The relationship between the entorhinal cortex (EC) and the hippocampus has been studied by different authors, who have highlighted the importance of grid cells, place cells, and the trisynaptic circuit in the processes that they regulate: the persistence of spatial, explicit, and recent memory and their possible impairment with ageing.
ObjectiveWe aimed to determine whether older age causes changes in the size and number of grid cells contained in layer III of the EC and in the granular layer of the dentate gyrus (DG) of the hippocampus.
MethodsWe conducted post-mortem studies of the brains of 6 individuals aged 56–87 years. The brain sections containing the DG and the adjacent EC were stained according to the Klüver-Barrera method, then the ImageJ software was used to measure the individual neuronal area, the total neuronal area, and the number of neurons contained in rectangular areas in layer III of the EC and layer II of the DG. Statistical analysis was subsequently performed.
ResultsWe observed an age-related reduction in the cell population of the external pyramidal layer of the EC, and in the number of neurons in the granular layer of the DG.
ConclusionOur results indicate that ageing causes a decrease in the size and density of grid cells of the EC and place cells of the DG.
La relación entre la corteza entorrinal y el hipocampo ha sido estudiada por diferentes autores, que han destacado la importancia de las células de cuadrícula, las células de posicionamiento y la conexión trisináptica, en los procesos que regulan; la persistencia de la memoria espacial, explícita y reciente y su posible afección con envejecimiento.
ObjetivoObservar si existen diferencias en el tamaño y número de células de cuadrícula contenidas en la lámina tres de la corteza entorrinal y en la capa granular del giro dentado del hipocampo de pacientes mayores.
MetodologíaRealizamos estudios post-mortem del cerebro de 6 sujetos de edades comprendidas entre los 56 y 87 años. Los cortes de cerebros que contenían el giro dentado (GD) del hipocampo y la corteza entorrinal (CE) adyacente se tiñeron con el método de Klüver- Barrera, después se midió, mediante el programa Image J, el área neuronal individual, el área neuronal total, así como el número de neuronas, contenidas en cuadrículas rectangulares a nivel de la lámina III del CE y la lámina II del GD y se lleva a cabo un análisis estadístico.
ResultadosSe ha observado una reducción de la población celular de la capa piramidal externa de la CE, así como de las neuronas de la capa granular del giro dentado relacionada con el envejecimiento.
ConclusiónNuestros resultados indican que el envejecimiento produce una disminución en el tamaño y la densidad neuronal en las células de cuadricula del córtex entorrinal y de posicionamiento del giro dentado.
The dentate gyrus (DG) is a hippocampal region that envelops the bumpy surface of the cornu ammonis and receives efferents from the pyramidal cells of that structure. It is medially separated from the parahippocampal gyrus by the hippocampal fissure and from the fimbria by the fimbriodentate sulcus (Figs. 1–5).1
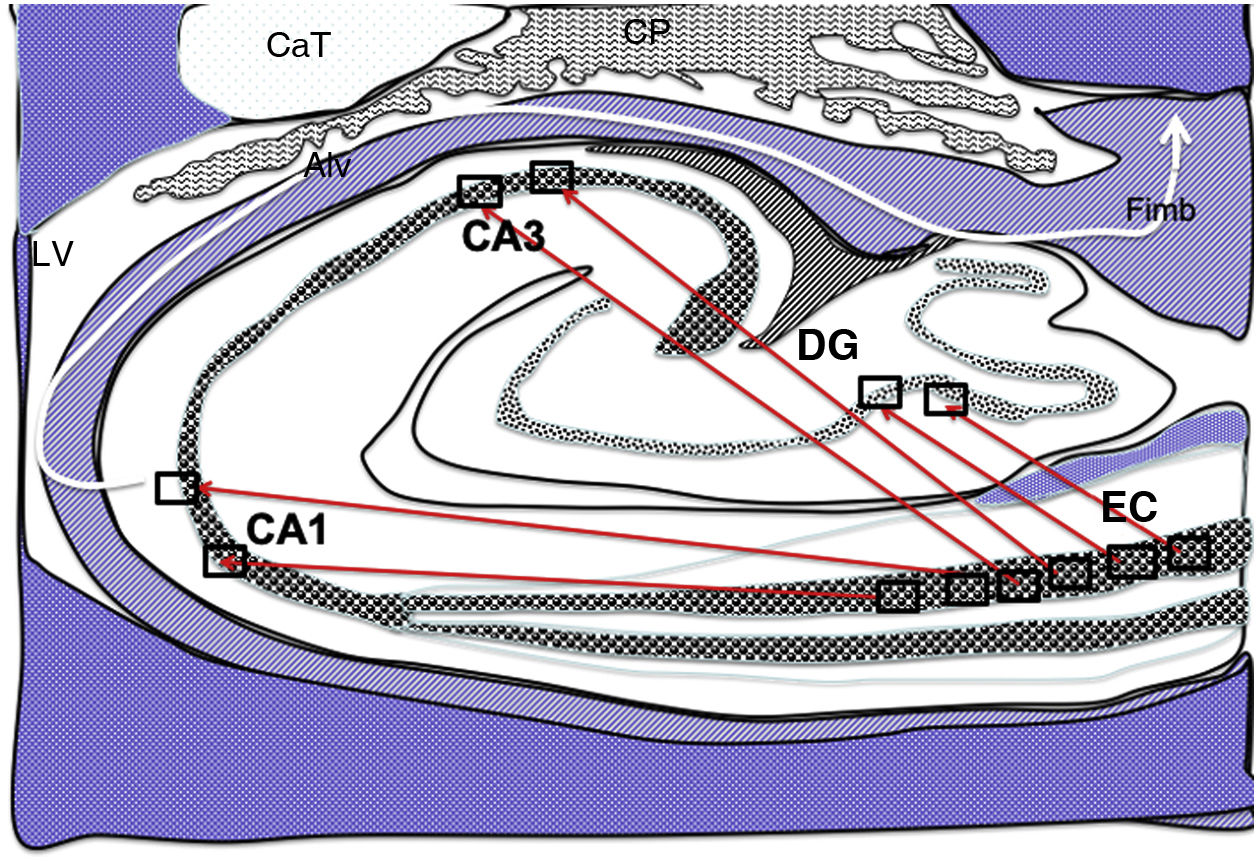
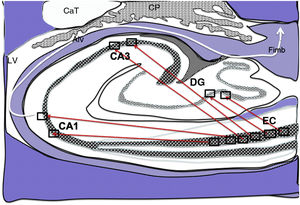
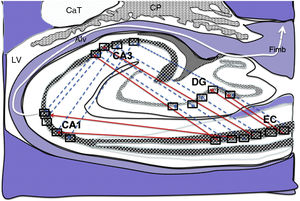
Diagram of a cross-section of the hippocampus at the level of the temporal horn of the right lateral ventricle. Arrows indicate direct connections (red solid arrows) from the grid cells of the entorhinal cortex to the place cells of the dentate gyrus, CA1, and CA3, and subsequently to the alveus and the fimbria (white arrow).26–30
Alv: alveus; CaT: tail of the caudate nucleus; CP: choroid plexi; DG: dentate gyrus; EC: entorhinal cortex; Fimb: fimbria; LV: lateral ventricle. Squares in the DG, CA3, and CA1 indicate the possible location of place cells; squares in the EC are the possible location of grid cells.
