarod. We used 2 habituation procedures: first, for 15minutes prior to the experiment, they were kept in their cages in the space where the tests were to be conducted; secondly, each rat was placed on the stationary rotarod for 2 to 3minutes. The assessment was conducted in a single session of 3 trials with 15-minute intervals between trials. At the beginning of the first trial, rats were placed in an individual lane of the rotarod; the rotarod was started at a constant speed of 4rpm and we checked that rats were able to walk on the rotarod for approximately 5seconds. In the test, the rotarod was started at a constant acceleration, increasing from 4 to 40rpm over 5minutes. We recorded both latency to fall and the rotation speed (rpm) at which the animal fell from the rotarod. After each trial, we cleaned the rotarod surfaces with 70% ethyl alcohol.
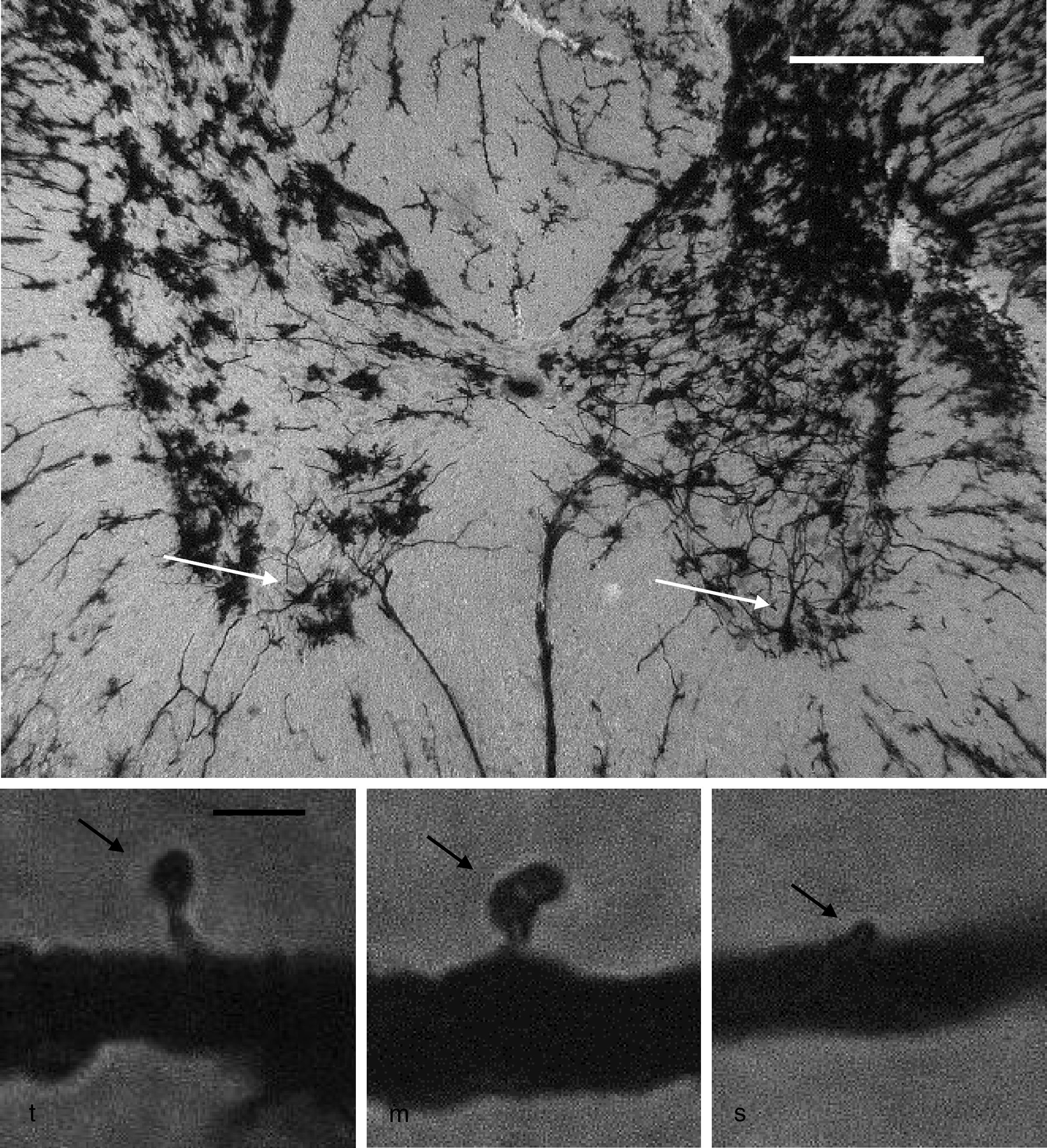
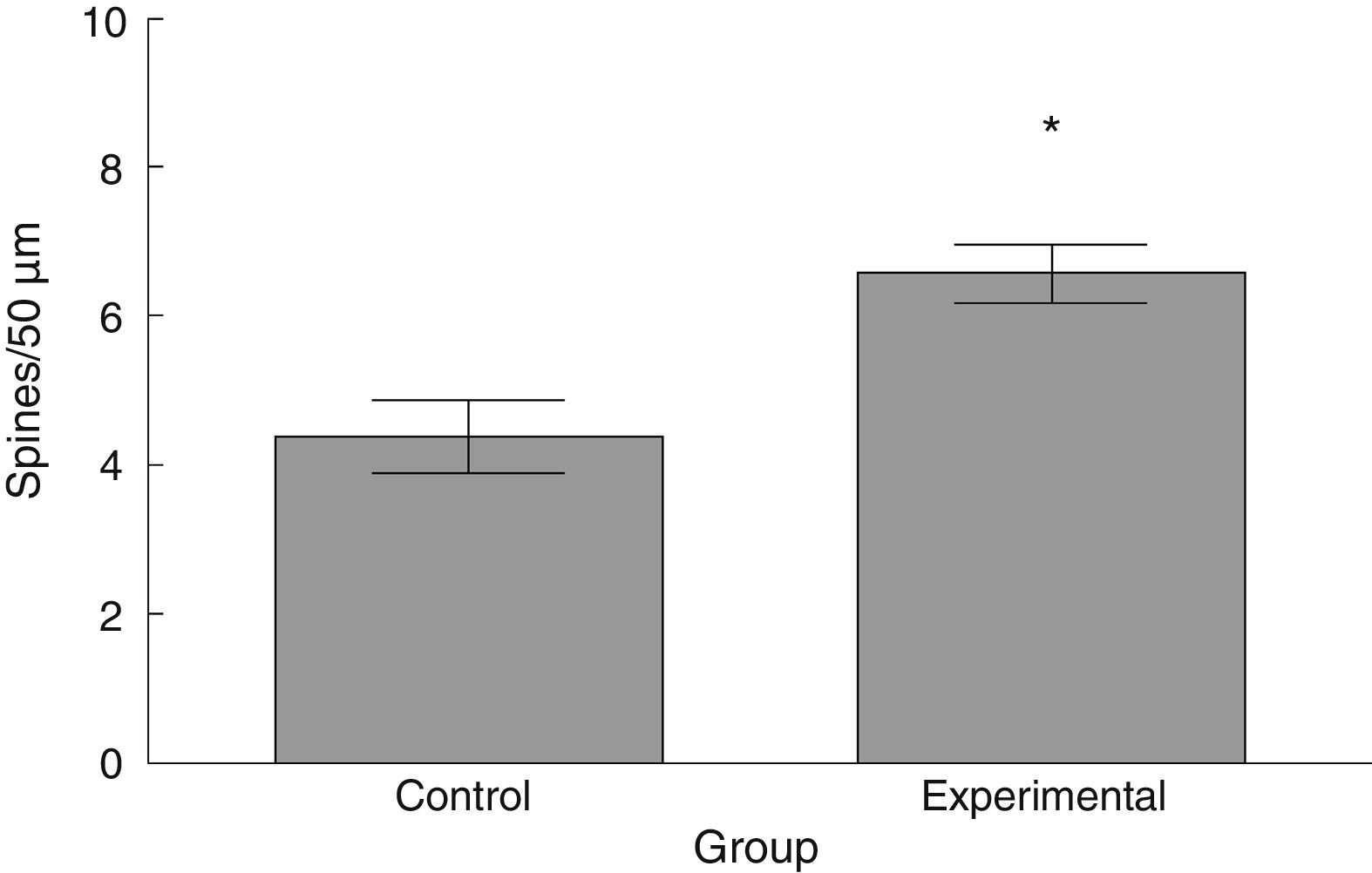
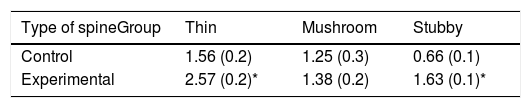
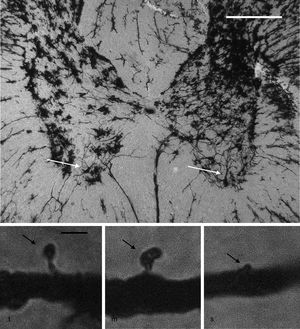
Neuronal cytoarchitectureWe randomly selected 6 animals from each group for the study of the neuronal cytoarchitecture. Animals were anaesthetised with intramuscular injection of 13mg/kg of xylazine followed by 80mg/kg of ketamine. They were immediately administered a transcardial perfusion of 200mL of phosphate buffer solution (pH 7.4; 0.1M), to which we added sodium heparin (1000IU/L) for anticoagulation and procaine hydrochloride (1g/L) for vasodilation.19 Immediately thereafter, we administered transcardial perfusion of 200mL of 4% formaldehyde in phosphate buffer solution (pH 7.4, 0.1M). We obtained 3-cm sections from between the thoracic and the lumbar regions of the spinal cord and preserved these in fresh 4% formaldehyde in phosphate buffer solution for at least 24hours. We dissected 4mm-thick tissue blocks, which were processed using the fast Golgi method20 for the neuronal cytoarchitecture study. Using 100μm thick horizontal slices, we selected 6 neurons per animal and recorded the number of dendritic spines, their density, and the proportional density of thin, mushroom, and stubby spines21 (Fig. 1).
