Although transcutaneous electrical nerve stimulation (TENS) has traditionally been used to treat pain, some studies have observed decreased spasticity after use of this technique. However, its use in clinical practice is still limited. Our purpose was twofold: to determine whether TENS is effective for treating spasticity or associated symptoms in patients with neurological involvement, and to determine which stimulation parameters exert the greatest effect on variables associated with spasticity.
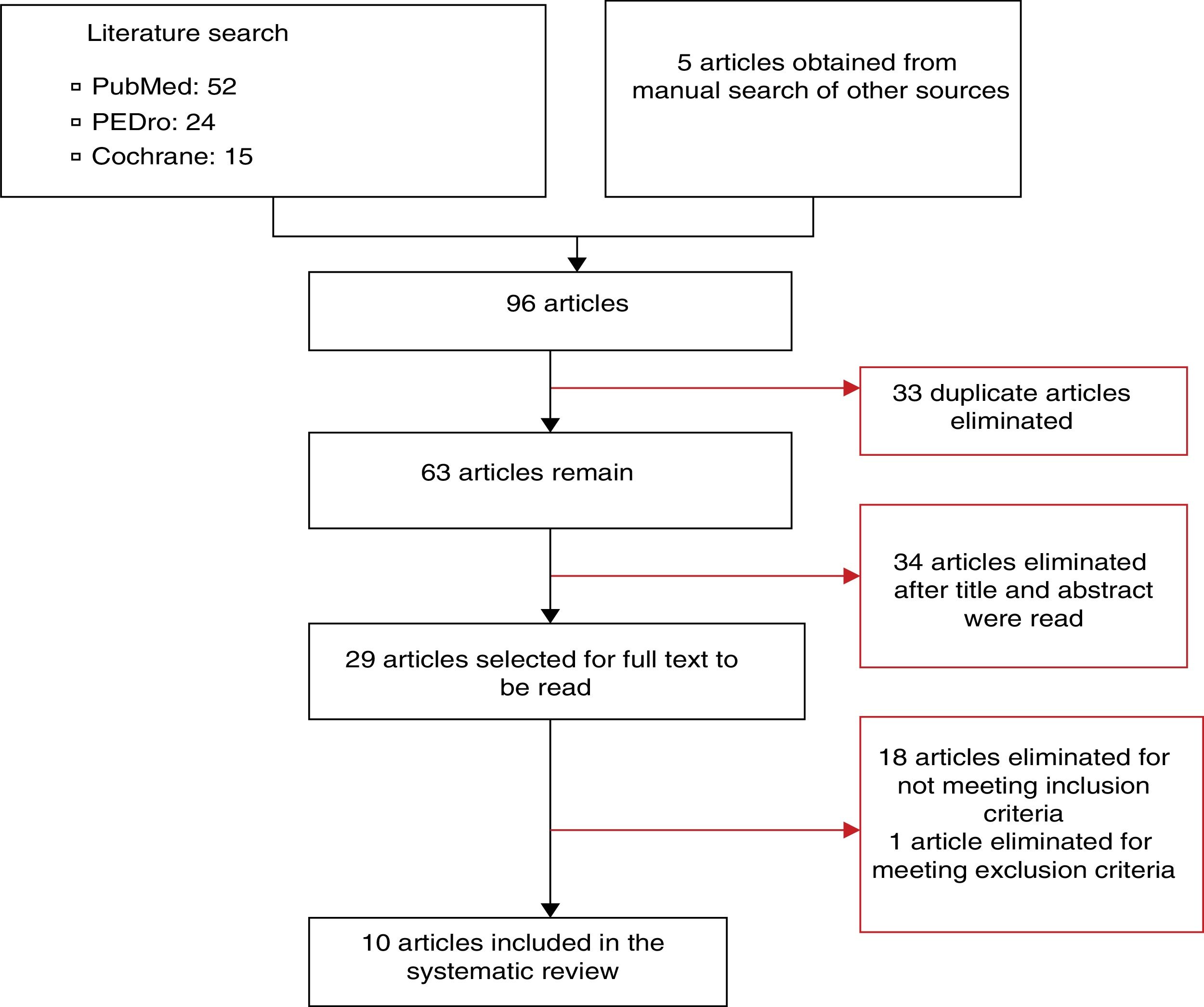
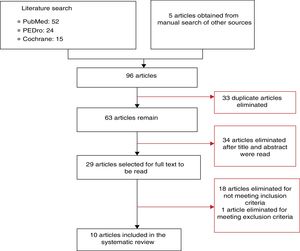
DevelopmentTwo independent reviewers used PubMed, PEDro, and Cochrane databases to search for randomised clinical trials addressing TENS and spasticity published before 12 May 2015, and selected the articles that met the inclusion criteria. Of the initial 96 articles, 86 were excluded. The remaining 10 articles present results from 207 patients with a cerebrovascular accident, 84 with multiple sclerosis, and 39 with spinal cord lesions.
