Cockayne syndrome (CS) is a rare autosomal recessive disease caused by biallelic mutations in ERCC6 (excision repair 6, chromatin remodeling factor) (CS type B [CSB]) or ERCC8 (excision repair 8, chromatin remodeling factor) (CS type A [CSA]) genes showing significant genotypic and phenotypic heterogeneity.1 It manifests as a progressive multisystem disorder, with multiorgan dysfunction related to defective DNA repair and transcription, characterized by psychomotor retardation, cerebral atrophy, microcephaly, mental retardation, sensorineural hearing loss, premature aging, cachectic dwarfism, and other defects such as cutaneous photosensitivity, kyphosis, ankylosis, and optic atrophy.1,2
In 2010, major diagnostic criteria (e.g., microcephaly) and minor diagnostic criteria (e.g., photosensitivity) were defined.3 These criteria can neither help in early disease detection nor are specific to CS.4,5 Most signs and symptoms in CS patients (e.g., cerebral atrophy) may also be present in mitochondrial diseases.6,7 The clinical and genetic spectrum of CS also keeps ever-expanding.4,8,9
We report a CSB case from India with axonal sensorimotor polyneuropathy caused by a de novo mutation c.422+5G>A in the ERCC6 gene, representing a novel phenotypic and genotypic variant, respectively.
A 14-year-old girl from rural West Bengal, India, born to second-degree consanguineous parents, was brought by her mother as an outpatient with complaints of failure to gain weight and height, poor scholastic performance, and progressive emaciation, easy sun-burning, and progressive freckling and tanning of the skin.
