In mammals, the preBötzinger complex (preBötC) is a bilateral and symmetrical neural network located in the brainstem which is essential for the generation and modulation of respiratory rhythm. Few human studies have addressed the preBötC and its relationship with neurological diseases has not been described. However, the possibility that the preBötC is important in neural control of breathing, and potentially involved in neurological diseases in humans, has been suggested based on pharmacological manipulation and lesion of the preBötC in animal models, both in vivo and in vitro.
MethodIn this review, we describe the effects of certain drugs on the inspiratory activity in vitro in a transverse slice that contains the preBötC, as well as some in vivo experiments. Drugs were classified according to their effects on the main neurotransmitter systems and their importance as stimulators or inhibitors of preBötC activity and therefore for the generation of the respiratory rhythm.
ConclusionClinical neurologists will find this information relevant to understanding how the central nervous system generates the respiratory rhythm and may also relate this information to the findings made in daily practice.
En mamíferos, el complejo preBötzinger (preBötC) es una red neuronal bilateral y simétrica localizada en el tallo cerebral, la cual es indispensable para la generación y modulación del ritmo respiratorio. En humanos existen pocos estudios acerca del preBötC y su relación con patologías neurológicas no ha sido descrita. Sin embargo, la importancia del preBötC en el control neural del ritmo respiratorio y su posible participación en enfermedades neurológicas en humanos ha sido sugerida gracias a la manipulación farmacológica y lesión del preBötC realizadas en modelos animales in vivo e in vitro.