ConclusionIn light of our results, we recommend TENS as a treatment for spasticity due to its low cost, ease of use, and absence of adverse reactions. However, the great variability in the types of stimulation used in the studies, and the differences in parameters and variables, make it difficult to assess and compare any results that might objectively determine the effectiveness of this technique and show how to optimise parameters.
Aunque tradicionalmente la estimulación eléctrica nerviosa transcutánea (TENS) se ha utilizado como tratamiento del dolor, algunos estudios han evidenciado una reducción de la espasticidad tras la aplicación de TENS. Sin embargo, su uso en la clínica aún está muy poco extendido. El objetivo de este estudio consiste en determinar si la estimulación TENS aplicada en pacientes con afectación neurológica resulta efectiva para tratar la espasticidad o alguno de sus síntomas asociados. Además, se pretende determinar los parámetros de estimulación que mayor efecto producen sobre las diferentes variables asociadas a la espasticidad.
DesarrolloSe buscaron ensayos clínicos aleatorizados relacionados con TENS y espasticidad encontrados en las bases de datos PubMed, PEDro y Cochrane con anterioridad al 12 de mayo de 2015. Dos revisores independientes realizaron las búsquedas y seleccionaron los estudios en función de los criterios de inclusión previamente establecidos. En la búsqueda inicial se encontraron un total de 96 artículos, de los cuales 86 fueron excluidos y 10 fueron seleccionados para analizar en esta revisión. Se presentan resultados en 207 sujetos con accidente cerebrovascular, 84 con esclerosis múltiple y 39 con lesión medular.
ConclusionesDebido a los resultados observados, su bajo coste, facilidad de aplicación y ausencia de efectos adversos, se recomienda la estimulación mediante TENS como tratamiento de la espasticidad. Sin embargo, la gran variabilidad existente entre las formas de estimulación, parámetros utilizados y variables analizadas, dificultan el análisis y la comparación de resultados que puedan determinar la eficacia objetiva de la técnica y la optimización de parámetros.
Spasticity is a sensory-motor disorder traditionally defined as “a motor disorder characterised by a velocity-dependent increase in the tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflexes.”1 Spasticity affects nearly 85% of patients with multiple sclerosis,2 around 35% of patients with chronic hemiparesis,3 and 65%-78% of patients with spinal cord injury.4 In Spain, spasticity is estimated to affect 300000 to 400000 people.5
Physiotherapy is the treatment of choice for spasticity, and is even used for prophylaxis. However, physiotherapy techniques are frequently ineffective or insufficient, and spasticity eventually interferes with patients’ quality of life. At this point, pharmacological treatment is started, with baclofen being the most frequently administered drug. Surgery is recommended for severe spasticity when physiotherapy and pharmacological treatment fail to control symptoms.6
Transcutaneous electrical nerve stimulation (TENS) is a treatment that applies high-frequency (50-150Hz) and low-frequency (below the motor threshold) electric currents onto the skin; this technique has traditionally been used for pain relief.7 The technique has been reported to have other effects, such as decreased amplitude of the H-reflex of the soleus muscle in healthy volunteers8 and even in neurologically impaired individuals. TENS has also been found to improve such clinical variables as balance, proprioception, and spasticity in patients with brain damage.9–11 However, this treatment is not widely used among neurological patients with spasticity.
Application of TENS requires physicians to consider numerous parameters, including electrode positioning, waveform, pulse frequency and width, intensity of the current, and session duration and frequency. Optimising these parameters is essential to achieving the desired effects. However, the literature reports great variability in the parameters used. This is one of the main reasons for the controversy over the effectiveness of TENS in patients with spasticity. Considering the limited effectiveness and the adverse effects of other treatments, TENS has numerous advantages: the devices are inexpensive, portable, and easy to use (even for patients themselves), and the treatment causes no adverse reactions. In the light of these qualities, TENS may be a valid treatment option for spasticity. This underscores the need for a comprehensive analysis of the available evidence on TENS for treating spasticity, including forms of application and the optimal parameters for greatest effectiveness.
The aim of this systematic review is to determine whether TENS is more effective than sham or alternative treatments for spasticity or any of its associated symptoms (spasms, clonus, etc.) when applied to patients with neurological disorders. As a secondary objective, we sought to determine the stimulation parameters with the greatest impact on different variables of spasticity.