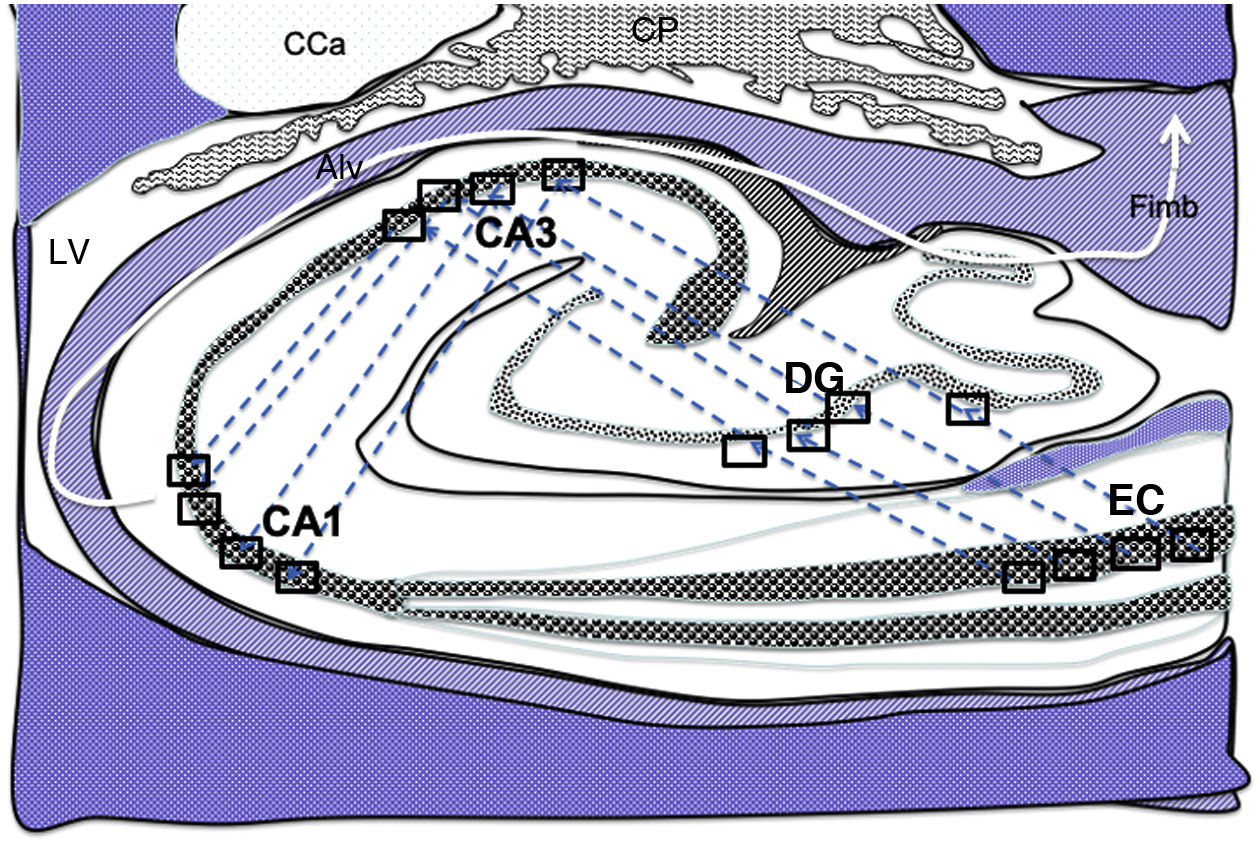
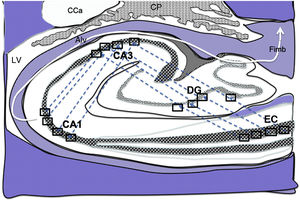
Diagram of a cross-section of the hippocampus at the level of the temporal horn of the right lateral ventricle. Arrows indicate indirect connections (blue dashed arrows) from the grid cells of the entorhinal cortex to the hippocampal place cells. These connections run first from the EC to the DG, then from the DG to CA1, from the CA1 to the CA3, and finally to the alveus and the fimbria (white arrows).31–34
Alv: alveus; CaT: tail of the caudate nucleus; CP: choroid plexi; DG: dentate gyrus; EC: entorhinal cortex; Fimb: fimbria; LV: lateral ventricle. Squares in the DG, CA3, and CA1 indicate the possible location of place cells; squares in the EC are the possible location of grid cells.