She was born at term with a weight of 3.0kg and no history of perinatal asphyxia. She had not attained menarche to date. Parents and younger male siblings had no similar problems. However, her elder brother has epilepsy, and her father has gross undiagnosed neuropsychiatric problems for which he was dependent on others’ assistance for activities of daily life.
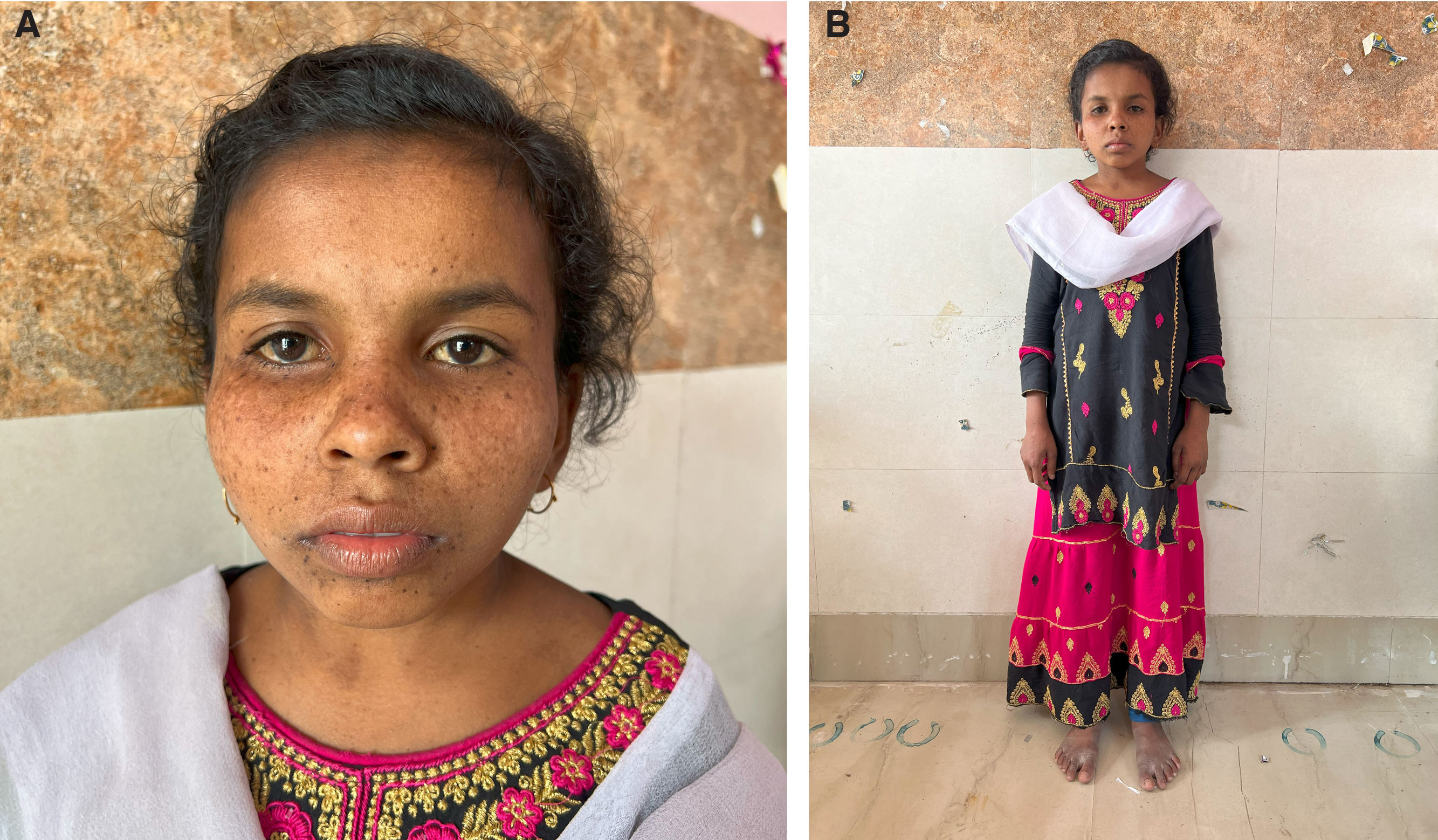
General examination revealed an inappropriate senile appearance with microcephaly, triangular face, deep-set sunken eyes, beaked nose, and micrognathia. The patient was cachectic, with short stature (Fig. 1), dry skin, hyperpigmentation, excessive freckling over the face (Fig. 2), extensive dental caries, and typical secondary sexual characteristics except for mildly underdeveloped breasts.
Examination reveals (A) a short-statured girl with extreme cachexia, microcephaly, senile look, loss of subcutaneous fat, and pinched nose, giving her a characteristic ‘Mickey Mouse’ appearance. The patient had hyperpigmented brown macules over the malar area with photosensitivity and scarring (B).
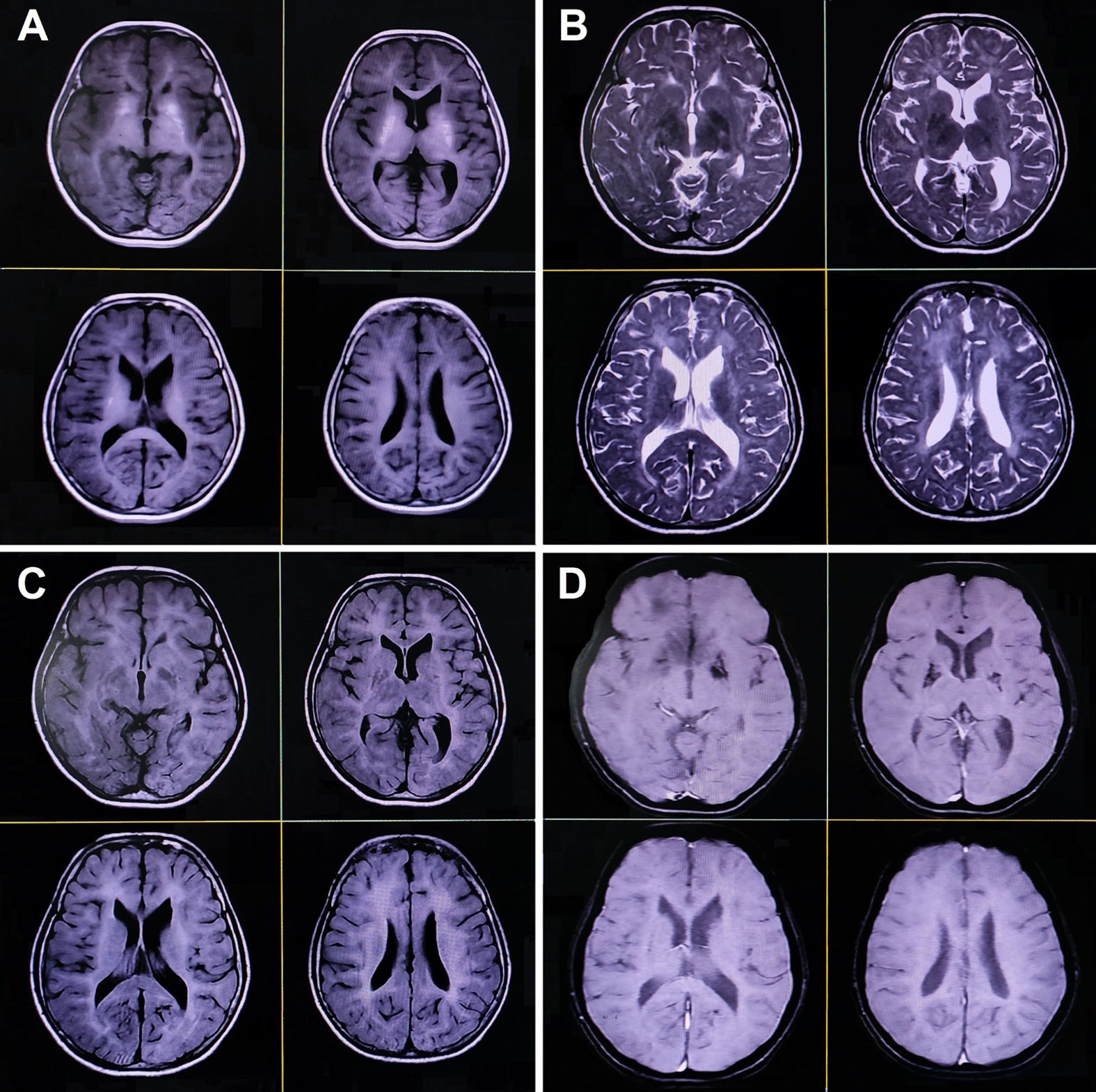
Magnetic resonance imaging of the brain reveals symmetrical hypomyelination of the hemispheric periventricular white matter with early ventricular dilatation, calcification of bilateral basal ganglia and thalamus, and dysgenesis of the corpus callosum (axial T1-weighted [A], T2-weighted [B], fluid-attenuated inversion recovery [C], and gradient echo sequences [D]).
A neurologic examination revealed she had mental retardation with an intelligence quotient of 80. Ophthalmological and fundus examinations were normal. There was no cranial nerve, motor, sensory, autonomic, or cerebellar deficit except for symmetric hyporeflexia-to-areflexia (more than lower limbs). Complete blood cell count and metabolic-endocrinological panel were within acceptable normal range. Pure-tone audiometry and