DesarrolloEn esta revisión describimos los efectos de algunos fármacos sobre la actividad inspiratoria in vitro en el modelo de rebanada transversal del tallo cerebral que contiene al preBötC, y algunos experimentos in vivo. La farmacología fue clasificada de acuerdo a los principales sistemas de neurotransmisión y a la importancia de los fármacos como estimuladores o inhibidores de la actividad del preBötC y por tanto de la generación del ritmo respiratorio.
ConclusionesEl neurólogo clínico encontrará está información relevante para entender cómo el sistema nervioso central genera el ritmo respiratorio y además podrá relacionar esta información con las observaciones hechas durante su práctica.
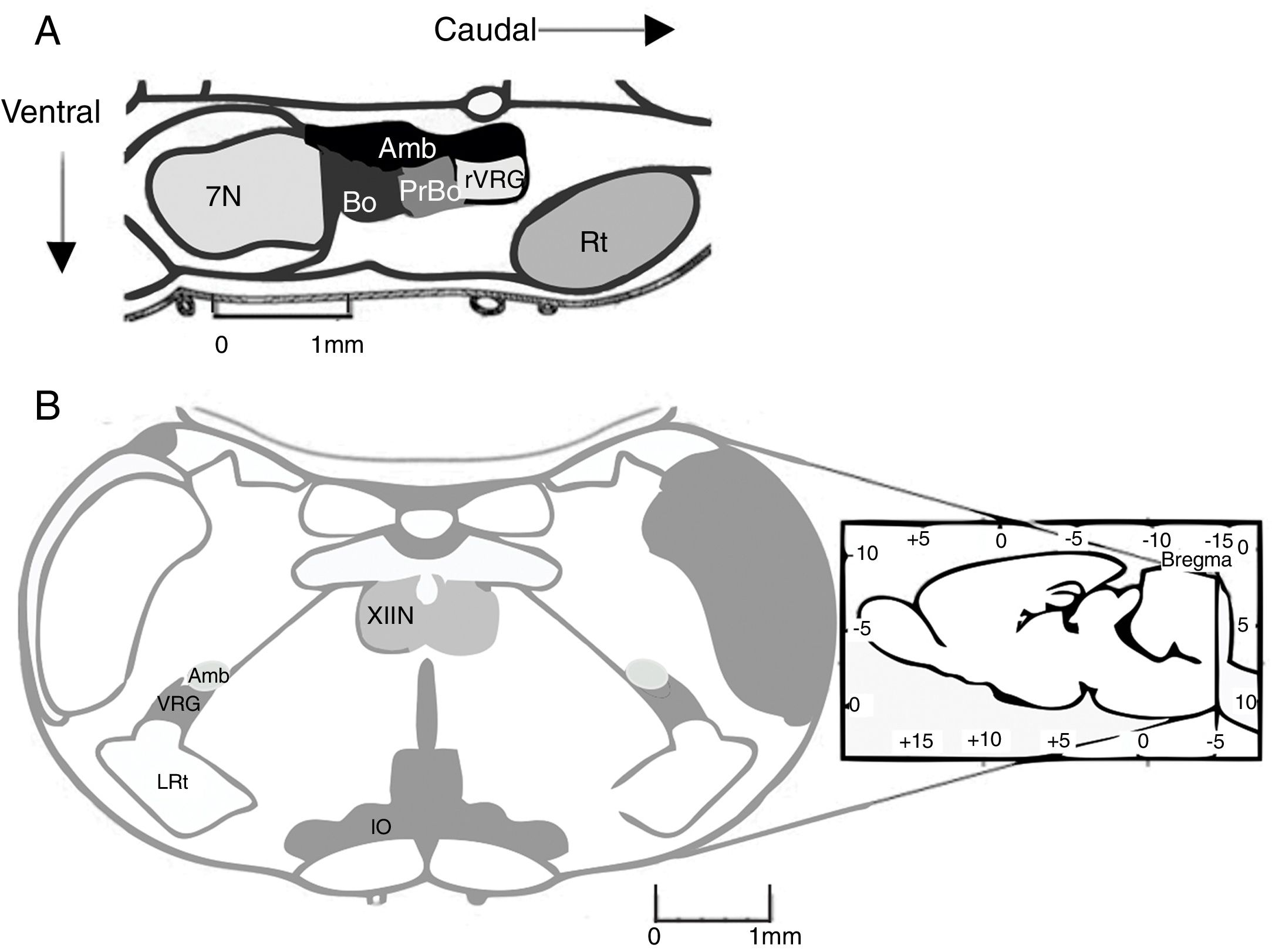
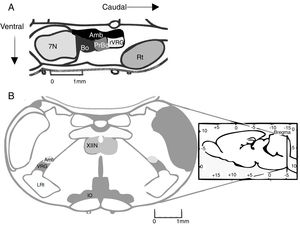
The pre-Bötzinger complex (preBötC) is a neural network responsible for inspiration during respiratory activity. In 1991, the preBötC was described as a region of the ventral medulla oblongata containing the neural pathway necessary to generate respiratory rhythm in neonatal rat brainstem preparations.1 The preBötC is a well-defined, bilateral, symmetrical region of the ventral respiratory group. It is located caudal to the facial nucleus and the Bötzinger complex (BötC), rostral to the lateral reticular nucleus, and ventral to the nucleus ambiguus1 (Fig. 1A). In humans, the preBötC is located ventral to the semi-compact division and caudal to the compact division of the nucleus ambiguus.2
(A) Location of the pre-Bötzinger complex in a sagittal section of the rodent brain (modified from Paxinos and Watson58). In mammals, the pre-Bötzinger complex is located in the ventrolateral medulla, at the level of the obex, caudal to the facial nucleus, rostral to the lateral reticular nucleus, and ventral to the nucleus ambiguus. (B) Coronal section showing the anatomical landmarks for locating the pre-Bötzinger complex. Amb: nucleus ambiguus; IO: inferior olivary nucleus; VRG: ventral respiratory group; XIIN: hypoglossal nucleus.