MethodsSearch strategyAlthough we used a standardised search protocol and followed the principles of the PRISMA statement,12 our article search and selection protocol were not registered. Two independent reviewers performed an online search on PubMed, CENTRAL, and PEDro. They also manually reviewed the references cited in the articles found with the protocol described above. The following keywords were used: transcutaneous electrical nerve stimulation, TENS, transcutaneous electric nerve stimulation, spasticity muscle, spastic, and spasticity. We also used the following MeSH terms for our literature search on PubMed and CENTRAL: transcutaneous electric nerve stimulation, and spasticity muscle. We searched for articles published up to 12 May 2015.
Study selectionOur study included randomised controlled trials published in English or Spanish and including patients with neurological disorders and spasticity; trials had to include at least one intervention group receiving TENS with surface electrodes, regardless of the area of application and the stimulation parameters used. Current intensity had to be low enough not to cause muscle contraction. Studies had to include variables quantifying spasticity or any of its associated symptoms (Ashworth Scale, H-reflex test, Penn Spasm Frequency Scale, clonus, Resistance To Passive Movement [REPAS] scale, etc.). Studies had to include a group receiving either sham stimulation or an alternative treatment for spasticity. We excluded all articles not applying TENS alone to any of the study groups, as studies of combined treatments do not allow us to assess the effects of TENS specifically. We also excluded the articles not specifying the pulse frequency, width, or intensity used.
The reviewers first read the titles of all the studies to exclude those articles not fitting the purpose of our study. They then read the abstracts of the selected articles and ruled out those studies not meeting the inclusion criteria. Full texts were read for all articles which met or potentially met the inclusion criteria. We used the PEDro scale, which has been shown to have good reliability,13 to evaluate the methodological quality of the studies.
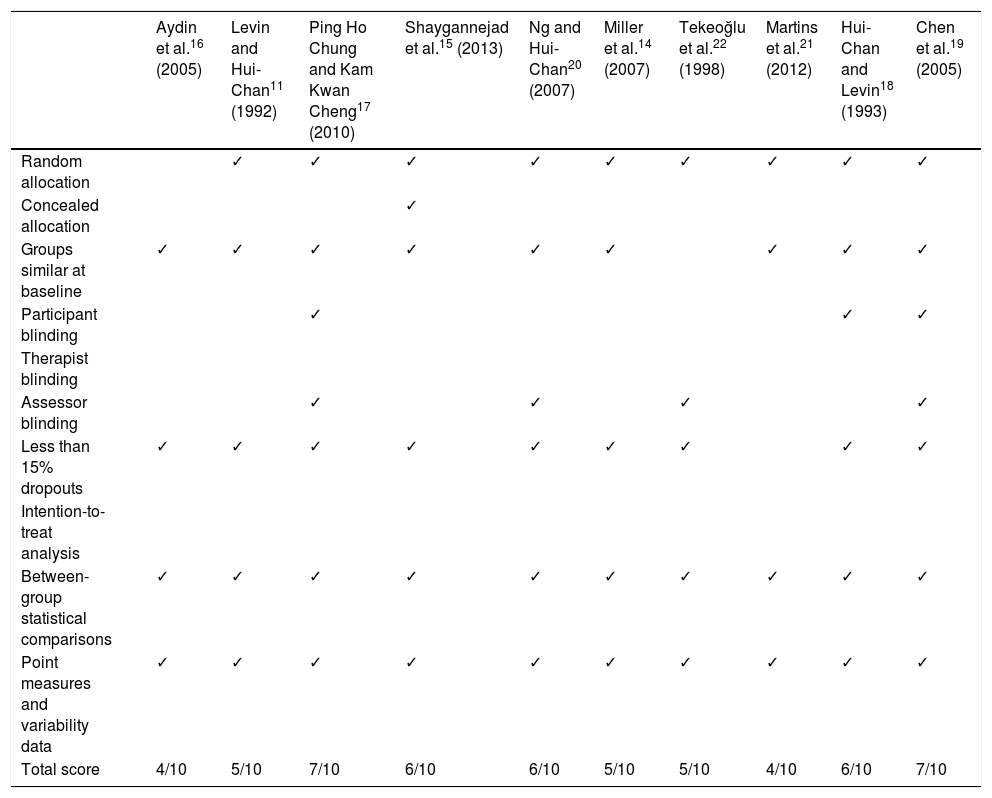
Synthesis of resultsThe article selection process is illustrated in Fig. 1. The initial literature search yielded 91 articles; 10 of these were finally included in our systematic review. The methodological quality of the selected articles is shown in Table 1. The most relevant characteristics of the articles are shown in Table 2.