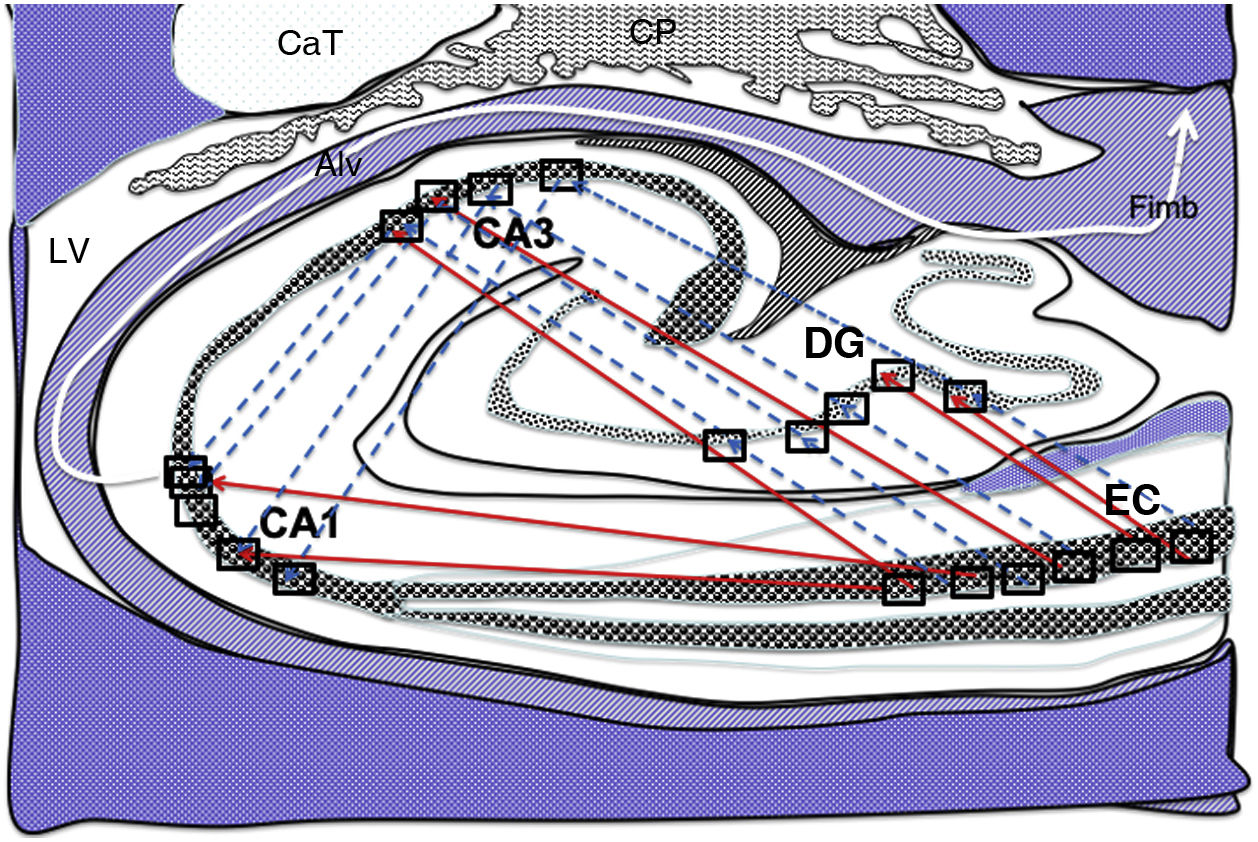
Diagram of a cross-section of the hippocampus at the level of the temporal horn of the right lateral ventricle. Arrows indicate both direct (red solid arrows) and indirect (blue dashed arrows) connections between grid cells of the entorhinal cortex and the place cells of the DG, CA1, and CA3.26–34
Alv: alveus; CaT: tail of the caudate nucleus; CP: choroid plexi; DG: dentate gyrus; EC: entorhinal cortex; Fimb: fimbria; LV: lateral ventricle. Squares in the DG, CA3, and CA1 indicate the possible location of place cells; squares in the EC are the possible location of grid cells.
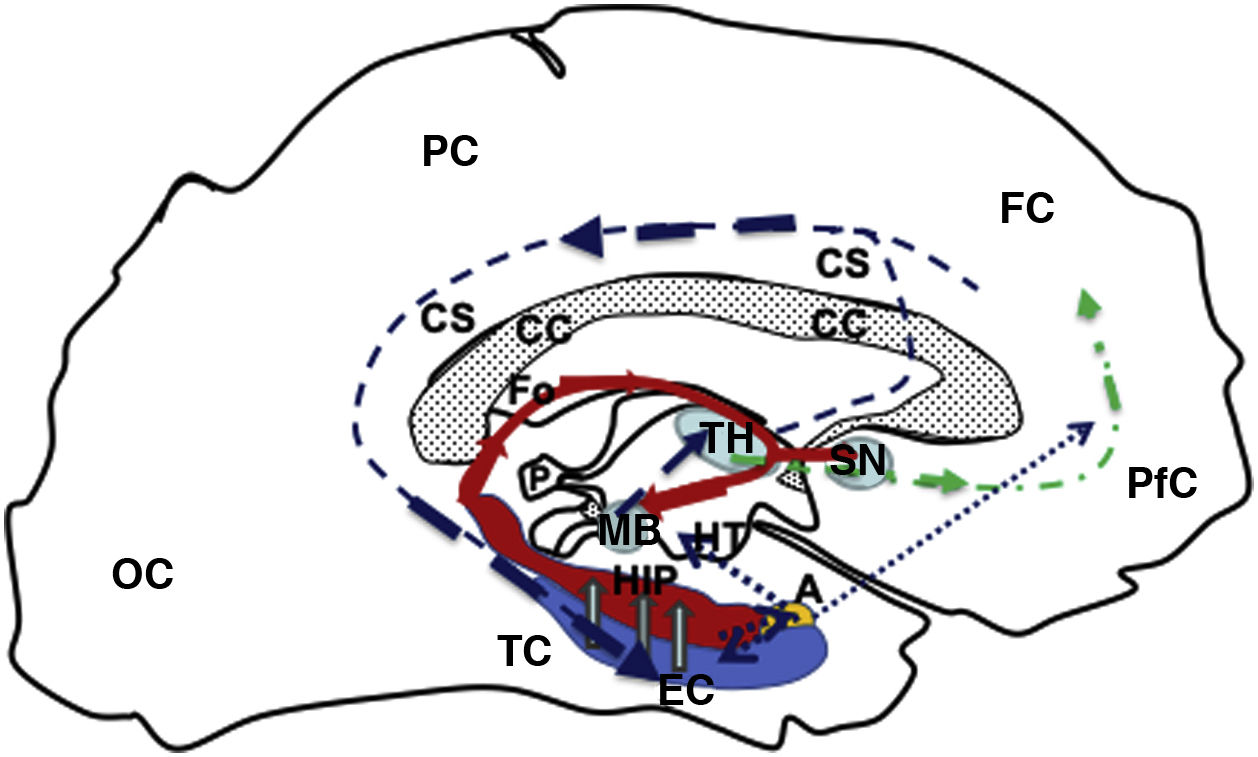
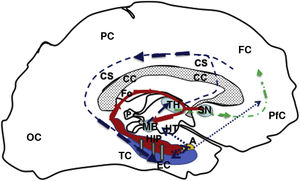
Diagram of a sagittal slice of the left telencephalon showing the Papez circuit. Modified from Kale and Frotscher.1
The circuit starts in the EC (blue), continuing (hollow grey arrows) towards the HIP (red), then via the fornix (red solid line) to the MB (blue), which is connected with the TH (blue) via the mammillothalamic tract (dark blue dashed line). From the TH, it connects with the PfC and CiC, and returns to the entorhinal cortex, closing the circuit (dark blue dashed line). Furthermore, the fornix (Fo) is connected with the contralateral Fo across the HC, and with the SN (ipsilateral and contralateral) via the WaC and PfC (green dashed and dotted line). Lastly, the hippocampus and entorhinal cortex connect with the amygdala, which in turn is connected with the HT and PfC (blue dotted line).