brainstem-evoked response audiometry studies were normal. A brain computed tomography scan revealed early cortical atrophy with extensive calcifications in the cerebellum, basal ganglia, and periventricular regions. Brain magnetic resonance imaging showed symmetrical (slightly parieto-occipital predominant) hypomyelination of the hemispheric periventricular white matter with early ventricular dilatation, basal ganglia, thalamus, and dentate calcification, dysgenesis of the corpus callosum, and mild cerebral and cerebellar atrophy with shrunken folia and enlarged cisterna magna (Fig. 2). Nerve conduction studies showed axonal sensorimotor polyneuropathy with a low amplitude of sensory nerves (right median 6.16μV; left median 6.97μV; right ulnar 4.59μV; left ulnar 5.31μV; right sural 1.89μV; left sural 1.48μV) and motor nerves (right median 2.4mV distal/2mV proximal; left median 1.7mV distal/1.2mV proximal; right ulnar 2.3mV distal/2mV proximal; left ulnar 2mV distal/1.6mV proximal; right tibial 1.3mV distal/1mV proximal; right common peroneal 1.2mV distal/1.0mV proximal; and left common peroneal without response). The amplitude of the left tibial nerve was normal (6.1mV). The latency and conduction velocity of all the nerves and needle electromyography findings were otherwise normal. The results of electrocardiography, 2D and M-mode echocardiography, and abdominal ultrasonography were also normal.
A homozygous splice site region variant c.422+5G>A in exon 2 of the ERCC6 gene was identified on whole exome sequencing. The observed variant has a minor allele frequency of 0.00000% in gnomAD exomes and genomes. A diagnosis of CSB was finally made. Genetic counseling was offered to her family. However, it could not be done due to a lack of financial resources.
