NEW DEVELOPMENTS IN HEADACHE AND INTRACRANIAL HYPERTE
Más datosTrigeminal neuralgia (TN) is a chronic neuropathic pain disorder affecting one or more branches of the trigeminal nerve. Despite its relatively low global prevalence, TN is an important healthcare problem both in neurology departments and in emergency departments due to the difficulty of diagnosing and treating the condition and its significant impact on patients’ quality of life. For all these reasons, the Spanish Society of Neurology’s Headache Study Group has developed a consensus statement on the management of TN.
DevelopmentThis document was drafted by a panel of neurologists specialising in headache, who used the terminology of the International Headache Society. We analysed the published scientific evidence on the diagnosis and treatment of TN and establish practical recommendations with levels of evidence.
ConclusionsThe diagnosis of TN is based on clinical criteria. Pain attributed to a lesion or disease of the trigeminal nerve is divided into TN and painful trigeminal neuropathy, according to the International Classification of Headache Disorders, third edition. TN is further subclassified into classical, secondary, or idiopathic, according to aetiology. Brain MRI is recommended in patients with clinical diagnosis of TN, in order to rule out secondary causes. In MRI studies to detect neurovascular compression, FIESTA, DRIVE, or CISS sequences are recommended. Pharmacological treatment is the initial choice in all patients. In selected cases with drug-resistant pain or poor tolerance, surgery should be considered.
La neuralgia del trigémino (NT) es un tipo de dolor neuropático que afecta a una o más ramas del nervio trigémino. Aunque su prevalencia poblacional es relativamente baja, la NT supone un problema muy importante tanto en las consultas de neurología como en las urgencias por la dificultad para el diagnóstico y tratamiento y el elevado impacto sobre la calidad de vida de las personas que la padecen. Por estos motivos, el Grupo de Estudio de Cefaleas de la Sociedad Española de Neurología ha elaborado un documento de consenso sobre el manejo de esta patología.
DesarrolloEste documento ha sido redactado por un comité de expertos utilizando la nomenclatura de la clasificación de la International Headache Society (IHS), analizando la evidencia científica publicada sobre diagnóstico y tratamiento y estableciendo unas recomendaciones prácticas con niveles de evidencia.
ConclusionesEl diagnóstico de la NT es clínico. La International Classification of Headache Disorders en su tercera edición (ICHD-3) clasifica el dolor atribuible a una lesión o enfermedad del nervio trigémino en NT y neuropatía trigeminal dolorosa. A su vez la NT puede dividirse en tres tipos principales según la etiología del dolor: clásica, idiopática y secundaria. Es recomendable la realización de una resonancia magnética (RM) craneal a todo paciente con diagnóstico clínico de NT para descartar causas secundarias. Para estudiar la presencia de una compresión neurovascular con RM se recomienda la aplicación de los protocolos de imagen FIESTA, DRIVE o CISS. El tratamiento inicialmente será farmacológico y en pacientes seleccionados con respuesta insuficiente o mala tolerancia a fármacos se debe valorar el tratamiento quirúrgico.
Trigeminal neuralgia (TN) is facial pain attributable to a lesion or disease of the trigeminal nerve, affecting at least one branch, and is characterised by brief pain attacks described as sharp, stabbing, or electric shock–like. Despite the existence of well-defined diagnostic criteria, many patients with intense facial pain with no clear underlying cause are misdiagnosed with TN. Conversely, TN is often mistaken for pain originating in the teeth, leading to unnecessary examinations and treatments.
The Spanish Society of Neurology’s Headache Study Group (GECSEN) drafted this consensus document on TN based on the results of a systematic literature review and our own clinical experience. The aim of this review was to provide a series of practical recommendations for the correct diagnosis and treatment of TN. Levels of evidence and grades of recommendation of therapeutic approaches are rated according to GECSEN’s 2020 clinical practice guidelines for headache.