A: amygdala; CC: corpus callosum; CiC: cingulate cortex; EC: entorhinal cortex; FC: frontal cortex; Fo: fornix; HC: hippocampal commissure; HIP: hippocampus; HT: hypothalamus; MB: mamillary body; OC: occipital cortex; P: pineal gland; PC: parietal cortex; PfC: prefrontal cortex; SN: septal nuclei; TC: temporal cortex; TH: thalamus; WaC: white anterior commissure.
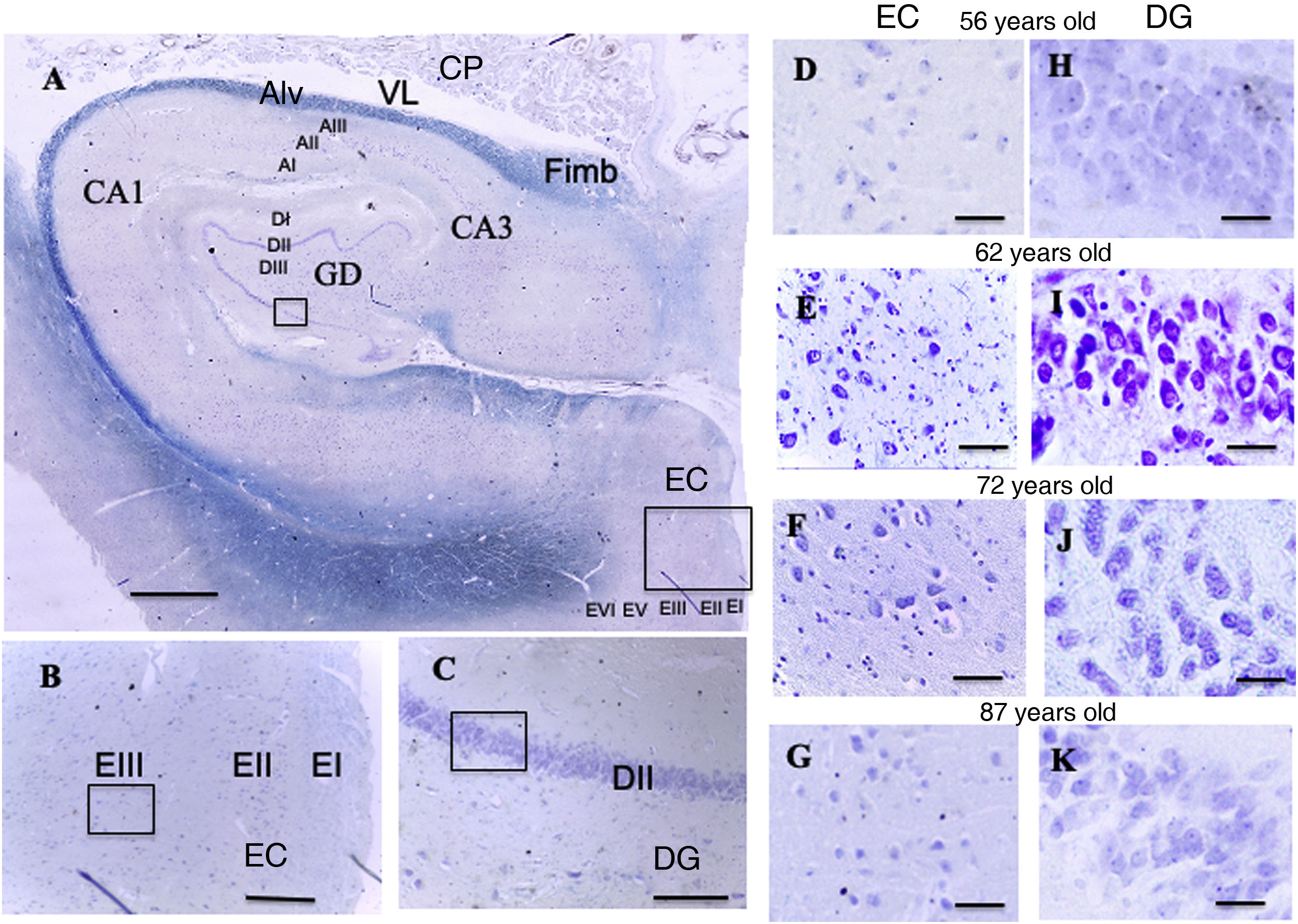
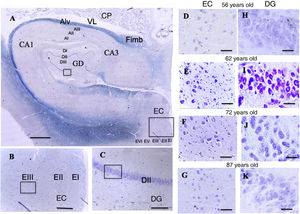
A: Cross-sectional slice of the hippocampus (dentate gyrus and cornu ammonis) and entorhinal cortex. Scale bar = 1000 µm. B: Detail of the EC in image A (scale bar = 250 µm); the small square shows the grid cells of lamina III. C: Detail of the DG in image A (scale bar = 250 µm); the small square shows the place cells of lamina III.
D, E, F, G: Photomicrographs of EC lamina III, showing the possible location of grid cells. H, I, J, K: Photomicrographs of DG lamina III showing the possible location of the place cells (scale bar = 50 µm).
AI: lamina I of the cornu ammonis; AII: lamina II of the cornu ammonis; AIII: lamina III of the cornu ammonis; Alv: alveus; CA1/CA3: subfields 1 and 3 of the cornu ammonis; CP: choroid plexi; DG: dentate gyrus; DI: lamina I of the DG; DII: lamina II of the DG; DIII: lamina III of the DG; EC: entorhinal cortex; EI: lamina I of the EC; EII: lamina II of the EC; EIII; lamina III of the EC; EV: lamina V of the EC; EVI: lamina VI of the EC; Fimb: fimbria; LV: lateral ventricle. Squares in the DG indicate the possible location of place cells; squares in the EC are the possible location of grid cells.
