Complex regional pain syndrome (CRPS) is a form of persistent pain which is disproportionate to the original lesion in terms of intensity and duration. There are 2 types of syndrome depending on the type of initial lesion, regardless of clinical or therapeutic factors. Type I, traditionally known as reflex sympathetic dystrophy, is triggered by trauma, mainly fractures but it can also occur after soft-tissue injury. Type II, previously known as causalgia, occurs after nerve lesions of varying intensity and diverse aetiology. The latter is differentiated from other peripheral neuropathies in that, although it initially appears in a specific location, it usually extends in a diffuse and imprecise manner as it progresses and shows an atypical temporal pattern; symptoms may be delayed for a month and it has distinctive clinical characteristics.1 Although their physiopathogenesis is different and they may represent different entities, both are diagnosed using clinical criteria which evaluate their characteristics. There are 2 main classifications. The traditional classification, established in 1994 by the International Association for the Study of Pain (IASP), was based on expert consensus to improve diagnosis and treatment of the disorder. This classification promoted the study and understanding of CRPS and offered common criteria for future studies.3 Despite its high sensitivity, it had poor specificity. Therefore, with a view to minimising its tendency to overdiagnosis and late diagnosis, a new set of clinical criteria was put forward in 2003: the Budapest criteria. These improved specificity (0.68 vs 0.41) without compromising sensitivity (0.99 vs 1) in addition to improving diagnostic consistency between clinicians (kappa index: 0.66–0.69). Whereas the traditional criteria had never been fully accepted, these were more widely used in the literature.2,3
CRPS is characterised by persistent burning pain, which is either spontaneous or exacerbated by mechanical or thermal stimuli and can vary in intensity. It presents with signs or symptoms of up to 4 different groups. According to the currently accepted criteria, patients must present symptoms from at least 3 of the 4 categories and at least one sign from one of the categories to be diagnosed with CRPS.1 These 4 categories are: (1) sensory alterations (allodynia and/or hyperalgesia for superficial and/or deep stimuli; they affect more than one nerve territory); (2) vasomotor alterations of both skin temperature and colour; (3) sudomotor alterations, including oedema and sweating disorders (sudomotor excess or deficiency, at rest or after physical effort); and (4) trophic changes in skin, nails, hair, or bones, or motor function alterations leading to reduced mobility, weakness, tremor, or dystonia.1,2
Several complications have been described, including infections, vascular changes in the subcutaneous tissue, atrophy, weakness, affective disorders, inappropriate drug use, and suicidal tendencies. Early diagnosis and appropriate treatment are therefore crucial.1
CRPS is a complex disorder whose aetiopathogenesis is not well understood. Some theories have proposed a connection between sustained inflammation and oxidative stress,4 or central and peripheral sensitisation,5,6 and it has also been suggested that CRPS may be an injury-triggered, regionally-restricted autoimmune disorder.7 There is no specific treatment for CRPS due to its complexity, the scarcity of data on the disorder, and the lack of clinical trials. Patients should therefore be treated following a multidisciplinary approach which includes not only pharmacological treatment but also physical, occupational, and psychotherapy, and even pain management programmes.
Pain is infrequently reported in the orofacial region despite the great variety of procedures performed in this zone. Likewise, trophic changes in this area are rare.8–11 We present the case of a patient with CRPS in the pretragal region after a maxillofacial intervention of the third lower molar.
Our patient, a 40-year-old female smoker, had a history of dyslipidaemia, psoriasis without arthropathy, de Quervain tenosynovitis, endometriosis, and hiatal hernia. She was receiving mirtazapine, vitamin B8, duloxetine, and omeprazole, and was under follow-up and psychiatric treatment due to anorexia nervosa. The patient attended our hospital due to a one-year history of facial pain. This started after pericoronitis due to semi-impacted tooth 48, which resulted in its extraction by luxation, prehension, and traction. She reported a persistent, burning pain of moderate intensity (VAS: 6–7/10) which lasted all day without exacerbations. Pain increased with palpation and jaw movements. She experienced hypoaesthesia, paraesthesia in the right lower maxillary region, and skin alterations in colour, vasomotor response (asymmetric flushing and sweating compared with the surrounding tissue), and at a trophic level (a subjective feeling of thinning tissue in the area). The patient had previously been treated with analgesics (paracetamol and tramadol), local injections, and muscle relaxants, but had experienced no substantial improvements in pain.