Above: panoramic microphotograph of the dorsal and ventral horns in a thoracic section from a rat spinal cord; the tissue was stained using a modified Golgi method. Arrows indicate motor neurons in the ventral horn containing the primary dendrites where dendritic spines were counted. Scale bar: 100μm. Below: representative photomicrographs showing a typical thin spine (t), mushroom spine (m), and stubby spine (s) (arrows), which were counted in our study. Scale bar: 2μm.
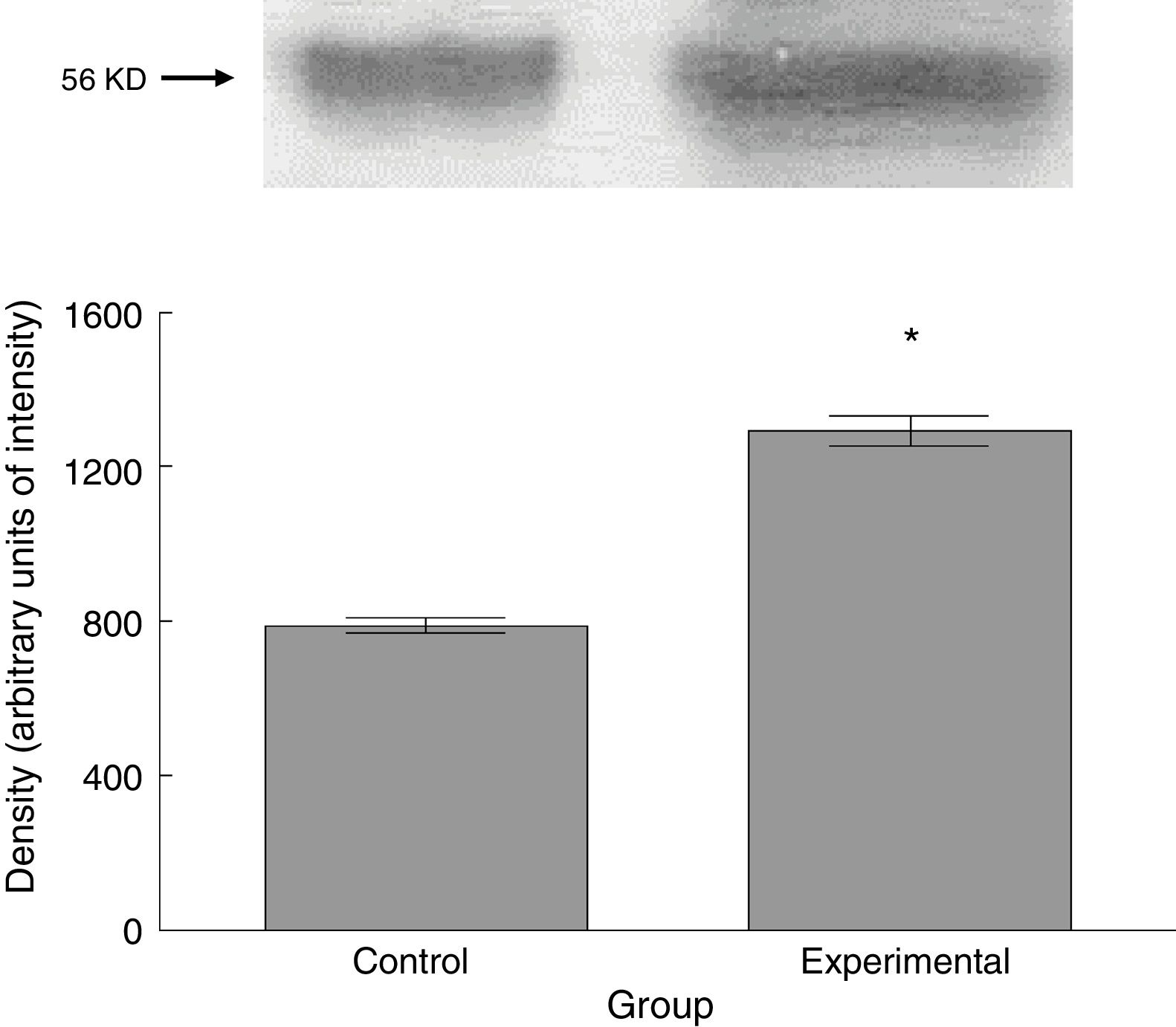
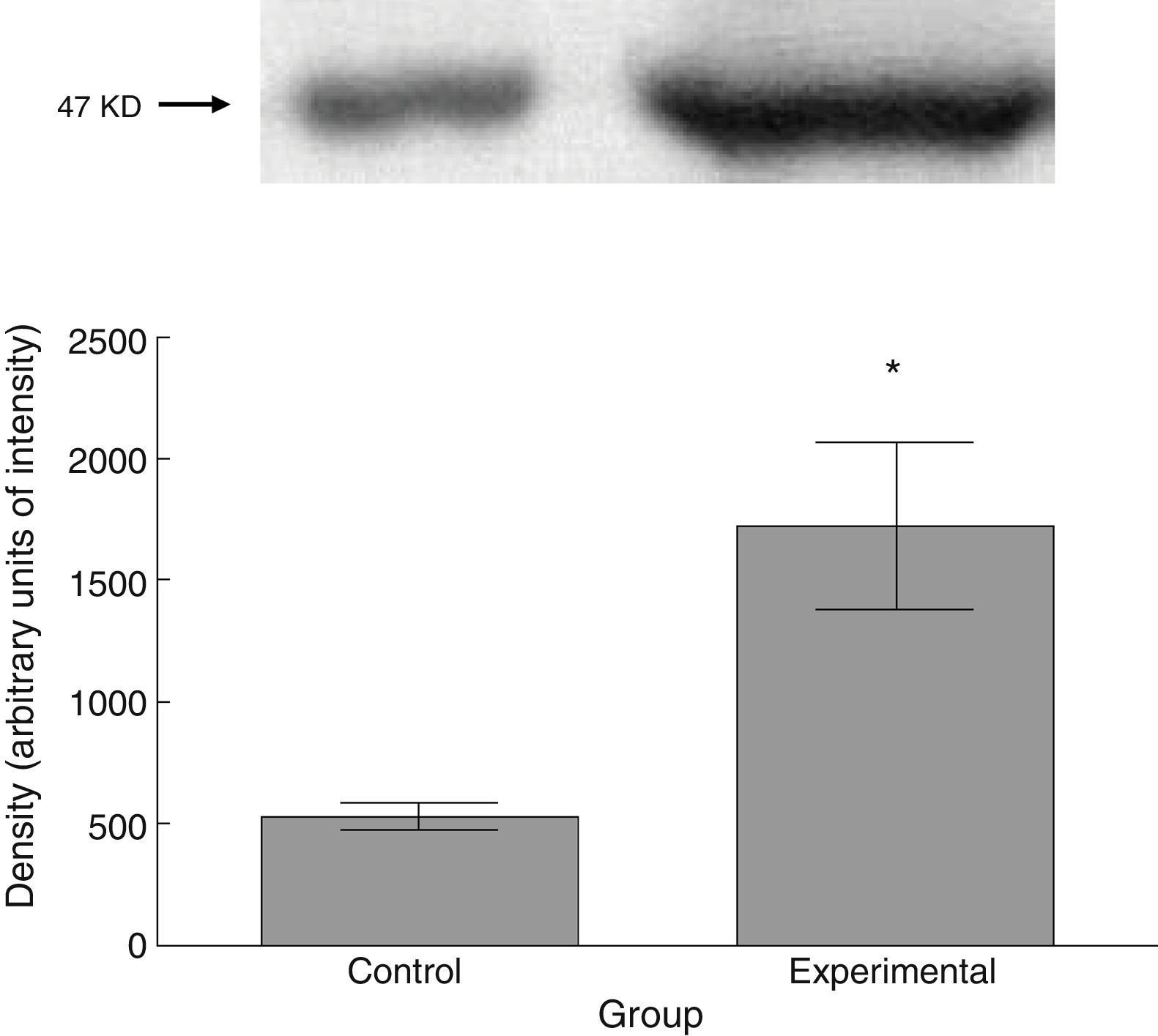
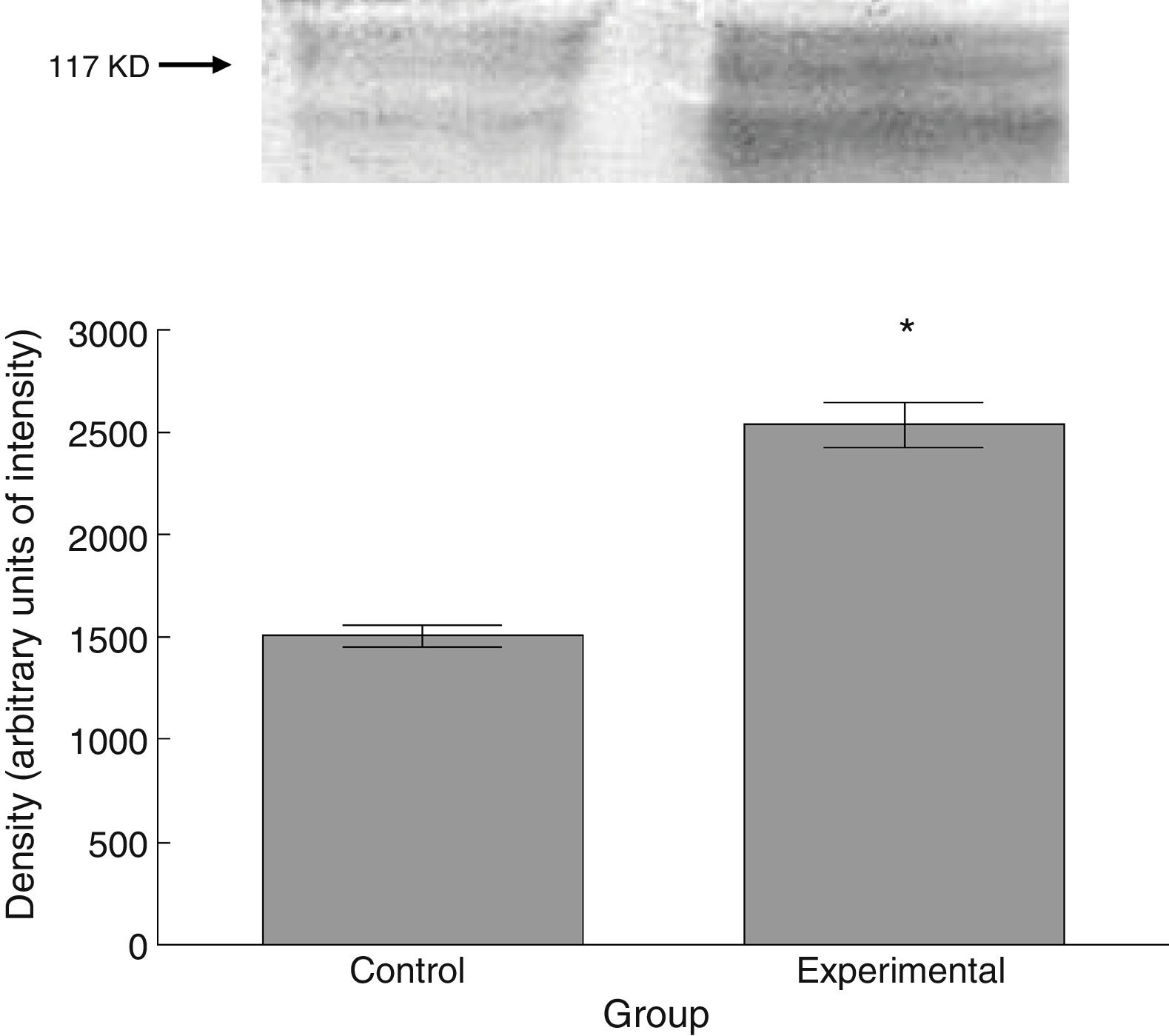
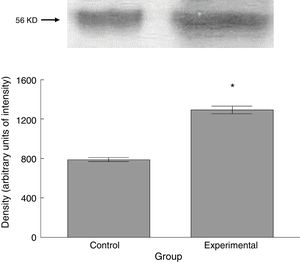
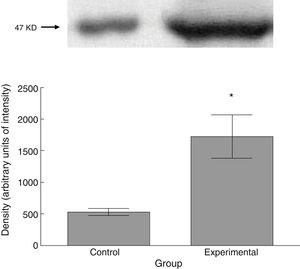
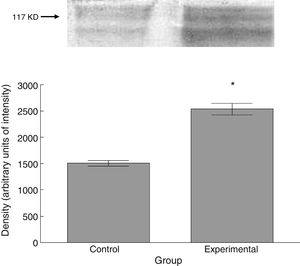
Six animals per group were euthanised by decapitation for protein quantification. We