In the DG, there is a phenomenon known as long-term potentiation that consists in the increase of synaptic connections following prolonged, repeated high-frequency stimulation.2 Together with the olfactory bulb, the DG is also one of the places where the phenomenon of adult neurogenesis occurs. Some studies suggest the hypothesis that new memories may preferentially use recently formed dentate cells, which constitutes a possible mechanism for distinguishing multiple demands from similar events or multiple information arrivals at the same location.3 The DG includes 3 layers (polymorphic, granule cell, and molecular layers), and participates in the trisynaptic circuit.4 It receives afferents from the entorhinal cortex (EC) through the perforant pathway (mainly from lamina III) as it does not receive afferents from other cortical structures.5,6 DG efferents include axons from granule cells known as mossy fibres, which connect with pyramidal cells in the hippocampal CA3 region and subsequently with the CA1 region through the Schaffer collateral pathway.7,8
Furthermore, it is reported that in the DG, one of the locations where adult neurogenesis takes place, glucocorticoids and the action of the sympathetic nervous system (stressful situation) inhibit the neurogenesis process.9 There is also evidence that neurogenesis is increased by aerobic exercise.10 The DG is also involved in spatial memory: destruction of 90% of granule cells in the DG of rats caused the animals to present great difficulty in navigating an environment they already knew.11 These granule cells would correspond with place cells (PC), which are crucial in behavioural discrimination of similar spatial memories, predicting that PC in the DG change and remap their relative spatial tuning for memory discrimination.12
Entorhinal cortexThe EC (Figs. 1–5) is located in the medial part of the temporal lobe, between the subiculum and the perirhinal cortex13,14 and represents the main source of afferents and efferents of the hippocampus.2,13,14 The EC is especially relevant in recent memory processes and time perception, in which connections from its external pyramidal layer (lamina III) play a significant role, as they are the place of the main efferents of the perforant pathway to the DG. In turn, the EC receives efferents from the prefrontal, perirhinal, and parahippocampal cortices.15 The deep layers, mainly layer V, receive one of the 3 main hippocampal efferents and, in turn, reciprocal connections from other cortical areas projecting to the superficial EC. Neurons in the lateral EC show limited spatial selectivity.16 Neurons in the medial EC present several “place fields,” adopting a rectangular or hexagonal pattern, and known as grid cells.17–19
It has been suggested that the hippocampus initiates systems-wide mnemonic processes by reactivating previously acquired spatial and episodic memory traces, which can recruit the entorhinal cortex as an initial stage in the redistribution of memory to other brain areas. Hippocampal reactivation occurs during sharp wave-ripples, in which synchronous network firing encodes sequences of places.20 The coordination of this synchronisation and coding has been studied by simultaneous recording of the assembly activity in the hippocampal CA1 region and the superficial layers of the medial EC. Thus, it has been shown that entorhinal cell clusters may reproduce trajectories independently in the hippocampus, where sharp waves with spatio-temporally random and diverse grid cell spikes are observed.21 This suggests that the hippocampus is not the only initiator of reactivation of spatial and episodic memory tracing.20–23
In everyday life, memories of previous experiences are needed to plan future behaviour. These memories are represented by the activity of specific neuron groups or engrams.24,25 Neuron engrams cluster during learning by synaptic modification, and reactivation of the engram represents memorised experience.24 Engrams of conscious memories are initially stored for several days in the hippocampus, and are subsequently transferred to cortical areas.25 In the hippocampal DG, granule cells clustered in PCs12 convert rich inputs from grid cells of the EC to a sparse output, which is sent to the pyramidal cell network, made up of highly interconnected PCs in the hippocampal CA3 region. Thus, synaptic groups throughout the trisynaptic circuit of the hippocampus are constantly reassigned to support the formation of dynamic representations in hippocampal areas based on a stable code provided by the DG.26
It should be noted that the EC is the location of grid cells, which send connections to the PC groups of the DG (Figs. 1–3); these connections emit electric pulses in a regular pattern to map the movement of an animal; the crucial trisynaptic connection in the Papez circuit starts in the EC (Fig. 4)1; and that these structures are part of the functional elements of spatial and recent memory. In the light of these considerations, the aim of this study is to analyse the brains of 6 patients aged between 56 and 87 years to observe variations in the neurons contained in rectangular areas of lamina III of the EC (where grid cells may be located) and lamina II of the DG (where PCs are located).
The size of these square or rectangular areas, where the grid cells of the EC would be located and which are sometimes stretched or compressed and their positioning in the DG, has been analysed by several authors27–29; considering the findings of these authors and that there is no clear and defined size, we decided to use rectangular areas measuring 200 × 250 µ2.
Material and methodsSamples were obtained from the brains of 3 men aged 56, 59, and 66 years and 3 women aged 62, 73, and 87 years, who donated their brains for teaching and research purposes to the department of human anatomy and embryology of Universidad de La Laguna (Tenerife, Spain). In accordance with the department’s protocols, corpses were perfused with 5% formaldehyde at 24 hours after death. Brains were immediately extracted by removing the cranial vault; we dissected 6 medial temporal lobes containing the EC and hippocampus. Each lobe was divided into coronal sections along the anteroposterior axis, through the head and body of the hippocampus and the underlying EC. The study was approved and supervised by the ethics committee of Universidad de La Laguna.
The extracted specimens were fixed in 5% formol, dehydrated, and embedded in paraffin. They were cut into 10-μm serial sections, which were grouped into 4 alternating series (A, B, C, and D). We analysed 2 series of samples with Klüver-Barrera staining,30 a method that combines 2 stains: cresyl violet (basic) and luxol fast blue (with affinity for lipids of the myelin sheath). Myelin is stained blue and nuclei and Nissl bodies are stained violet. Image acquisition was performed with a Leica DM 1000 photomicroscope. All images were processed with Adobe Photoshop. We also used the Image J software (Java) for the analysis (cell counting, perimeter calculation, individual neuronal area, and total neuronal area of each grid) of the neurons contained in 7 grids measuring 200 × 250 µm, from several rostrocaudal sections from each specimen, at the level of lamina II of the EC and the granular layer of the DG.