Methodological quality of the articles included in the systematic review, according to the PEDro scale.
| Aydin et al.16 (2005) | Levin and Hui-Chan11 (1992) | Ping Ho Chung and Kam Kwan Cheng17 (2010) | Shaygannejad et al.15 (2013) | Ng and Hui-Chan20 (2007) | Miller et al.14 (2007) | Tekeoğlu et al.22 (1998) | Martins et al.21 (2012) | Hui-Chan and Levin18 (1993) | Chen et al.19 (2005) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Random allocation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Concealed allocation | ✓ | |||||||||
| Groups similar at baseline | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Participant blinding | ✓ | ✓ | ✓ | |||||||
| Therapist blinding | ||||||||||
| Assessor blinding | ✓ | ✓ | ✓ | ✓ | ||||||
| Less than 15% dropouts | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Intention-to-treat analysis | ||||||||||
| Between-group statistical comparisons | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Point measures and variability data | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Total score | 4/10 | 5/10 | 7/10 | 6/10 | 6/10 | 5/10 | 5/10 | 4/10 | 6/10 | 7/10 |
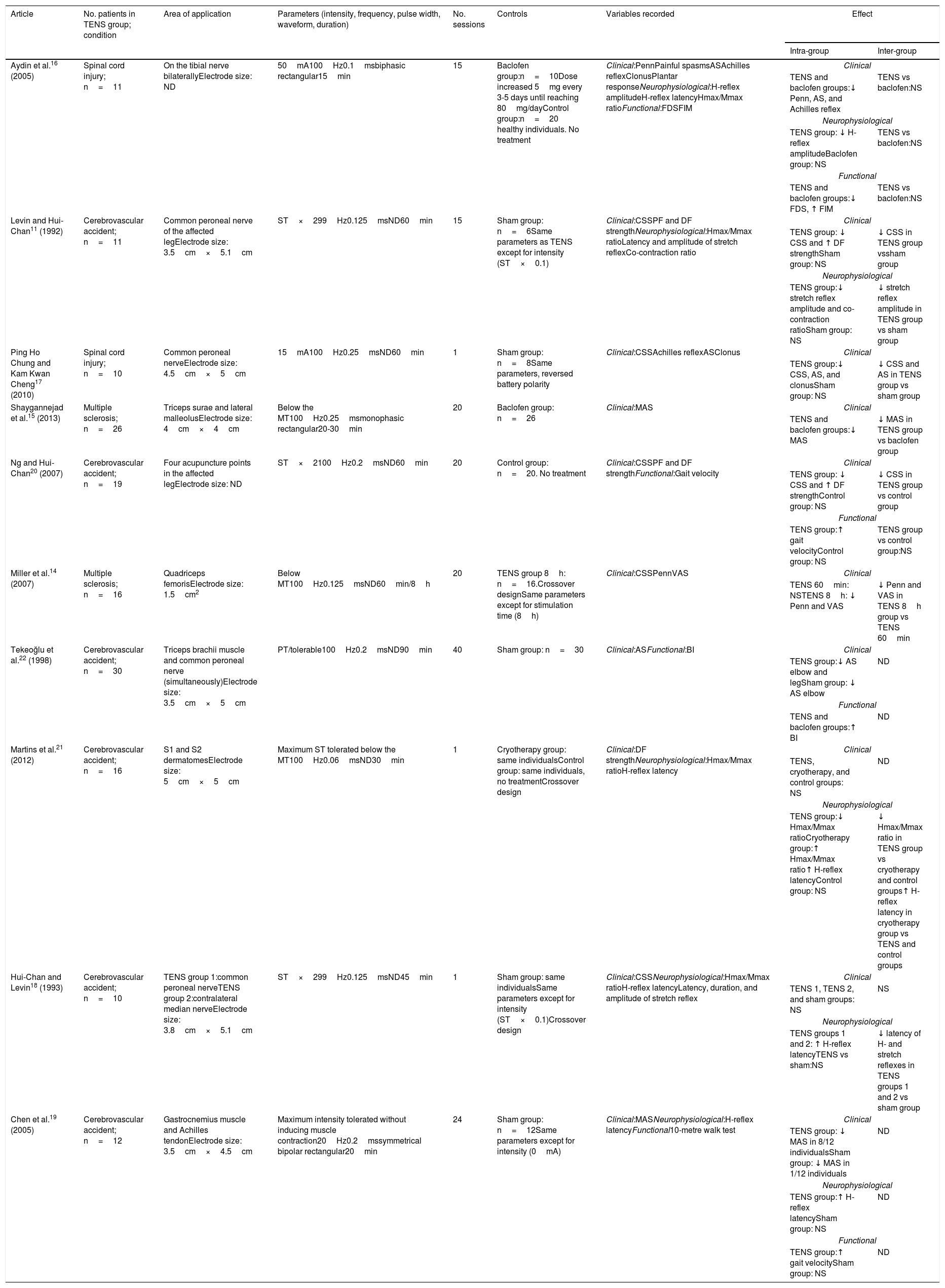
Main characteristics of the studies included in the literature review.