obtained 3-cm sections of thoracic and lumbar spinal cord to be processed for quantification of the proteins β III tubulin (56kD), synaptophysin (47kD), and spinophilin (117kD), using the Western blot technique. For the analysis, we used digital images of the resulting membrane from a photo documentation system; the data obtained are reported in arbitrary units of intensity.
Statistical analysisThe t test for independent samples was used to analyse the results of behavioural, morphological, and molecular studies.
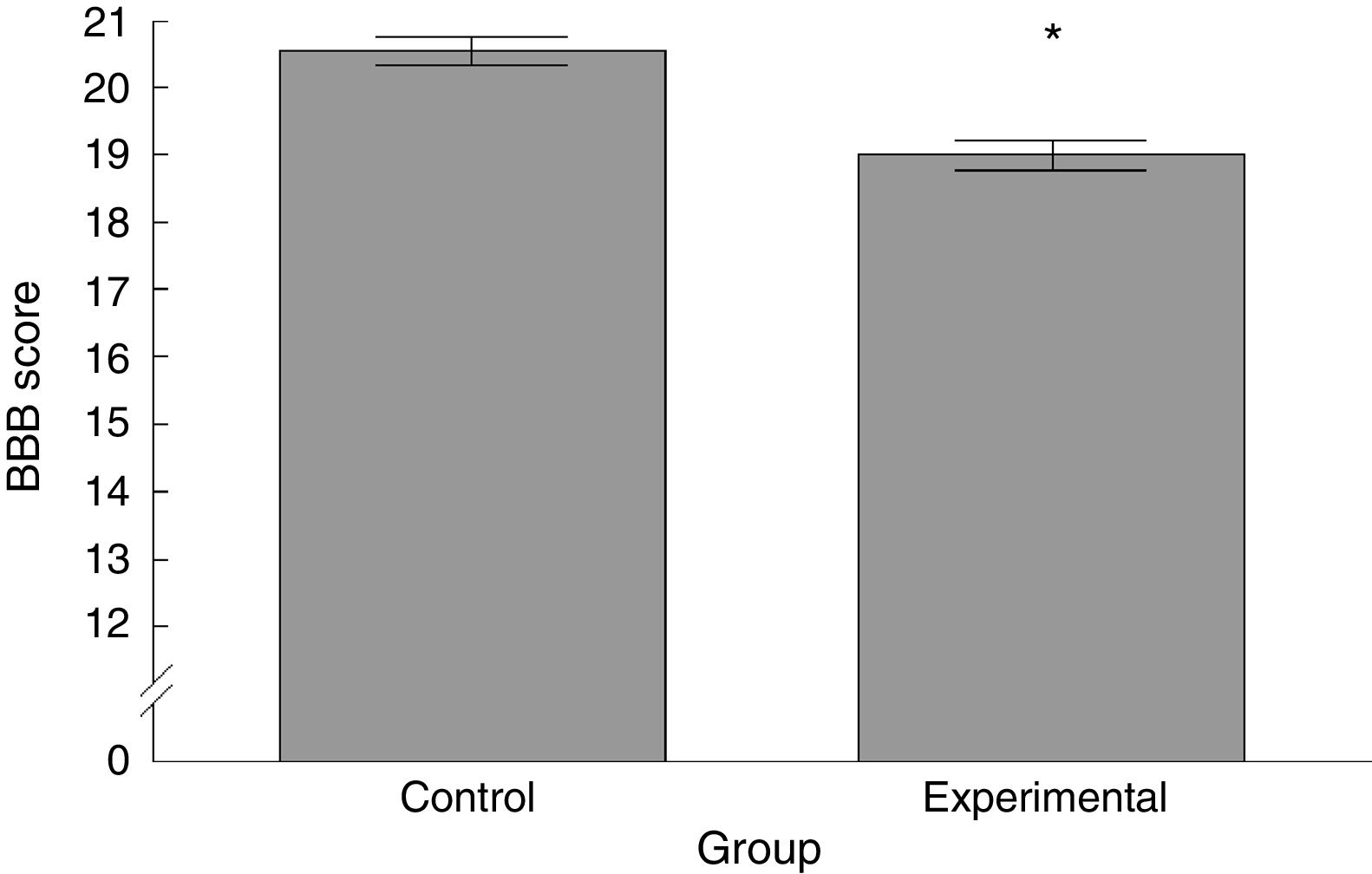
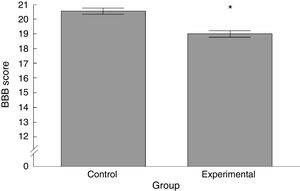
ResultsBasso, Beattie, Bresnahan functional scaleWe observed significant differences between the 2 groups analysed, with the experimental group scoring lower than the control group (t=4.924, P<.0001) (Fig. 2).