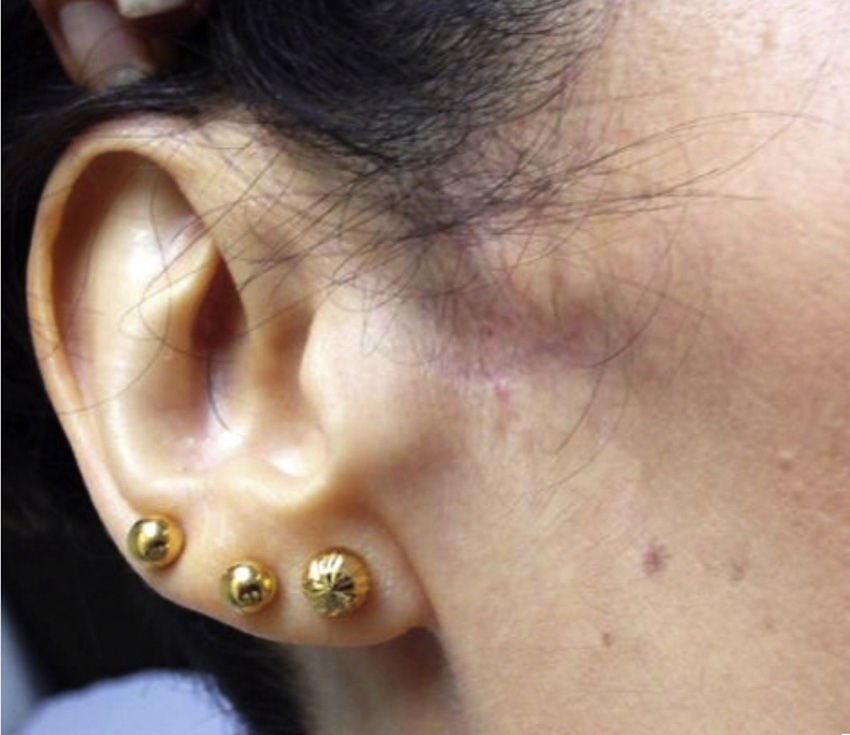
The examination revealed a decreased range of mouth opening; the temporomandibular joint made no clicking sounds when testing the end feel. Facial asymmetry was observed; the patient presented local atrophy and cutaneous changes in the affected area, where skin was paler, atrophic, hairless, and cooler than the surrounding facial tissue (temperature was not measured). Palpation revealed a decrease in subcutaneous tissue (Fig. 1). The patient displayed hyperalgesia in the pin-prick test and allodynia to both superficial and deep stimuli and to mandibular movement. The affected areas were the periauricular and perioral regions, the ramus of the mandible down to the chin, the lower labial mucosa and lip, the buccal mucosa, gingiva, teeth, and the anterior tongue. Motor functions of the tongue and the area supplied by the fifth cranial nerve were normal; the remaining results of the examination were also normal.
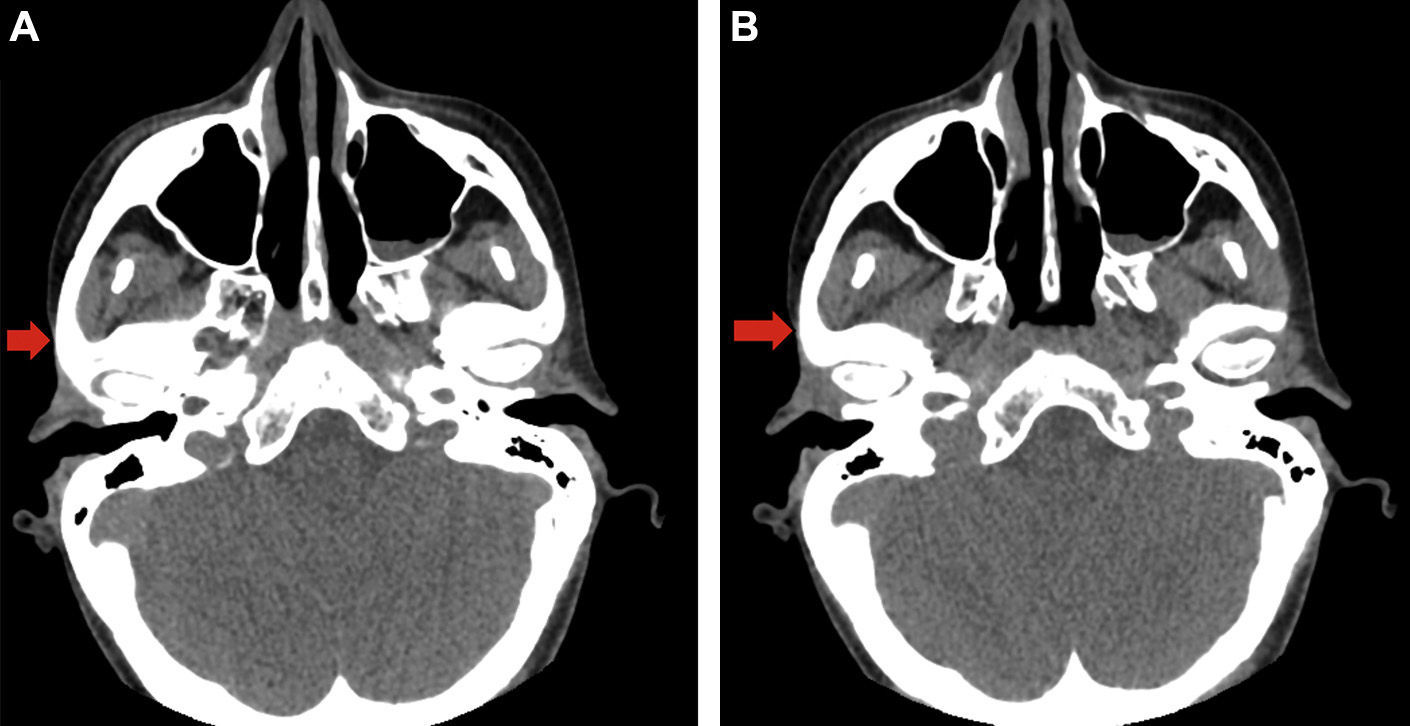
A head CT scan revealed a decrease in fat and subcutaneous tissue compared to the contralateral side (Fig. 2A and B).