| Article | No. patients in TENS group; condition | Area of application | Parameters (intensity, frequency, pulse width, waveform, duration) | No. sessions | Controls | Variables recorded | Effect | |
|---|---|---|---|---|---|---|---|---|
| Intra-group | Inter-group | |||||||
| Aydin et al.16 (2005) | Spinal cord injury; n=11 | On the tibial nerve bilaterallyElectrode size: ND | 50mA100Hz0.1msbiphasic rectangular15min | 15 | Baclofen group:n=10Dose increased 5mg every 3-5 days until reaching 80mg/dayControl group:n=20 healthy individuals. No treatment | Clinical:PennPainful spasmsASAchilles reflexClonusPlantar responseNeurophysiological:H-reflex amplitudeH-reflex latencyHmax/Mmax ratioFunctional:FDSFIM | Clinical | |
| TENS and baclofen groups:↓ Penn, AS, and Achilles reflex | TENS vs baclofen:NS | |||||||
| Neurophysiological | ||||||||
| TENS group: ↓ H-reflex amplitudeBaclofen group: NS | TENS vs baclofen:NS | |||||||
| Functional | ||||||||
| TENS and baclofen groups:↓ FDS, ↑ FIM | TENS vs baclofen:NS | |||||||
| Levin and Hui-Chan11 (1992) | Cerebrovascular accident; n=11 | Common peroneal nerve of the affected legElectrode size: 3.5cm×5.1cm | ST×299Hz0.125msND60min | 15 | Sham group: n=6Same parameters as TENS except for intensity (ST×0.1) | Clinical:CSSPF and DF strengthNeurophysiological:Hmax/Mmax ratioLatency and amplitude of stretch reflexCo-contraction ratio | Clinical | |
| TENS group: ↓ CSS and ↑ DF strengthSham group: NS | ↓ CSS in TENS group vssham group | |||||||
| Neurophysiological | ||||||||
| TENS group:↓ stretch reflex amplitude and co-contraction ratioSham group: NS | ↓ stretch reflex amplitude in TENS group vs sham group | |||||||
| Ping Ho Chung and Kam Kwan Cheng17 (2010) | Spinal cord injury; n=10 | Common peroneal nerveElectrode size: 4.5cm×5cm | 15mA100Hz0.25msND60min | 1 | Sham group: n=8Same parameters, reversed battery polarity | Clinical:CSSAchilles reflexASClonus | Clinical | |
| TENS group:↓ CSS, AS, and clonusSham group: NS | ↓ CSS and AS in TENS group vs sham group | |||||||
| Shaygannejad et al.15 (2013) | Multiple sclerosis; n=26 | Triceps surae and lateral malleolusElectrode size: 4cm×4cm | Below the MT100Hz0.25msmonophasic rectangular20-30min | 20 | Baclofen group: n=26 | Clinical:MAS | Clinical | |
| TENS and baclofen groups:↓ MAS | ↓ MAS in TENS group vs baclofen group | |||||||
| Ng and Hui-Chan20 (2007) | Cerebrovascular accident; n=19 | Four acupuncture points in the affected legElectrode size: ND | ST×2100Hz0.2msND60min | 20 | Control group: n=20. No treatment | Clinical:CSSPF and DF strengthFunctional:Gait velocity | Clinical | |
| TENS group: ↓ CSS and ↑ DF strengthControl group: NS | ↓ CSS in TENS group vs control group | |||||||
| Functional | ||||||||
| TENS group:↑ gait velocityControl group: NS | TENS group vs control group:NS | |||||||
| Miller et al.14 (2007) | Multiple sclerosis; n=16 | Quadriceps femorisElectrode size: 1.5cm2 | Below MT100Hz0.125msND60min/8h | 20 | TENS group 8h: n=16.Crossover designSame parameters except for stimulation time (8h) | Clinical:CSSPennVAS | Clinical | |
| TENS 60min: NSTENS 8h: ↓ Penn and VAS | ↓ Penn and VAS in TENS 8h group vs TENS 60min | |||||||
| Tekeoğlu et al.22 (1998) | Cerebrovascular accident; n=30 | Triceps brachii muscle and common peroneal nerve (simultaneously)Electrode size: 3.5cm×5cm | PT/tolerable100Hz0.2msND90min | 40 | Sham group: n=30 | Clinical:ASFunctional:BI | Clinical | |
| TENS group:↓ AS elbow and legSham group: ↓ AS elbow | ND | |||||||
| Functional | ||||||||
| TENS and baclofen groups:↑ BI | ND | |||||||
| Martins et al.21 (2012) | Cerebrovascular accident; n=16 | S1 and S2 dermatomesElectrode size: 5cm×5cm | Maximum ST tolerated below the MT100Hz0.06msND30min | 1 | Cryotherapy group: same individualsControl group: same individuals, no treatmentCrossover design | Clinical:DF strengthNeurophysiological:Hmax/Mmax ratioH-reflex latency | Clinical | |
| TENS, cryotherapy, and control groups: NS | ND | |||||||
| Neurophysiological | ||||||||
| TENS group:↓ Hmax/Mmax ratioCryotherapy group:↑ Hmax/Mmax ratio↑ H-reflex latencyControl group: NS | ↓ Hmax/Mmax ratio in TENS group vs cryotherapy and control groups↑ H-reflex latency in cryotherapy group vs TENS and control groups | |||||||