Statistical analysisWe calculated the mean (and standard deviation) for normally distributed variables and the median (and interquartile range) for non–normally distributed variables. The Mann-Whitney U test was used for comparisons between the EC and the DG. To study the progression of the cell count as a function of area (EC or DG), sex, and age (< 65 or ≥ 65 years), we applied a general linear model with Poisson distribution and logarithmic link, whereas for the individual area and total neuronal area, we used a general linear model. In both models, we included the significant interactions between the different factors.
ResultsQualitative resultsDentate gyrus (Fig. 5, A and C)In the granule cell layer in the patients aged between 56 and 62 years, we observed a high density of granule cells with nuclei occupying a large part of the cytoplasm, as well as a central or peripheral nucleolus, with little variation between patients (Fig. 5, H and I).
In the lamina II of the patients aged between 66 and 87 years, we observed great density of granule cells showing large nuclei occupying a large part of the cytoplasm, with nucleoli showing a central or peripheral distribution. We observed small qualitative differences in these patients, which were more pronounced in the eldest patient (87-year-old woman), particularly in the granule cell layer, which qualitatively showed lower density of granule cells, with nuclei occupying a large part of the cytoplasm, and central nucleoli showing a preferentially central distribution (Fig. 5, J and K).
Entorhinal cortex (Fig. 5, A and B)In the patients aged between 56 and 62 years, we observed that the external pyramidal cell layer (layer III) contains groups of medium- and large-sized pyramidal neurons with a prominent nucleus occupying almost the entire cytoplasm, with disperse chromatin fibres and a single nucleolus in a central position. Small neurons were less densely distributed between them. We observed no qualitative differences when comparing ages within this group (Fig. 5, D and E).
In the youngest patient of the group aged between 66 and 87 years, similarly to the younger group, the external pyramidal layer showed a relatively low density of medium-sized neurons, with well-delimited nuclei occupying a large part of the cytoplasm. The other 2 patients showed more qualitative variations compared to younger patients: the external pyramidal layer in the 73-year-old woman showed a smaller population of pyramidal cells, whereas the 87-year-old woman showed a poorly delimited nucleus, with both central and peripheral location on different occasions (Fig. 5, F and G).
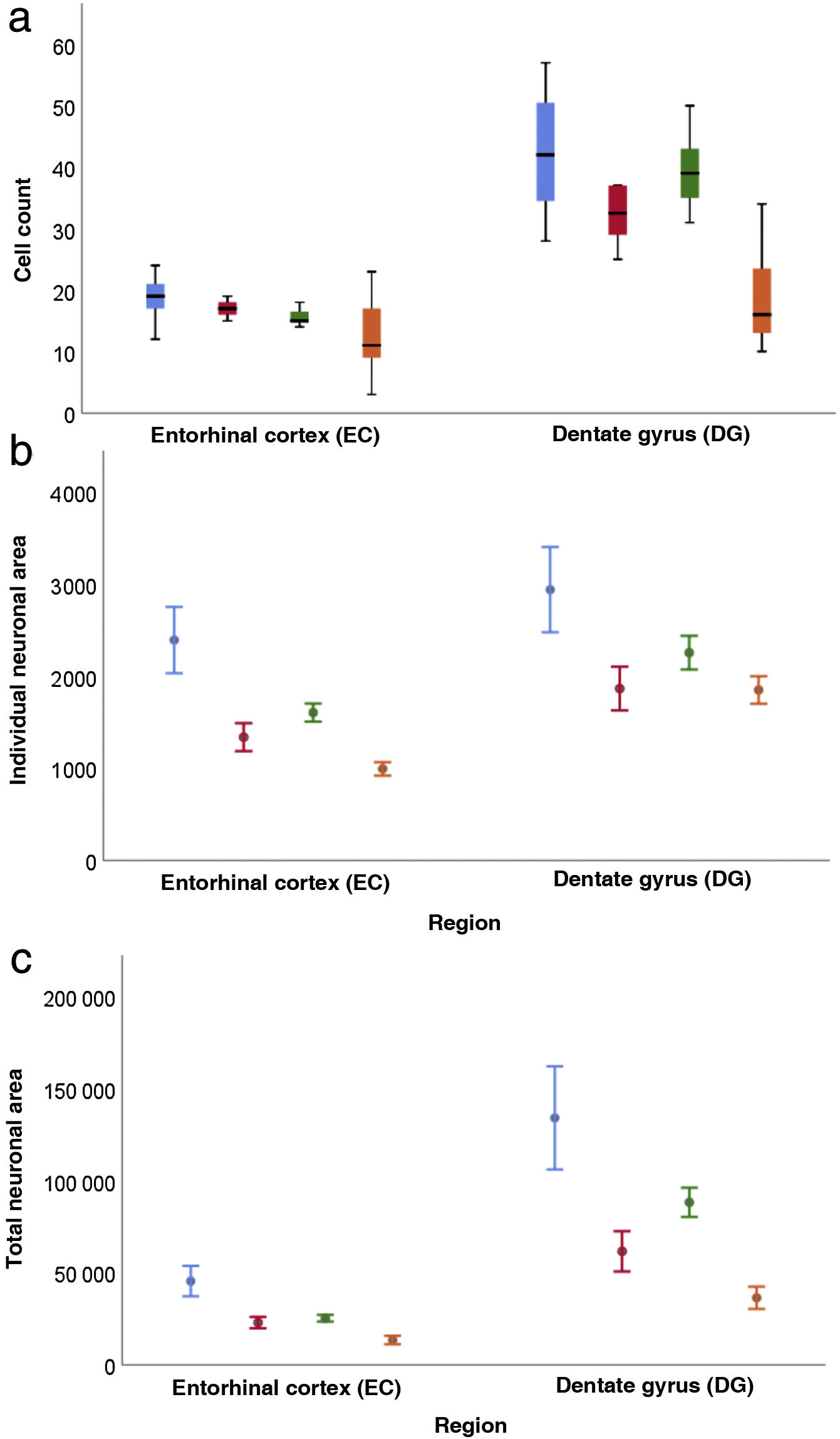
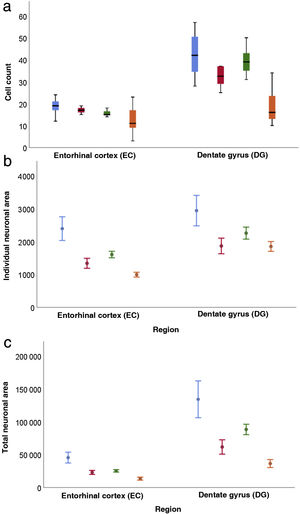
Quantitative resultsOur sample includes 3 men and 3 women with a mean age of 67.5 (11.36) years, with 3 of them being older than 65 (Table 1). Table 2 shows the descriptive statistical analysis of cell count, individual neuronal area, and total neuronal area in the 2 regions studied. In all these variables, values in the DG were significantly higher than in the EC (P < .001).