The patient was diagnosed with probable type II CRPS secondary to a surgical lesion of the right inferior alveolar nerve; we started treatment with gabapentin and referred her to the rehabilitation team for assessment.
Although in our case the nerve lesion was not diagnosed based on electroclinical findings, the clinical diagnosis was clear based on the patient's history of surgery and the fact that the affected area and the surgically treated area coincided. The topography of symptoms could have indicated neuralgia of a terminal branch of the trigeminal nerve; however, as symptoms progressed, sensory alterations involved not only the inferior alveolar nerve but also the territories innervated by the lingual, mental, and auriculotemporal nerves, all of which are branches of the mandibular nerve. The clinical characteristics of her pain and the accompanying symptoms led us to diagnose her with type II CRPS, since she met both the Budapest criteria and the IASP criteria.1 Our patient tried a wide range of treatments, none of which have been proven effective to date. Several factors may have played a role in pain chronification, including a history of psychiatric disease, polytherapy, delayed diagnosis (one year), an initially unidisciplinary follow-up, and infrequent follow-up visits.
This case is interesting because of the facial involvement; although the location of CRPS is not fully defined, it has only rarely been reported in the facial region. Of the few studies which describe involvement of the trigeminal region, some report CRPS secondary to mandibular nerve injury.8,9 Other studies have described the appearance of trophic changes (atrophic lingual papillae, ulcers),8 which are very infrequent and whose existence has even been questioned.10–12 Most published cases lack detailed descriptions of vasomotor, motor, and trophic changes. Likewise, the type of fibres involved (transmitting tactile and temperature information) or the severity of hypoesthesia are not usually described.8
As mentioned previously, despite the high frequency of orofacial procedures and trauma CRPS rarely involves the orofacial region, contrary to what occurs with neuropathic pain such as trigeminal neuralgia. The presence of trophic changes and skin atrophy are not usually described in orofacial CRPS8–11; the same is true for motor alterations, which are especially difficult to define due to the complexity of monitoring motor function. The prognostic factors of this disorder are unclear since the data reported in the literature are contradictory or have insufficient statistical power; to date, cold skin temperature and sensory disturbances are the factors most frequently associated with a poor prognosis.13
We still do not know what treatment is most effective due to the low prevalence of CRPS. The literature reports several cases that were refractory to a wide range of treatments, including neuromodulators (carbamazepine, amitriptylin), occupational therapy, physical therapy, rehabilitation, transcutaneous electrical nerve stimulation, morphine derivatives, stellate ganglion block (the stellate ganglion may be useful for diagnosis in some cases), and ketamine.11,14 Conducting controlled clinical trials is not easy due to the small number of cases of CRPS. In addition, the available data are not sufficient to determine treatment guidelines.6,13
This case provides new data about an infrequent and probably underdiagnosed disorder, whose diagnosis to date is based on clinical criteria. This approach has an acceptable negative predictive value but only a moderate positive predictive value.13,15 Complementary tests, though scarcely used, have proven to be useful in the differential diagnosis6,16; however, they are not employed consistently even though some of them, such as ultrasonography, can be easily accessed. Many questions remain about CRPS. We still do not know its physiopathogenesis or whether there are 2 different entities with similar pain features, and to date there is no specific treatment for the disorder due to the lack of clinical trials.
Please cite this article as: García-Azorín D, Ortega Suero G, Liaño Sánchez T, Marcos Dolado A. Síndrome de dolor regional complejo tipo ii facial con cambios tróficos documentados. Neurología. 2016;31:212–214.