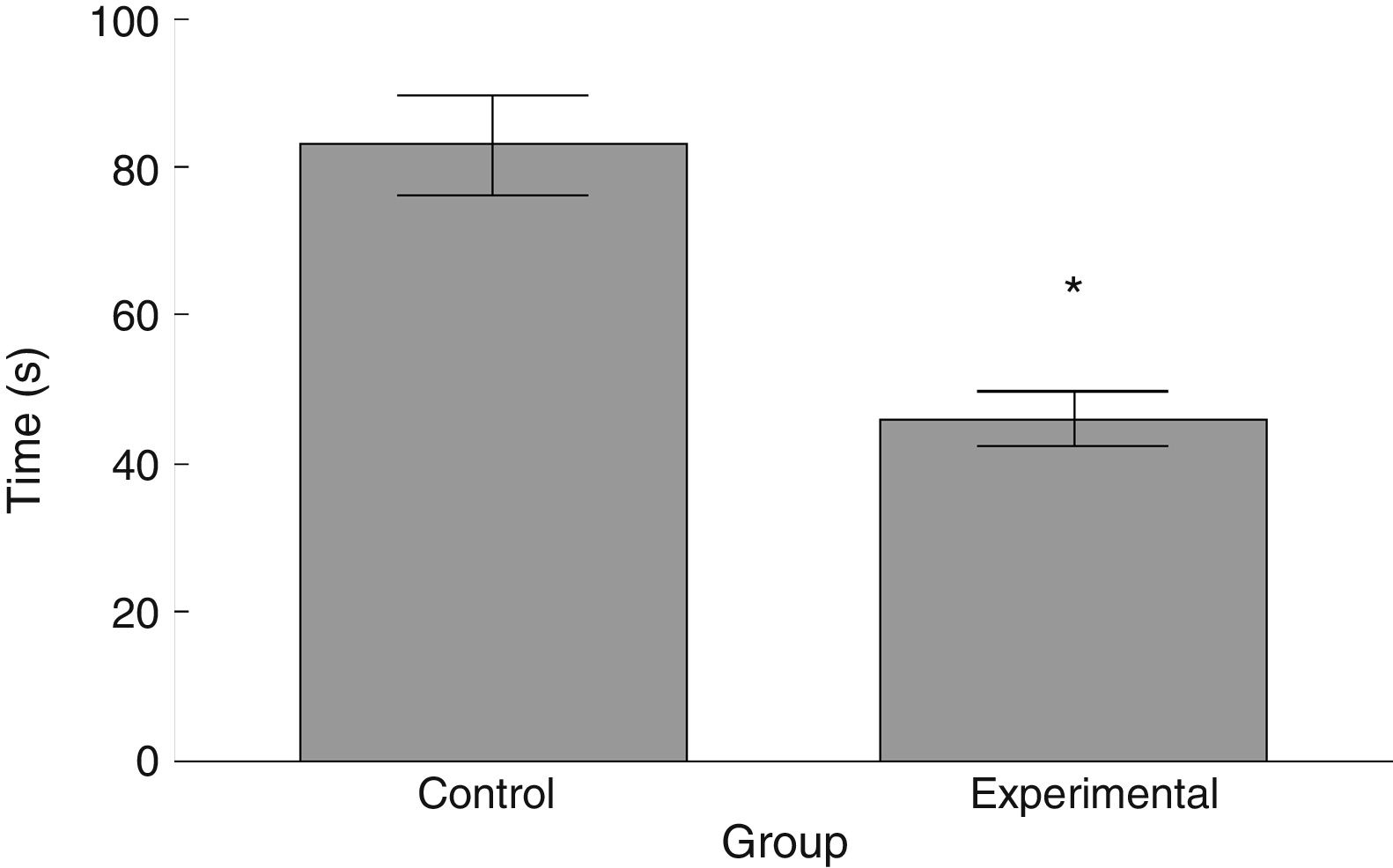
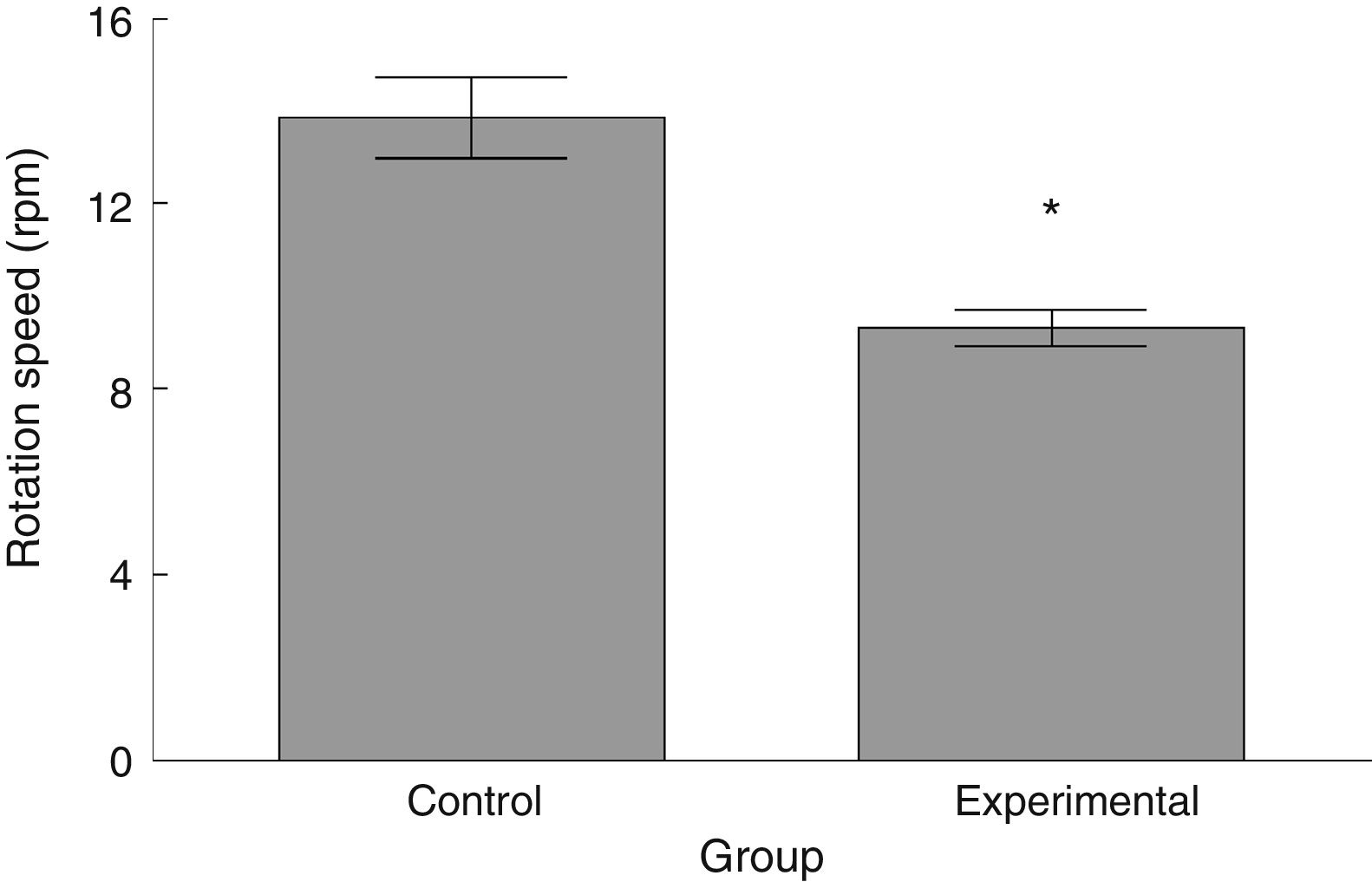
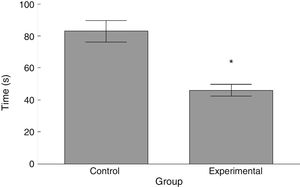
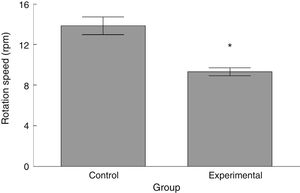
RotarodLatency to fall in the experimental group was lower than in the control group (t=4.883, P<.0001) (Fig. 3). Experimental animals fell from the rotarod at a lower rotation speed than did control animals (t=4.747, P<.0001) (Fig. 4).