In adult rats, the preBötC extends 300-400μm rostrocaudally.1 Each preBötC has independent rhythmic activity. However, they communicate with one another bidirectionally via synaptic connections which synchronise their activity.3 In vivo studies have shown that preBötC neurons project to the contralateral preBötC, the ipsi- and contralateral BötC, the caudal ventral respiratory group, the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG), the parahypoglossal nucleus, the solitary nucleus, the parabrachial nucleus/Kölliker-Fuse nucleus, and the periaqueductal grey.4
In addition to its role in respiratory rhythm generation, the preBötC is essential for generating the respiratory pattern.5 Peptidergic modulation of the preBötC by such chemosensitive nuclei as the RTN/pFRG, and possibly the solitary nucleus, is essential for sighing.6
Respiratory rhythm generation: from in vitro preparations to electrophysiological recordingThe generation of respiratory rhythm in mammals has been studied in vivo in different animal models, including cats, goats, rats, and mice.1,7–9 The most frequently used models for studying respiratory rhythm in vitro are brainstem/spinal cord preparations (or en bloc preparations) and rhythmic, transverse slice preparations from neonate rats and mice.9 Rhythmic, transverse slice preparations, described by Smith et al.1 in 1991, contain the preBötC, hypoglossal nucleus, and hypoglossal nerve (CN XII), which register and integrate data on the rhythmic motor activity of respiration. This activity is similar to that generated by the neurons of the respiratory central pattern generator, that is the preBötC. In en bloc preparations, the rhythmic activity of the phrenic nerve is similar to preBötC activity.
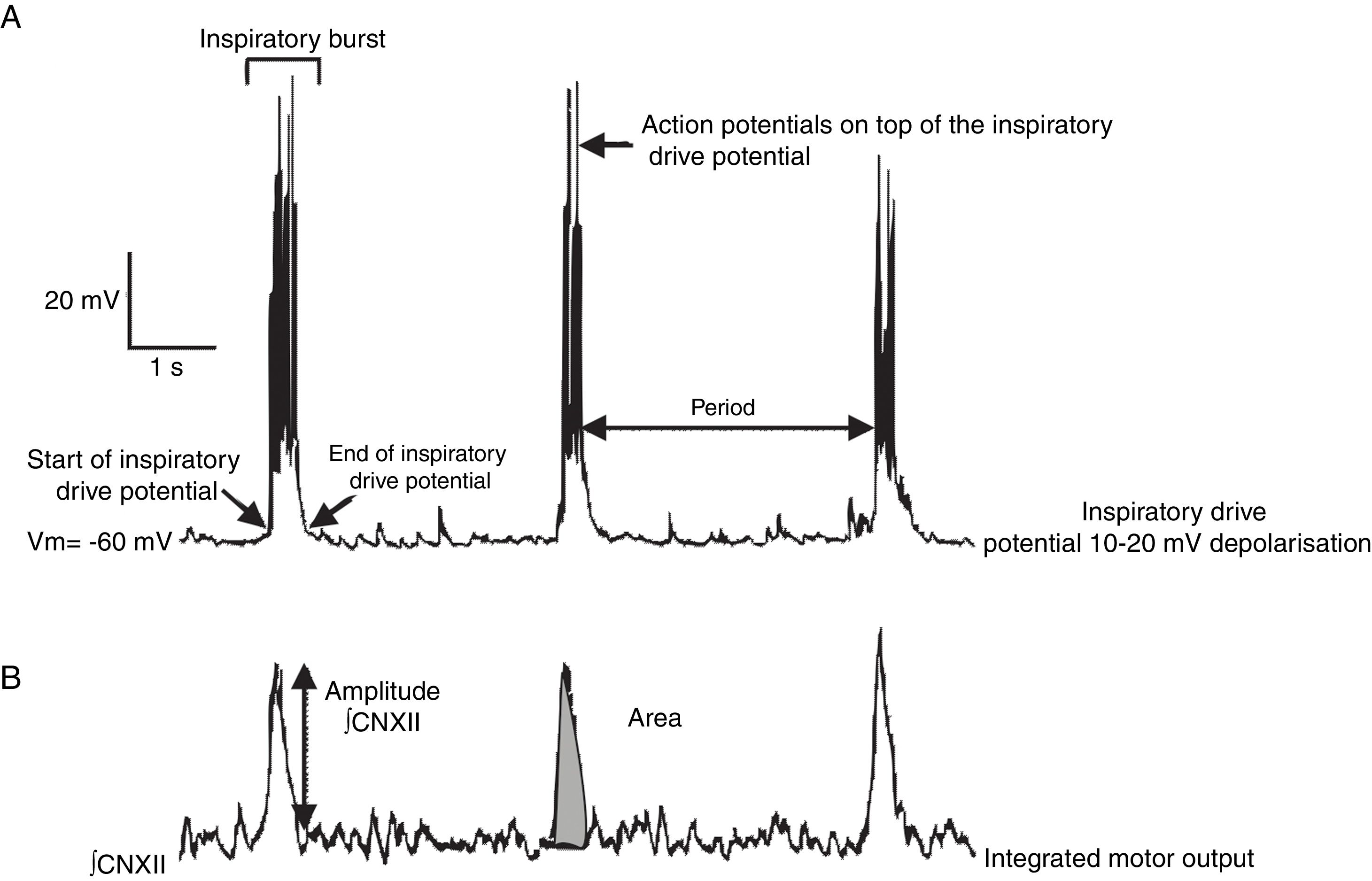
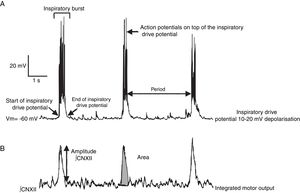
Previous studies have addressed the advantages and limitations of studying respiratory rhythm in vitro, and proposed a methodology for obtaining rhythmic, transverse slices.10 Using a vibratome, the brainstem is sliced into serial, transverse sections under a microscope until such anatomical landmarks as the nucleus ambiguus and the inferior olivary nucleus are revealed (Fig. 1B). At this point, a 500-600μm transverse slice is obtained. With this preparation, we can register rhythmic activity of preBötC neurons either directly, using the single-cell patch clamp technique to study synaptic interactions of the neural network, or indirectly, by recording the activity of CN XII roots, which transmit signals from hypoglossal nucleus motor neurons excited by preBötC neurons (Fig. 2). The rhythmic activity of CN XII is usually integrated (∫CNXII). Once baseline activity has been established, pharmacological agents can be added to the bathing medium or injected near the neuron under study.