CS is a progressive multisystem disease with a specific cellular defect in transcription-coupled repair.1,2 There are various degrees of severity: the “classical” or moderate (type I CS), the early-onset or severe (type II), and the mild or late-onset (type III).1,2 The most severe cases (CS type II) often occur because of CSB mutations, whereas CSA mutations are usually linked to the milder form, CS type I.1,2 However, this association is not always straightforward, e.g., CSB mutations can also trigger the mild form.1,2 These overlapping subtypes constitute a continuum without clear limits. Two-thirds of the patients are linked to mutations in the CSB (ERCC6) gene (chromosome 5q11) and one-third in the CSA (ERCC8) gene (chromosome 10q11).3,8
CS belongs to the family of pathologies related to the nucleotide excision repair pathway. This pathway removes ultraviolet radiation and chemically induced DNA lesions.10 Deficiencies in nucleotide excision repair proteins can cause various diseases, ranging from ultraviolet radiation-sensitive syndrome to cancer-predisposing xeroderma pigmentosum, trichothiodystrophy, and severe premature aging diseases such as CS.10 Its incidence in Europe is approximately one case per 200,000 births.11
ERCC6 encodes a DNA-binding protein that is important in transcription-coupled excision repair. Mutations in this gene are associated with CSB and cranio-oculo-facio-skeletal syndrome.1 Phenotype/genotype correlation is difficult to establish.1,12 Clinical photosensitivity is detected in only a fraction of patients, with various mutations, and is mainly uncoupled from the severity of the disease.1,7
Phenotypic variability represents a challenge for establishing a diagnosis and supportive treatment. Besides, patients have a short life span (generally less than five years for the severe forms and 16 years for the moderate phenotypes)13. Therefore, next-generation sequencing technologies play a crucial role in the molecular diagnosis of such cases.1
CS epidemiological data in India are grossly inadequate. In a recent prospective study conducted in six Indian families, one family had a homozygous mutation in ERCC8, and the other five had homozygous mutations in ERCC6. Novel variants in ERCC6 were identified in four families, but no one had our novel mutation.9 The peripheral nervous system often gets involved in some DNA repair disorders. CSA and CSB are classically linked to progressive demyelinating-type polyneuropathy that correlates with clinical severity.14
There are only three previously reported possible CS cases with axonal polyneuropathy,15,16 but genetic testing was not performed in two of them.15 In the third case,16 mutation analyses demonstrated one novel heterozygous missense mutation in CSB, c.3806A>C (p.D1269A).16 In this case,16 nerve conduction studies demonstrated an axonal sensory (with no motor component) polyneuropathy involving only lower limbs (no response of both sural nerves). The rest of the nerves’ latency, amplitude, and conduction velocity was normal (sensory: right median and superficial peroneal; motor: right common peroneal and tibial). In addition, this patient developed a mild coarse postural tremor bilaterally and a shuffling gait with bradykinesia, which could have originated from basal ganglia lesions, neuropathy, or both, successfully managed with oral carbidopa–levodopa.16 By contrast, our patient had an axonal sensorimotor polyneuropathy involving the upper and lower limbs with no tremor.
Our patient would be the first reported confirmed CSB case with axonal sensorimotor polyneuropathy caused by a de novo ERCC6 mutation c.422+5G>A, constituting a novel phenotypic and genotypic variant, respectively.
The current case also highlights the importance of (1) knowledge regarding phenotypic and genotypic CS heterogeneity; (2) carefully understanding/interpreting neuroimaging characteristics of adolescent white matter disease patterns; and (3) asking for electrodiagnostic and genetic tests in case of a strong clinical suspicion of CSB for diagnostic confirmation.
Early recognition of clinical conundrums could help achieve a prompt diagnosis, proper supportive management, and genetic counseling. Prenatal diagnosis can be achieved only if molecular diagnosis is established in the proband. Finally, further prospective studies are warranted to analyze symptoms and prognosis associated with this novel ERCC6 gene mutation, including axonal sensorimotor polyneuropathy as a phenotypic marker.
CRediT authorship contribution statementAll authors contributed significantly to the creation of this manuscript; each fulfilled criterion as established by the ICMJE.
Informed consentWritten informed consent was obtained from the patient's parents prior to participation in the study (consent for research).
FundingNone declared.
Declaration of competing interestThe authors report no relevant disclosures.
J. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD – platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451).





![Magnetic resonance imaging of the brain reveals symmetrical hypomyelination of the hemispheric periventricular white matter with early ventricular dilatation, calcification of bilateral basal ganglia and thalamus, and dysgenesis of the corpus callosum (axial T1-weighted [A], T2-weighted [B], fluid-attenuated inversion recovery [C], and gradient echo sequences [D]). Magnetic resonance imaging of the brain reveals symmetrical hypomyelination of the hemispheric periventricular white matter with early ventricular dilatation, calcification of bilateral basal ganglia and thalamus, and dysgenesis of the corpus callosum (axial T1-weighted [A], T2-weighted [B], fluid-attenuated inversion recovery [C], and gradient echo sequences [D]).](https://static.elsevier.es/multimedia/02134853/unassign/S0213485325000131/v2_202502170403/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