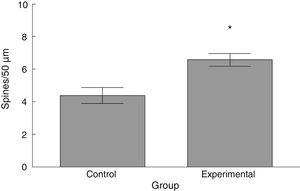
The density of dendritic spines was higher in the experimental group than in the control group (t=−3.508, P<.006) (Fig. 5). Specifically, we observed more thin (t=−2.624, P<.02) and stubby spines (t=−4.447, P<.001) in the experimental group than in the control group. We observed no significant differences between groups in the proportion of mushroom spines (Table 1).
The experimental group showed higher levels of β III tubulin (t=−11.9, P<.0001) (Fig. 6), synaptophysin (t=−3.451, P<.006) (Fig. 7), and spinophilin (t=−8.370, P<.0001) (Fig. 8).
Several demyelinating diseases of the corticospinal tract progress with neurological damage and motor impairment.4,5 Our study assessed motor function and the underlying plasticity of thoracic/lumbar spinal cord motor neurons after experimental M1 injury.
The functional neurological assessment using the BBB scale and the rotarod revealed impaired motor performance. This was observed concomitantly with an increased numerical density of dendritic spines, specifically thin and stubby spines.
The increased number of dendritic spines observed in the spinal cord motor neurons following M1 injury may be interpreted as a compensatory plastic response that, in the case of functional spines,22–24 would represent an increase in the associative capacity of afferent synaptic inputs25 and eventually promote functional recovery.26
Various studies using different experimental models of motor injury,27–30 including spinal cord lesions,16 have shown that not only is the numerical density of dendritic spines important as a plastic response to an altered synaptic microenvironment, but also that variations in the proportional density of the different types of spines also represent critical plastic events.
Thin spines have classically been associated with the acquisition of new information31,32 due to their characteristic fast processing of afferent synaptic inputs.33,34 Although the synaptic information processed in the spinal cord is not related to learning, the underlying electrophysiological phenomena share common mechanisms.28 In this study, such bioelectrical phenomena constitute a spontaneous plastic response to help acquire the decreased chemical information from those nervous fibres that: (a) remain viable after the lesion; (b) result from subsequent axonal sprouting; or (c) are the result of both events. In fact, previous studies report an increase in β III tubulin levels, as observed in our study, after an injury; this would reflect the sprouting of new axon terminals.35 In any case, the increased density of thin spines may be interpreted as a spontaneous plastic response to experimentally induced Wallerian degeneration, tending to compensate for the reduced transmission of afferent inputs to the spinal cord motor neurons.
As with thin spines, the number of stubby spines also increased after the experimental lesion. Stubby spines lack a neck, which confers them the functional characteristic of providing little resistance to calcium-mediated current36; according to circumstantial evidence,21,32,37,38 the functional activity of this type of spines would consist in regulating postsynaptic neuronal excitability. Thus, the proportional increase in stubby spines suggests, on the one hand, that the afferent excitatory activity to spinal motor neurons may have increased after the lesion to the corticospinal tract, and on the other hand, that there is a plastic response that tends to regulate the bioelectrical homeostasis of motor neurons. This proposal is supported by the greater expression of synaptophysin, a marker of the release of the neurotransmitter into the intersynaptic space,39 in tissues from animals in the experimental group. Furthermore, increased synaptophysin levels would correlate with an increase in spinophilin expression, which would indicate the presence of a greater number of dendritic spines,40 as we observed in our study.
The study did not find changes in the proportional density of mushroom spines. Synaptic transmission mediated by this type of spines is slower than in any other type of spines,31 since they are potentiated by afferent stimulation.41 Based on the above, functional activity of this type of spines has been linked to long-term information storage.31
The fact that mushroom spines did not undergo any changes suggests that plastic processes in the dendritic spines of spinal motor neurons do not tend to consolidate the afferent synaptic inputs, which would maintain the latent capacity to make dynamic adjustments to synaptic information, represented by the predominance of thin spines. In that case, in a bioelectrical microenvironment regulated by the high density of stubby spines, this could enable more effective patterns of motor activity to be established in early rehabilitation treatment of patients with this type of motor neuron alterations.
FundingThis study was financed by the Health Research Fund of the Mexican Institute of Health, with registry number FIS/IMSS/PROT/G11-2/1028.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martínez-Torres NI, González-Tapia D, Flores-Soto M, Vázquez-Hernández N, Salgado-Ceballos H, González-Burgos I. Espinogénesis en motoneuronas de la médula espinal tras la lesión farmacológica de la corteza motora de ratas. Neurología. 2021;36:119–126.