Types of neurons in the preBötCThe preBötC is a heterogeneous network of interneurons.11 In rats, it contains around 1000 neurons on each side12; approximately 600 of these neurons express the neurokinin-1 receptor (NK1R), which binds to substance P.13 Some neurons express somatostatin, a neuropeptide involved in modulating respiration. Immunoreactivity to NK1R or to somatostatin is used as a marker for preBötC neurons.14–16 Autopsy studies of the brains of patients with multiple system atrophy, Lewy body dementia, or Perry syndrome have revealed significantly fewer NK1R-immunoreactive neurons in the region of the preBötC in these patients than in patients who died due to other causes. This suggests a high likelihood that the respiratory alterations associated with the disorders mentioned above are caused by the loss of neurons regulating respiratory rhythm.17–20
The preBötC also contains subpopulations of glutamatergic, glycinergic, GABAergic, and glycinergic-GABAergic neurons.16,21 As a result of synaptic interactions between preBötC neurons, each neuron generates inspiratory rhythmic activity in the form of action potentials on top of 10-20mV synchronic depolarisation lasting 0.3-0.8s; this is known as inspiratory drive potential22 (Fig. 2). Despite being rhythmic, most preBötC neurons are not considered pacemaker neurons23 since rhythmic activity stops in the absence of synaptic connectivity. Lastly, the preBötC also contains silent neurons, which do not fire action potentials even though they are located in the same neuroanatomical area. The preBötC contains no expiratory neurons. During pulmonary ventilation, inspiration is an active process involving muscle contraction, whereas expiration is passive.24 Expiration becomes active in such processes as coughing, sneezing, and physical exercise. It has been suggested that active expiration is controlled by the RTN/pFRG.25,26
Pharmacology of glutamate receptorsGlutamate is the main excitatory neurotransmitter in the preBötC.21,27 PreBötC neurons express ionotropic (N-Methyl-d-aspartate [NMDA], α-amino-3-hydroxy-5-methyl-4-isoxazole propionate [AMPA], kainate) and metabotropic glutamate receptors (mGluR).28–30
AMPA receptor (AMPAR) activation and modulation is essential for respiratory rhythm generation. Applying the AMPAR agonist to the preBötC increases rhythm frequency by depolarising rhythmogenic neurons,29,31 whereas applying the antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)-quinoxaline (NBQX) completely abolishes respiratory activity.28 However, removal of extracellular Mg2+ to relieve the voltage-dependent block of NMDA receptors (NMDAR) maintains respiratory rhythm even at high NBQX concentrations, since the postsynaptic current generated by NMDARs substitutes that of AMPARs.28
Despite being present in preBötC neurons, NMDARs are not essential for respiratory rhythm generation or drive transmission in the neonate: dizocilpine hydrogen maleate (MK-801) has been found to have no effect on respiratory rhythm in in vitro rat preparations.28 Furthermore, mouse preparations lacking the NMDAR 1 subunit (NMDAR1−/−), which is necessary for generating functional NMDARs, display similar respiratory rhythm to that of controls.32