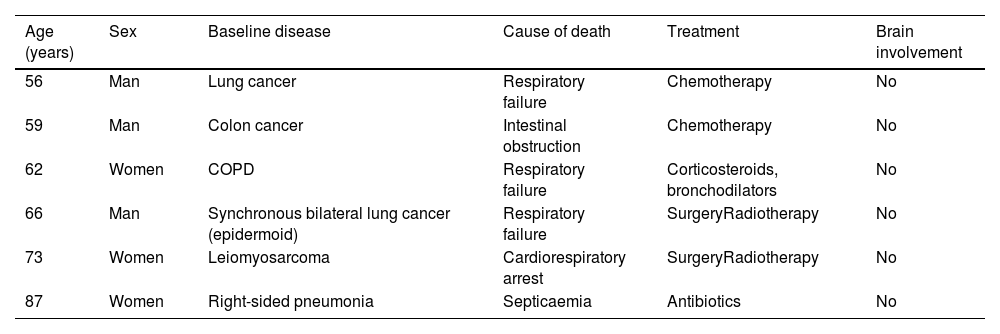
Distribution of cases according to age, sex, baseline disease, cause of death, treatment, and possible brain involvement.
| Age (years) | Sex | Baseline disease | Cause of death | Treatment | Brain involvement |
|---|---|---|---|---|---|
| 56 | Man | Lung cancer | Respiratory failure | Chemotherapy | No |
| 59 | Man | Colon cancer | Intestinal obstruction | Chemotherapy | No |
| 62 | Women | COPD | Respiratory failure | Corticosteroids, bronchodilators | No |
| 66 | Man | Synchronous bilateral lung cancer (epidermoid) | Respiratory failure | SurgeryRadiotherapy | No |
| 73 | Women | Leiomyosarcoma | Cardiorespiratory arrest | SurgeryRadiotherapy | No |
| 87 | Women | Right-sided pneumonia | Septicaemia | Antibiotics | No |
COPD: chronic obstructive pulmonary disease.
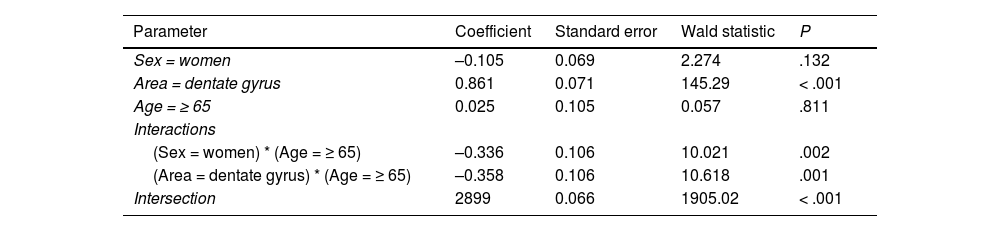
Regarding cell count (Table 3), the difference between the DG and the EC was smaller among patients older than 65 (P = .001); a decrease was also observed in both regions among women older than 65 (P = .002) (Fig. 6a).
Log-linear model for cell count.
| Parameter | Coefficient | Standard error | Wald statistic | P |
|---|---|---|---|---|
| Sex = women | –0.105 | 0.069 | 2.274 | .132 |
| Area = dentate gyrus | 0.861 | 0.071 | 145.29 | < .001 |
| Age = ≥ 65 | 0.025 | 0.105 | 0.057 | .811 |
| Interactions | ||||
| (Sex = women) * (Age = ≥ 65) | –0.336 | 0.106 | 10.021 | .002 |
| (Area = dentate gyrus) * (Age = ≥ 65) | –0.358 | 0.106 | 10.618 | .001 |
| Intersection | 2899 | 0.066 | 1905.02 | < .001 |
Both in the DG and the EC, the individual neuronal area (Table 4) presented the same structure depending on sex and age, with higher values in the DG (P = .002) and lower values in women (P = .045) and patients older than 65 years (P < .001) (Fig. 6b).
General linear model for individual neuronal area.
| Parameter | Coefficient | Standard error | 95% confidence interval | P |
|---|---|---|---|---|
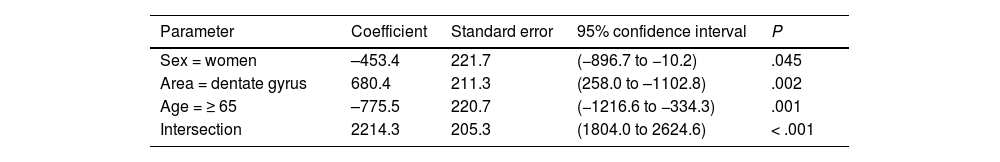
| Sex = women | –453.4 | 221.7 | (−896.7 to −10.2) | .045 |
| Area = dentate gyrus | 680.4 | 211.3 | (258.0 to –1102.8) | .002 |
| Age = ≥ 65 | –775.5 | 220.7 | (−1216.6 to −334.3) | .001 |
| Intersection | 2214.3 | 205.3 | (1804.0 to 2624.6) | < .001 |
Table 5 presents our results on total neuronal area, which includes the combination of results for individual neuronal area and cell count. Values were higher in the DG than in the EC (P < .001), and lower in women than in men (P = .034); the difference between DG and EC was smaller among patients older than 65 years (P = .020) (Fig. 6c).