| Hui-Chan and Levin18 (1993) | Cerebrovascular accident; n=10 | TENS group 1:common peroneal nerveTENS group 2:contralateral median nerveElectrode size: 3.8cm×5.1cm | ST×299Hz0.125msND45min | 1 | Sham group: same individualsSame parameters except for intensity (ST×0.1)Crossover design | Clinical:CSSNeurophysiological:Hmax/Mmax ratioH-reflex latencyLatency, duration, and amplitude of stretch reflex | Clinical | |
| TENS 1, TENS 2, and sham groups: NS | NS | |||||||
| Neurophysiological | ||||||||
| TENS groups 1 and 2: ↑ H-reflex latencyTENS vs sham:NS | ↓ latency of H- and stretch reflexes in TENS groups 1 and 2 vs sham group | |||||||
| Chen et al.19 (2005) | Cerebrovascular accident; n=12 | Gastrocnemius muscle and Achilles tendonElectrode size: 3.5cm×4.5cm | Maximum intensity tolerated without inducing muscle contraction20Hz0.2mssymmetrical bipolar rectangular20min | 24 | Sham group: n=12Same parameters except for intensity (0mA) | Clinical:MASNeurophysiological:H-reflex latencyFunctional10-metre walk test | Clinical | |
| TENS group: ↓ MAS in 8/12 individualsSham group: ↓ MAS in 1/12 individuals | ND | |||||||
| Neurophysiological | ||||||||
| TENS group:↑ H-reflex latencySham group: NS | ND | |||||||
| Functional | ||||||||
| TENS group:↑ gait velocitySham group: NS | ND | |||||||
AS: Ashworth scale; BI: Barthel index; CSS: Composite Spasticity Scale; DF: dorsiflexion; FDS: functional disability scale; FIM: Functional Independence Measure; MAS: Modified Ashworth Scale; MT: motor threshold; ND: no data; NS: not significant; Penn: Penn Spasm Frequency Scale; PF: plantar flexion; ST: sensory threshold; VAS: visual analogue scale for perceived spasticity.
The studies selected for this review included a total of 84 patients with multiple sclerosis,14,15 39 with spinal cord injury,16,17 and 207 with cerebrovascular accidents, of which 147 were chronic11,18–21 and 60 acute.22 Hemiparesis secondary to a cerebrovascular accident was the most frequently studied condition; furthermore, TENS was most effective in these patients.
Forms of stimulation and stimulation parametersMost studies used pulse frequencies ranging between 99 and 100Hz11,18 and a pulse width of 0.1-0.25ms, except for the study by Martins et al.,21 who used a 0.06ms pulse width. Current intensity was subjective and adapted to the patients’ sensation. In most cases, stimulation caused a tolerable tingling sensation below the motor threshold, which did not cause pain. Electrodes were most commonly located along the trajectory of the nerve, with the common peroneal nerve being the most frequently studied.11,17,18,22 Most studies evaluated the effectiveness of the technique based on clinical variables.11,16,17,22 Other studies have reported positive results with direct stimulation of the spastic muscle14,15,19; the effectiveness of stimulating the antagonist muscle is more controversial.22
Three studies evaluated the effects of TENS after a single session,17,18,21 with conflicting results. Other studies applied intervention programmes of 1511,16 or 20 sessions,14,15,20 demonstrating positive effects for different variables. All studies applied sessions lasting at least 15minutes. Sessions lasted 15-30minutes in 4 studies,15,16,19,21 45-60minutes in 5 studies,11,14,17,18,20 and over 90minutes in only 2 studies.22 One study even applied continuous sessions of TENS lasting 8hours; 8-hours sessions were found to be more effective than one-hour sessions.14
Variables recordedDue to the wide range of variables recorded in the different studies, we categorised them as clinical, neurophysiological, and functional variables; this approach has been used by previous authors.23
Clinical variablesAll articles evaluating spasticity from a clinical viewpoint used the Ashworth Scale or the Modified Ashworth Scale, either in isolation for one or several joints or as a part of the Composite Spasticity Scale (CSS). Ping Ho Chung et al.17 reported lower CSS scores in patients treated with TENS than in those receiving the sham treatment. Other studies using the Ashworth Scale or its modified version have shown that TENS has similar16 or more beneficial effects15 than baclofen, a drug commonly used to treat spasticity.