It has been suggested that group I mGluRs contribute to inspiratory drive potential generation: mGluR1 regulates K+ conductance and mGluR5 uses intracellular Ca2+ as a second messenger and acts via inositol 1,4,5-trisphosphate receptors (IP3R).30,33 Selective mGluR1 antagonist LY36738 and mGluR5 antagonist 6-methyl-2-(phenylethynyl) pyridine (MPEP) significantly reduce the amplitude and area of the inspiratory drive potential without affecting respiratory motor output.33 The general group II mGluR antagonist (RS)-1-Amino-5-phosphonoindan-1-carboxylic acid (APICA) reduces the frequency of CN XII motor output without affecting amplitude.33
Capsaicin, the active ingredient of chilli peppers, evokes glutamate and substance P release. This compound depletes substance P and prevents reaccumulation of the neuropeptide in peripheral sensory neurons.34 Applying capsaicin to transverse slices decreases the frequency of respiratory rhythmic activity measured in ∫CNXII in a dose- and time-dependent manner. Respiratory rhythm probably halts because capsaicin evokes glutamate release and substance P depletion in fibres within the preBötC.27
Pharmacology of GABA receptorsThe anaesthetic sevoflurane may either inhibit or stimulate inspiratory neurons via type A GABA receptors (GABAAR): at low concentrations (0.23 and 0.47mM), sevoflurane stimulates preBötC activity, whereas at concentrations of 0.75mM and higher, it inhibits preBötC activity.35 Microinjection of the GABAAR agonist muscimol into the bilateral preBötC halts the rhythm in CN XII; unilateral microinjection does not have this effect.36 There is a transitional period in the development of respiratory motor control between rat postnatal days P10 and P15; GABAergic neuron inhibition increases during this period. In fact, sensitivity to pentobarbital increases at age P14 in the preBötC, and decreases at ages P11 to P15 in hypoglossal nucleus motor neurons, being the lowest in P14 rats. These changes in sensitivity to pentobarbital point to alterations in the expression of GABAAR subunits.37 This differential expression may be a key factor in compensating for or preventing increased GABAergic inhibition and, consequently, respiratory depression during this developmental period. Bicuculline, a GABAB receptor agonist, causes no changes in the respiratory activity of neonatal rats.38
Cumulative evidence suggests that the involvement of the GABAergic system in respiratory rhythm generation may be age-dependent: while it does not seem to play a relevant role in preBötC rhythmogenesis at young ages, at ages older than P10 it may modulate respiratory depression.
Pharmacology of glycine receptorsGlycine receptors (GlyR) are ligand-activated Cl− ion channels mediating fast inhibitory neurotransmission in the brainstem and spinal cord.39 The GlyR antagonist strychnine has been used to abolish GlyR-mediated spontaneous currents and as a molecular target for volatile anaesthetics.35,38 For example, applying strychnine before sevoflurane antagonises the depressor effect of the latter on preinspiratory neurons, whereas co-applying it with sevoflurane (0.47mM) significantly increases preinspiratory neuron activity.35 This antagonist has not been demonstrated to play a critical role in respiratory rhythm generation, however.