Anatomy and pathophysiologyAnatomy of the trigeminal nerveThe trigeminal is a mixed nerve, although sensory fibres are clearly predominant. Nuclei extend throughout the brainstem, from the midbrain to the first cervical spinal segments. The motor nucleus and chief sensory nucleus are located in the middle pons, and the spinal nucleus, which receives information on pain and temperature, extends from the pons to the upper spinal cord. The motor root originates in the masticator motor nucleus, emerging from the lateral pons, anteromedial to the sensory root, and joining the third branch of the nerve. It subsequently traverses the skull through the foramen ovale to innervate the muscles of mastication (masseter, temporalis, and medial and lateral pterygoid muscles), as well as other muscle groups with different functions (mylohyoid, anterior belly of the digastric, tensor veli palatini, and tensor tympani). The sensory root thickens, forming the Gasserian ganglion, which contains the somas of sensory neurons.1
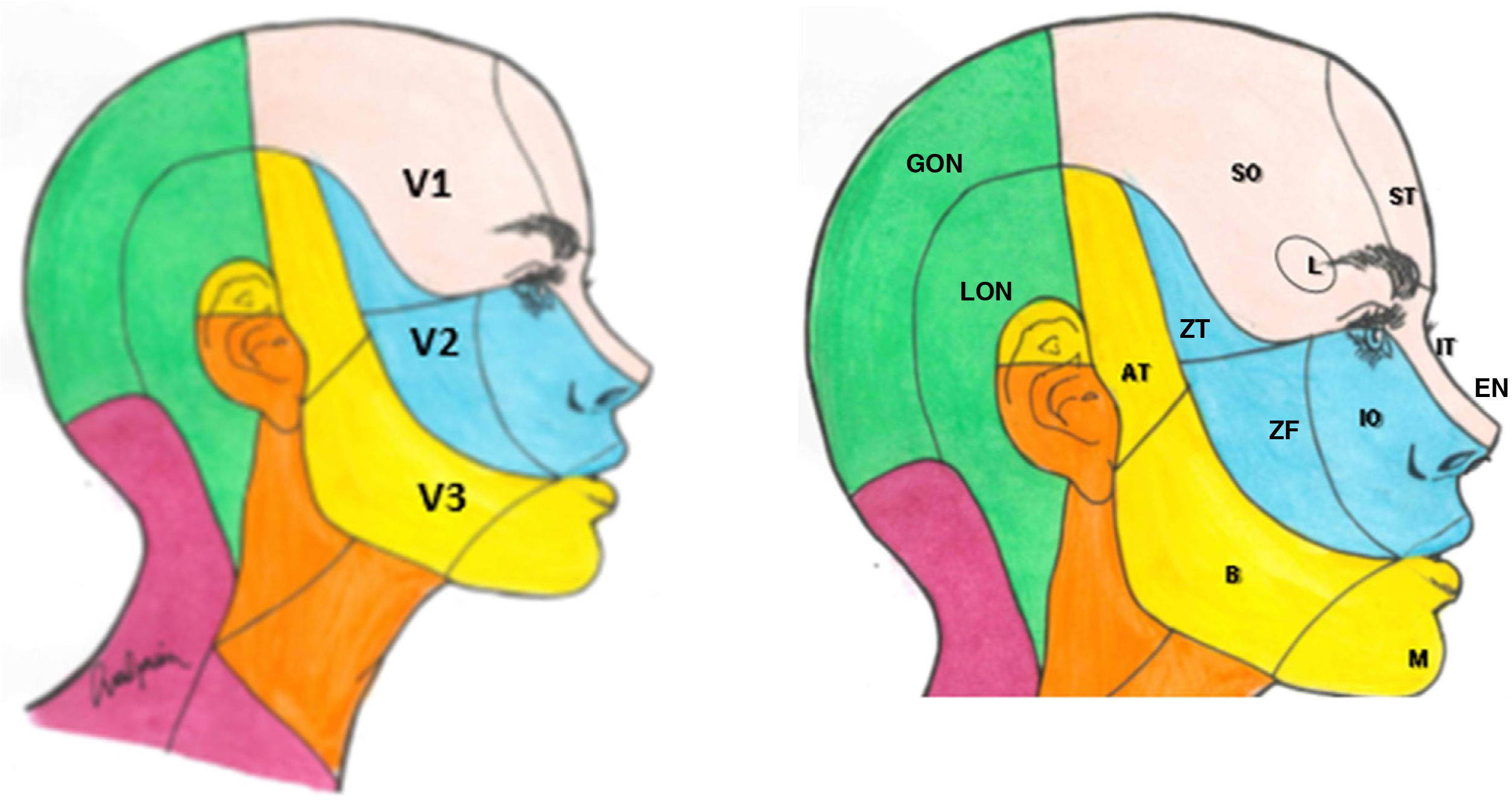
The sensory component has 3 divisions: the ophthalmic (V1), maxillary (V2), and mandibular branches (V3). The ophthalmic branch innervates the skin of the upper part of the nose (bridge, sides, lateral wall of the nasal cavity, and septum), forehead, upper eyelid, orbit, and lacrimal gland. The maxillary branch innervates the zygomatic area, nasal wings, upper lip, the gums of the upper dental arch, palate, nasopharynx, posterior nasal cavity, and the meninges of the anterior and middle cranial fossa. Finally, the mandibular branch innervates the buccal mucosa, temple and lateral scalp, external auditory meatus, tympanic membrane, temporomandibular joint, mandible and lower dental arch, the 2 anterior thirds of the tongue, lower lip, and chin (Fig. 1).1,2
Dermatomes of each branch of the trigeminal nerve.
