Wernicke's encephalopathy (WE) is a neurological disease caused by thiamine deficit. In some autopsy series, its incidence rates range from 0.4% to 2.8%,1 and prevalence is especially high among susceptible populations, including patients with alcoholism. It is estimated that 70% of all cases are not diagnosed until severe complications have appeared; the mortality rate approaches 17%.
Although alcoholism is its most frequent cause, WE has also been described in patients with malnutrition, hyperemesis, a history of bariatric surgery, cancer, and parenteral nutrition. It may not be detected in these situations, which can lead to serious complications.2 This letter presents an exceptional case of WE secondary to hyperthyroidism and consumption of foods rich in thiaminase.
We present the clinical case of a 54-year-old woman referred to the Emergency Department with a 3-week history of asthenia and vomiting. During that time she had been ingesting little more than water and tea. The patient reported episodic palpitations, heat intolerance, dyspnoea, and double vision that had appeared 3 days previously, in addition to gait instability. Personal history was anodyne; she lived alone and neither the patient nor family members reported alcohol abuse.
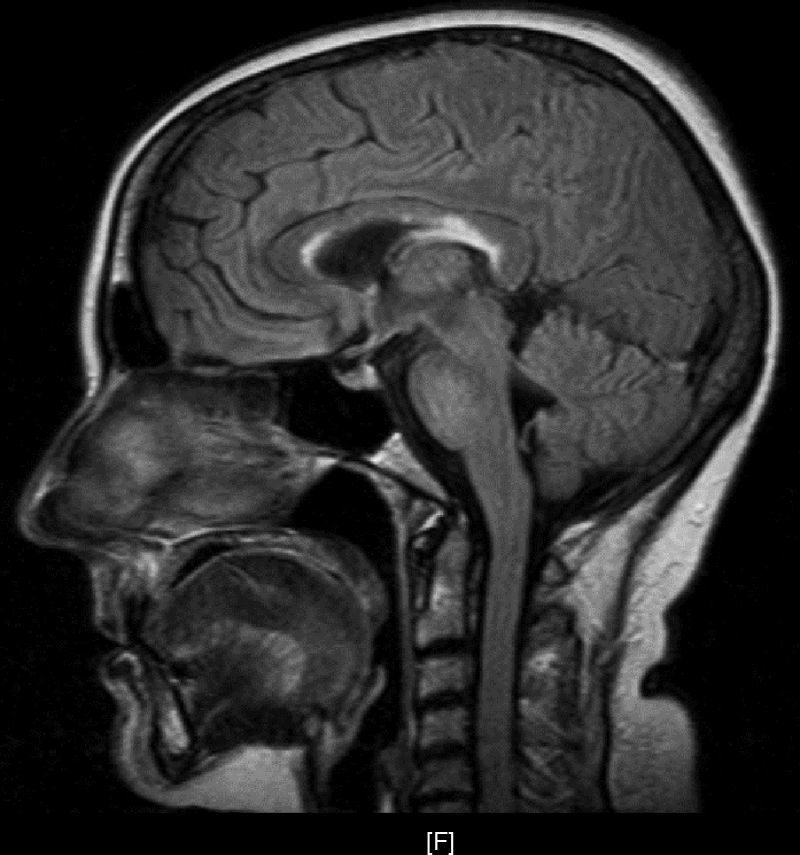
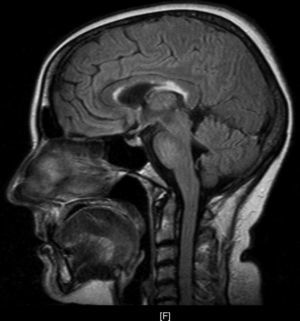
Upon her arrival at the Emergency Department, BP was 154/106 and heart rate was 150bpm. The patient presented convergent strabismus with bilateral miosis, bilateral sixth cranial nerve palsy, and horizontal nystagmus, plus ataxia and abolished patellar reflexes. In the first hours following admission, as the patient was being treated with glucose solution, her level of consciousness decreased and the ataxia and ophthalmoparesis worsened. Complementary testing revealed the following values: K 2.8mmol/L, Ca 11.72mg/dL, GOT 193U/L, GPT 199U/L, PTH 9, TSH<0.05, T4L 2.01; antithyroid peroxidase AB>600; tumour markers and HIV, HVA, HVB, and HVC serology tests all negative. ECG showed supraventricular tachycardia with a rate of 140bpm. Ultrasound revealed a thyroid of borderline size with no nodules. Echocardiography, abdominal ultrasound, gastroscopy, and brain and thoracic-abdominal CTs showed no significant findings. The MRI scan (Fig. 1) revealed deep periventricular alterations, demyelinating lesions in the white matter in predominantly frontal bilateral locations, and well-circumscribed plaques of demyelination in the corpus callosum and pons. All of these signs are compatible with WE.
The patient's clinical condition improved with parenteral thiamine plus metamizole and beta-blockers to correct thyroid alterations. Nystagmus and dyplopia resolved and ataxia improved progressively; the patient achieved full recovery. Water–electrolyte imbalances, transaminase levels, and thyroid hormone levels all reverted to normal and the lesions visible in imaging studies resolved almost completely. Symptoms were compatible with WE due to hyperthyroid crisis and consumption of thiaminase-rich beverages.
WE consists of the classic triad of ophthalmoparesis with ataxia and confusional syndrome, although only a third of all patients present the 3 signs simultaneously. Altered mental status presents in 82% of all patients, with manifestations that include disorientation, apathy, lethargy, confusion and agitation, memory impairment, and even stupor and coma. In contrast, ophthalmoparesis presents in 29% of cases and includes horizontal nystagmus, paralysis of the lateral rectus muscles with diplopia and internal strabismus, conjugate gaze palsy, ptosis in rare cases, and miosis in long-standing processes. Ataxia appears in 23% of cases with unstable tandem gait, short strides, and increased base of support. Other signs may include polyneuropathy as a manifestation of nutritional deficiency, and tachycardia with postural hypotension due to autonomous nervous system disorders or cardiac beriberi. Diagnosis is basically clinical, and the disease is reversible with administration of thiamine. MR imaging studies provide additional information with a low sensitivity of about 53% and a higher specificity of 93%. Studies show increased T2-weighted signal intensity in the periventricular area, mamillary bodies, and periaqueductal grey matter, as well as demyelinating lesions in the cortex and corpus callosum.3
Treatment consists of administering intravenous thiamine. Ophthalmoparesis was the first manifestation to improve (resolving over a few hours except for nystagmus), followed by ataxia within a few days (40% recovery) and state of consciousness (improving in 2–3 weeks). Our review of the literature yielded few cases of Wernicke's encephalopathy associated with or secondary to hyperthyroidism.4–9 Most cases were associated with hypercatabolism and increased nutritional needs. Thiaminases are known to be present in raw fish, shellfish, tea, and coffee. These enzymes act as natural thiamine antagonists and may have contributed to the development of WE in our patient, who had been consuming little more than tea in the preceding weeks.10
In conclusion, the case was diagnosed as WE secondary to hyperthyroidism that was probably exacerbated by the thiaminases present in tea. The case we present is interesting as it demonstrates that symptoms compatible with WE may develop without the classic antecedents of malnutrition or alcoholism.
Pleas cite this article as: Ventura A, et al. Encefalopatía de Wernicke secundaria a hipertiroidismo e ingesta de productos ricos en tiaminasas. Neurología. 2013;28:257–9.