Pharmacology of acetylcholine receptorsCholinergic neurotransmission alterations in the brainstem affect respiratory motor patterns both in vitro and in vivo.40 PreBötC neurons react to local acetylcholine (ACh) and carbachol, and to bath application of muscarine. Muscarine increases the frequency, amplitude, and duration of CN XII motor output and induces seizure-like activity during the expiratory periods. These effects are blocked by atropine.40 ACh and nicotine modulate respiratory patterns by activating nicotinic ACh receptors (nAChR) in the preBötC.41 Nicotine activates the α4 subunit of nAChRs, promoting excitatory glutamatergic input to preBötC inspiratory neurons and depolarising them. The acetylcholinesterase inhibitor neostigmine increases ACh levels, excites inspiratory neurons, and increases respiratory frequency and amplitude and induces tonic activity in CN XII.42
Unilateral microinjection of the acetylcholinesterase inhibitor physostigmine into the preBötC increases the frequency of ∫CNXII rhythmic activity; ipsilateral injection induces tonic activity, increases the amplitude and duration of ∫CNXII bursts, and reduces respiratory cycle periods and period variability.43 Furthermore, 4-Diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP), an M3 muscarinic ACh receptor selective antagonist, blocks carbachol-induced currents, increases membrane conductance, and blocks all the effects of physostigmine except for increased respiratory frequency.40,43 In the presence of both dihydro-β-erythroidine and 4-DAMP, physostigmine induces the opposite effects, which suggests that cholinergic transmission in the preBötC regulates respiratory frequency, whereas in CN XII it regulates tonic activities and increases the amplitude and duration of ∫CNXII inspiratory bursts in neonate rats.43
Pharmacology of serotonin receptorsSerotonin (5-Hydroxytryptamine or 5-HT) plays a key role in modulating the respiratory pattern by acting on different receptor subtypes. Systemic administration of 8-hydroxy-2-diproplyaminotetralin hydrobromide (8-OH-DPAT) to dogs decreases inspiratory and expiratory duration by 40%, but has no effect on the amplitude of phrenic nerve discharges.44 In an in vivo study, 8-OH-DPAT was found to modulate respiratory rhythm by activating 5-HT receptors in regions other than the preBötC and, in general, outside the respiratory column; this points to the involvement of such nearby structures as the raphe nucleus, RTN, parabrachial nucleus/Kölliker-Fuse nucleus, and the solitary nucleus.44
In vitro, bath administration of 5-HT or direct injection of 5-HT into the preBötC increases respiratory rhythm frequency.45,46 The effect of 5-HT on rhythmic activity is mimicked by agonist R(–)2-(2,5-dimethoxy-4-iodophenyl) hydrochloride and blocked by 5-HT2 receptor antagonist ketanserine.45,47
Pharmacology of adrenergic receptorsNorepinephrine is a catecholamine that regulates respiratory activity.48 Loss of noradrenergic innervation is linked to Alzheimer disease.49 Applying norepinephrine to slice preparations from mice with acute intermittent hypoxia alters the modulatory response of the respiratory network, probably due to increased synaptic inhibition in the preBötC.48 These alterations may be prevented by blocking synaptic inhibition before inducing acute intermittent hypoxia; respiration cannot be restored when synaptic inhibition is blocked after induction of the disease. The above suggests that synaptic transmission of the respiratory rhythm generating network undergoes subtle changes, such as endogenous neuromodulator release.
Pharmacology of purinergic receptorsHomeostasis of different functions of the neural respiratory network is maintained via adenosine signalling action on P1 purinergic receptors and ATP signalling action on P2 purinergic receptors.50 P2 receptor signalling plays a major role in central ventilatory response to hypoxia.51 The networks generating inspiratory rhythm in the preBötC are excited by activation of the P2 receptor subtype P2Y.52
Adenosine has been found to significantly reduce inspiratory frequency in mouse preparations. No effect on baseline inspiratory frequency has been observed in rat preparations.50 Local administration of ATP in rat preparations causes a rapid-onset, short-duration increase in inspiratory frequency, but has no significant effects when applied to mouse preparations.50,53
Some researchers have studied the modulatory effects of P2Y1 receptors on phrenic nerve (C4) and CN XII motor output in the presence of the agonist MRS2365, which increases inspiratory burst amplitude at CN XII. MRS2365 produces similar effects on C4 at concentrations 10 times higher.52 Applying MRS2365 to the preBötC for 10seconds in mouse preparations increases ∫CNXII frequency and duration compared to baseline values.50,53 Whole-cell recordings show that CN XII motor neurons are more sensitive than phrenic nerve motor neurons to P2Y1 receptor modulation.52
Pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid 4-sodium reduces the magnitude of ATP-induced currents.51 Evidence suggests that ATP signalling indirectly modulates the preBötC, modifying respiratory frequency.