TENS was found to be superior to the sham treatment in 311,17,20 of the 5 studies using the CSS. In a study by Ng and Hui-Chan,20 CSS scores decreased faster in patients treated with TENS than in controls.
Only 3 studies have evaluated the effects of TENS on strength in spastic patients; their results for intra- and intergroup comparisons are controversial.19–21 However, no study directly demonstrated that TENS increases the strength of plantar flexor or dorsiflexor muscles significantly more than does sham treatment.
Neurophysiological variablesAll the studies assessing neurophysiological variables assessed the H-reflex or one or more of its parameters. H-reflex amplitude was only evaluated by Aydin et al.16; these authors report a decrease in this variable after treatment with TENS, with results similar to those of baclofen. However, they found no significant differences when the H-reflex was normalised to the M-wave (Hmax/Mmax ratio); similarly, Levin and Hui-Chan11 observed no changes in this variable after stimulating the common peroneal nerve. Martins et al.21 did observe a significant decrease in the Hmax/Mmax ratio in patients treated with TENS compared to controls and patients receiving cryotherapy. Only the studies by Hui-Chan and Levin18 and Chen et al.19 showed an increase in H-reflex latency after treatment with TENS compared to sham stimulation.
Other studies also measured the latency and amplitude of the stretch reflex. Levin and Hui-Chan11 observed that stretch reflex latency was longer in patients treated with TENS than in the sham group; the difference was not significant, however, due to the high variability in this measure. The researchers did observe a decrease in stretch reflex amplitude after 3 weeks of treatment. Another study by the same researchers found an increase in the latency of the stretch reflex in patients treated with TENS compared to those receiving a sham treatment, and only a non-significant decrease in stretch reflex amplitude.18
Functional variablesFour of the studies selected used some type of functional scale or assessed functional variables. The variables analysed differ between studies, however. Aydin et al.16 used a functional disability scale and the Functional Independence Measure, observing a significant increase in both variables in patients treated with TENS; this effect was similar to that caused by baclofen. Ng and Hui-Chan20 found that TENS combined with task-oriented training achieved greater gait velocity than no treatment, TENS alone, and task-oriented training alone. In contrast, Chen et al.19 report a decrease in gait velocity in the 10-metre walk test in patients treated with TENS compared to those receiving sham stimulation. Lastly, Tekeoğlu et al.22 used the Barthel Index, reporting higher scores in both the TENS and the control groups. The patients treated with TENS did score higher than controls on some specific parts of the scale.
DiscussionMost of the studies included in this review show TENS to be effective for improving at least some variables, with some authors finding the effectiveness of TENS to be similar16 or superior15 to that of baclofen. This suggests that the technique may be a valid treatment option for patients with spasticity. However, the great variability in the form of stimulation, the parameters used, and the variables analysed makes it difficult to analyse and compare results to objectively determine the effectiveness of the technique and to identify the optimal parameters for treating spasticity. Based on the findings of our literature review, we would highlight a number of considerations regarding the use of TENS for the treatment of spasticity.