General linear model for the total neuronal area.
| Parameter | Coefficient | Standard error | 95% confidence interval | P |
|---|---|---|---|---|
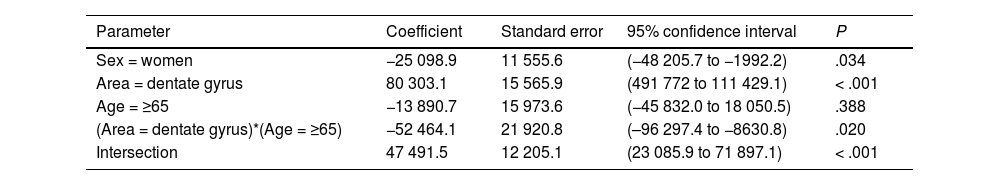
| Sex = women | −25 098.9 | 11 555.6 | (−48 205.7 to −1992.2) | .034 |
| Area = dentate gyrus | 80 303.1 | 15 565.9 | (491 772 to 111 429.1) | < .001 |
| Age = ≥65 | −13 890.7 | 15 973.6 | (−45 832.0 to 18 050.5) | .388 |
| (Area = dentate gyrus)*(Age = ≥65) | −52 464.1 | 21 920.8 | (–96 297.4 to −8630.8) | .020 |
| Intersection | 47 491.5 | 12 205.1 | (23 085.9 to 71 897.1) | < .001 |
These findings should unquestionably be considered with caution due to the limited number of samples and the narrow age range of our series.
DiscussionDiseases affecting memory and spatial navigation currently have a considerable impact on public health systems, which tends to grow as the population ages. As a result, it is essential to focus on researching these brain regions,31 such as the EC, hippocampus, and grid and place cells.
Grid cells, located in layer III (external pyramidal layer) of the EC,27,32 are the origin of afferents to PCs located in the DG and cornu ammonis of the hippocampus. However, the location and connections of these cells are subject to debate. Thus, on the one hand, PCs are located in the granule cell layer of the GD and in the pyramidal cell layers of the CA3, CA2, and CA1 regions, which are the destination of direct connections from the grid cells of the EC (Fig. 1).26,27,32,33 On the other hand, this contradicts other findings34–37 that support indirect connections such as the perforant pathway, trisynaptic connection, and the Papez circuit, in which connections would run between the EC and the DG, the DG and the CA3 region, and between the CA3 region and the CA1 region, via the Schaffer collateral pathway (Fig. 2).31–34 Indirect connections are the most broadly accepted theory; we may assert that both groups of authors are right, although indirect connections are more abundant than direct connections (Fig. 3). In any case, age-related changes occurring in the DG and the CA3 region may explain the increase in alterations associated with ageing, which also contribute to cognitive decline. However, it is yet to be determined whether this increase in the susceptibility to such alterations as seizures or cognitive impairment is the cause or the effect of age-related changes in these structures.38–42 For instance, a study using MRI techniques observed that atrophy in the CA1 region and DG is more marked during ageing.38
Previous studies38–42 partially agree with our own, as we observed a significant decrease in the number and size of granule cells of the DG. Another study using diffusion MRI described increased atrophy in the CA2 and CA3 regions and the DG in line with age.42 However, another study reports that this atrophy was observed in the subiculum but not in the DG or the CA1 region.43
There is no doubt that the majority of authors have observed an association between advanced age and decreased size or involvement of the CA3 region and the DG.38,43,44 Therefore, the changes observed may contribute to the memory deficits observed with age, affecting the processing of new and recent information but reinforcing the processing of previously stored information.
Our results in the DG may confirm the findings described by several other authors in the different parts of the hippocampus. Therefore, ageing clearly seems to affect the DG and the hippocampus in general, causing a decrease in the area occupied by PCs in the areas studied.
In Alzheimer disease, a progressive neurodegenerative disease responsible for 60% to 80% of all cases of dementia in the elderly population worldwide, getting lost is one of the first cognitive symptoms observed in these patients. Early onset of memory impairment initially occurs in the EC and subsequently extends to the hippocampus, which makes the location of the entorhinal-hippocampal connection, ie from grid cells to PCs, the most vulnerable region; marked neuronal loss of both grid cells and PCs has been described in mouse models of Alzheimer disease.45 Furthermore, it has been shown in recent decades that postnatal neurogenesis46,47 is a process that lasts until adulthood and persists into old age, even in patients with Alzheimer disease.48 Furthermore, voluntary physical exercise increases neurogenesis in the DG in adult rats.10,49 Very importantly, newly-formed neurons have been shown to migrate to injured areas, which are the origin of nerve pathways, playing an important role in learning and memory processes.50 Considering both our results and those of previous authors, and regardless of the degree of neurogenesis in our patients, we may assert that there is a quantitative neuronal loss in advanced ages.
The location of hippocampal cells represents a model of how the brain builds cognitive representations and of how these representations support complex behaviour, learning, and memory.16,49,50 In this line, studies with rats have shown that several of these individual units of location, the hippocampal neurons, are mainly related to the grid cells of the medial EC.16,51 Furthermore, based on the results of experiments with robotic platforms, an entorhinal-hippocampal model has been proposed that can successfully build cognitive maps; neurobiological experiments have also inspired the development of additional cognitive mapping systems.49,50 Furthermore, it has been suggested that these grid cells from neuron populations in the lateral EC inherently represent time through experience coding. This representation of episodic time may be integrated with spatial inputs from the medial EC of the hippocampus, enabling the hippocampus to store a unified representation of what, where, and when.15
There is currently growing debate on the effect of ageing on the EC, as until recently, studies on ageing placed greater emphasis on the DG and the different regions of the cornu ammonis. In our study, we aimed to describe the effects of ageing not only in the DG, but also to emphasise effects on the EC (layer III), which is the origin of efferents from the trisynaptic pathway and/or Papez circuit. Thus, an age-related decrease in the connections between the EC and the DG is reported, as a consequence of the loss of density of dendritic spines of granule cells in the DG44 (location of the afferents of the perforant pathway). Another study in humans using MRI reported that the thickness of the EC varies throughout life: it initially increases, reaching its peak at 44 years, and subsequently decreases with age.52
The results of these studies on the decrease in EC connections and thickness would be supported by our study, which shows a high sensitivity of neurons in the EC to ageing, with clear qualitative alterations in an 87-year-old patient. We also observed quantitative alterations revealing a decrease in the number of neurons in the grids studied, in which the compensatory neuronal hypertrophy did not prevent the loss of neuronal density in grids.
ConclusionsWe may conclude that, in our cases, qualitative and quantitative alterations were observed both in the EC and the DG, peaking at the eldest age studied (87 years). These alterations are located in the rectangular areas studied, which may correspond to the grid cells of the EC and PCs of the DG. In general, we may assert that our findings, in a limited sample, are reasonably consistent with those reported in the literature on the negative effects of ageing on the EC and DG.
FundingThis study received funding from the Canarian Institute of Research and Science (INIPRO). Spain; project no. 03/14
Conflicts of interestThe authors have no conflicts of interest to declare.