Pharmacology of opioid receptorsDirect injection of μ-opioid receptor (μOR) agonists into the preBötC alters respiratory rhythm. It has been suggested that rhythmogenic neurons (also known as type 1 neurons) expressing NK1R and μOR play a relevant role in respiratory rhythm generation, since they modulate respiratory frequency.14
Bath application of μOR agonist (D-Ala2, N-MePhe4, Gly5-ol)-enkephalin to slice preparations decreases respiratory frequency at CN XII.54 Injecting the μOR agonist directly into the preBötC activates K+ conductance, inducing neuron hyperpolarisation.14 These findings are clinically relevant. Epidural or intrathecal injection of morphine, which activates μORs, is commonly used for long-term postoperative analgesia. However, the drug can cause respiratory depression. A potential explanation for this adverse effect is the fact that morphine-containing CSF activates μOR in preBötC neurons, inhibiting their activity and altering respiratory rhythm.
Other drugsSome compounds do not act on a specific, known receptor, but do alter respiratory rhythm. This is the case with creatine, an endogenous compound synthesised from arginine and glycine and stored mainly in skeletal muscle tissue. Creatine supplementation prevents neurodegenerative diseases, whereas creatine deficiency in the CNS is associated with intellectual disability.55,56
Wilken et al.57 studied the effects of creatine on inspiratory burst amplitude and duration in hypoxic slice preparations, and on ATP levels in anoxic slice preparations containing preBötC neurons. The researchers used the whole-cell patch clamp technique and recording of CN XII motor output to analyse slices from mice of different postnatal ages (P0-P5 and P6-P13), which were fed normal food (controls) or administered creatine (2g/kg/day, nutrition group), and slices incubated in creatine for 3hours (200μM; incubation group). In P0-P13 mouse slices undergoing 30-minute anoxia, the amplitude and duration of inspiratory bursts increased in both the control and the nutrition groups compared to pre-anoxia values.57 ATP values remained constant in slices pretreated with creatine and decreased in control slices. These findings suggest that creatine may counteract hypoxia-induced energy depletion.57
ConclusionMultiple pharmacological experiments have been conducted to study central respiratory rhythm generation. However, the receptors and endogenous signalling pathways necessary and sufficient for respiratory rhythm remain unknown. A clear distinction should be made between rhythm generation and rhythm modulation mechanisms in the study of rhythmogenesis. Although we know which types of neurons are present in the preBötC, it is as yet unclear how they interact to generate inspiratory activity. Pharmacology has increased our understanding of preBötC organisation. However, no drug is free of undesired effects: even at adequate concentrations, they may affect systems other than those under study. Further research should address drug specificity and any potential interactions with non-target receptors. As shown in the present review, non-selective drugs are still being used. These generate an observable and measurable response, although they also make it difficult to understand the particular molecular mechanisms involved in rhythmogenesis. The drug concentrations used in experimental studies are considerably different from the concentrations proposed by such organisations as the International Union of Basic and Clinical Pharmacology Committee on Receptor Nomenclature and Drug Classification for activating the desired receptors. Future studies should thoroughly analyse the interaction between receptors from different systems rather than from a single system.
Few studies have explored the preBötC in humans and non-human primates. Despite the scarcity of data, the available evidence suggests a direct correlation between death due to respiratory failure in patients with advanced-stage neurodegenerative diseases and the quantity of neurons in the preBötC.
FundingThe study has received funding from CONACyT (CONACYT-128392, CONACYT-153627) and a doctoral scholarship (CONACyT-515158).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Muñoz-Ortiz J, Muñoz-Ortiz E, López-Meraz L, Beltran-Parrazal L, Morgado-Valle C. El complejo pre-Bötzinger: generación y modulación del ritmo respiratorio. Neurología. 2019;34:461–468.