The most frequent approach involves high-frequency stimulation (around 100Hz) over the trajectory of the nerve.11,14–18,20–22 This type of stimulation is thought to activate mainly large-calibre afferent fibres, increasing presynaptic inhibition of the hyperactive stretch reflex10,11 and disinhibition of voluntary commands to the motor neurons of the paretic muscle.11 Quasi-experimental studies not included in this review also show the effectiveness of high-frequency TENS compared to frequencies of 2Hz.24 However, the improvements in spasticity observed via clinical, neurophysiological, and functional variables after treatment with 20-Hz pulses suggest the involvement of other mechanisms that should also be considered.19
Current intensity is a key factor in the effectiveness of TENS. Most studies do not objectively specify the intensity used; rather, they use subjective and ambiguous expressions of perceived sensation (“below the motor threshold,”15,21 “bearable pain threshold,”22 or “twice the sensory threshold”18,20), or express intensity in absolute values.16 However, expressing current intensity in this way may induce error since it depends on the area covered by the electrodes. Future studies should express intensity as current density (mA/cm2) in order to allow comparison of TENS dosage.25 Likewise, most of the studies included in our review do not specify the waveform used.11,14,17,18,20–22 This parameter is responsible for the subjective sensation of electric current that is used in many studies to determine current intensity. Monophasic waveforms, which have a polar effect, are more poorly tolerated as they cause more irritation than biphasic waveforms, which have no electrochemical effect.7
Although insufficient data are available to perform a thorough analysis, session number and duration seem to be key factors in the effectiveness of the intervention. In general terms, effectiveness is directly correlated with the number of sessions. This hypothesis is supported by the results of the study by Aydin et al.,16 who observed a decrease in Ashworth Scale scores after 15 sessions, but not after the first 15-minute session. According to the studies included in our review, session duration seems not to have any impact on treatment effectiveness. However, Miller et al.14 did observe effects after 8hours of continuous stimulation that were not seen after a one-hour session. Further research is necessary to ascertain the role of session frequency and duration on the effectiveness of the technique.
Despite the wide range of variables analysed in the studies included in our review, it is clear that the Ashworth Scale, or its modified version, is instrumental in the clinical assessment of spasticity. The scale has good sensitivity to change but also has limitations: it only quantifies hypertonia, disregarding velocity dependence.23 Complementary tools should be used to quantify other symptoms of spasticity; these include the CSS for patients with brain damage18,20 and scales evaluating spasms and clonus in patients with spinal cord injury.16 Evidence of the effectiveness of TENS for the latter 2 symptoms is limited, however.16,17,22
Neurophysiological variables are recorded to determine and understand the action mechanism of the intervention. The H-reflex is the most frequently analysed in patients with spasticity, and is used as an indirect measure of alpha motor neuron excitability. Spastic patients tend to show shorter latencies than healthy individuals.11 Latencies are also shorter in the affected limb than in the non-affected limb in patients experiencing cerebrovascular accidents.21 H-reflex amplitude and the Hmax/Mmax ratio are also increased in these patients.11,21 Decreased H-reflex amplitude suggests decreased spinal hyperexcitability. Our review found contradictory results on the role of the H-reflex, however.11,16,21 Specific neurophysiological studies should be performed to determine the role of TENS on the mechanisms of spinal inhibition.
Although functional scales do not directly quantify spasticity, they are useful for determining the impact of the disorder on activities of daily living. Improvements in functional disability scale and Functional Independence Measure scores,16 gait velocity,19,20 or Barthel Index scores22 after treatment with TENS reveal that the intervention has an indirect positive effect on spasticity, and consequently on patients’ quality of life. These studies also show that the effectiveness of TENS increases with the number of sessions (at least 15). Session duration has less of an effect; benefits have been observed in patients undergoing sessions lasting as little as 15minutes16 and up to 90minutes.22 These findings should be interpreted with caution, however, as functional variables are evaluated in only 4 studies, none of which reported greater functional improvements in the TENS group compared to controls.
None of the articles reviewed report adverse reactions to TENS. As with pain relief,7 we may conclude that this technique is safe for the treatment of spasticity. One limitation of this systematic review is the exclusion of quasi-experimental studies of great clinical and neurophysiological value.10,26 We included only clinical trials in order to maximise the methodological quality of the review. Another limitation of the study is that we did not analyse the duration of treatment effects. This is further complicated by the great variability in session number and duration and the fact that numerous articles report only short-term effects. In any case, the effects of TENS on spasticity are believed to be short-lasting (minutes or hours); the impact of the intervention may be prolonged with additional sessions.
ConclusionMost of the studies analysed show the effectiveness of TENS for improving most of the variables recorded; the effects of the technique are in some cases similar or superior to those of pharmacological treatment. Given its low cost and ease of use and the absence of adverse reactions, TENS should be regarded as a treatment option for reducing spasticity in neurological patients. The efficacy of TENS for improving some variables is controversial, probably due to the variability in stimulation parameters. These observations call for specific, standardised analyses of applications and stimulation parameters to optimise treatment outcomes.
FundingThe authors have received no public or private funding for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernández-Tenorio E, Serrano-Muñoz D, Avendaño-Coy J, Gómez-Soriano J. Estimulación eléctrica nerviosa transcutánea como tratamiento de la espasticidad: una revisión sistemática. Neurología. 2019;34:451–460.