V1: first branch (ophthalmic); V2: second branch (maxillary); V3: third branch (mandibular).
AT: auriculotemporal nerve; B: buccal nerve; EN: external nasal nerve; GON: greater occipital nerve; IO: infraorbital nerve; IT: infratrochlear nerve; L; lacrimal nerve; M: mental nerve; LON: lesser occipital nerve; SO: supraorbital nerve; ST: supratrochlear nerve; ZF: zygomaticofacial nerve; ZT: zygomaticotemporal nerve.
Fibres are classed as nociceptive (Aδ and C fibres) and low-threshold mechanoreceptor fibres (Aα and Aβ fibres). C fibres are small, unmyelinated fibres with a slow conduction speed, whereas Aδ fibres are thinly myelinated, medium-sized, and present a higher conduction velocity. Both types can be stimulated by mechanical, thermal, or chemical stimuli. Aα and Aβ proprioceptive fibres are larger, myelinated fibres with fast conduction, and are stimulated by painless or proprioceptive stimuli.3
Pathophysiology. Neurovascular conflictThe near-homogeneity of the symptoms of TN, regardless of the cause, points to a lesion to the nerve root involving its entry into the pons (extra-axial) or the nerve tract (intra-axial). Numerous trigeminal nerve alterations have been described secondary to compression by vascular structures, including focal demyelination of the nerve root at its entry into the pons, axonal atrophy, and damage to Schwann cells/oligodendrocytes and to myelin.3 Although several pathophysiological hypotheses have been suggested, the most widely accepted is the one proposed by Devor et al.,4 which attempts to link the paroxysmal nature of pain to structural alterations. According to that theory, TN is caused by focal demyelination at the root entry zone (REZ) in the pons. The REZ includes the transition zone (or Obersteiner-Redlich zone), the site where central myelin (synthesised by oligodendrocytes) changes to peripheral myelin (synthesised by Schwann cells). This segment of the REZ, measuring 2–2.5 mm, is particularly susceptible to damage secondary to extrinsic compression (vascular loop, meningioma, etc).5 Local nerve compression at the transition zone induces focal demyelination of proprioceptive fibres, which transmit tactile stimuli from the skin or mucosa of the face, promoting contact between exposed axons and the unmyelinated axons of the nociceptive fibres. This contact causes ephaptic transmission of action potentials between fibres. This cross-membrane transmission, with recruitment of proximal bundles of fibres (firing), between fibres carrying light touch information and those transmitting nociceptive information, may be the reason for which pain attacks are triggered after tactile stimulation of the face.6 According to a second hypothesis, pain results from changes in energy and mitochondrial metabolism secondary to focal demyelination, affecting the function of the sodium-potassium pump, which is more concentrated at the nodes of Ranvier. This would result in sustained depolarisation and, consequently, continuous neuronal hyperexcitability triggering ephaptic transmission of action potentials.5,7
The great intensity and hyperacute nature of pain attacks may be explained by the presence of prolonged discharges by the soma of sensory neurons, triggered by tactile stimulation, with spread to the adjacent neurons. Ephaptic impulses may also explain the concomitant continuous pain observed in some patients, although this may also be related to alterations to the central pain processing system, with impairment of descending pain inhibitory mechanisms. In fact, patients with TN present changes in the blink reflex and in brainstem evoked potentials.
EpidemiologyEpidemiological studies are scarce, and generally include small samples. The prevalence of TN is estimated at 0.3%,8 with an incidence rate of 12.6 cases/100 000 person-years.9 Incidence increases with age, with rates of 17.5 cases/100 000 person-years in the population aged 60–69 years and 25.6 cases among those aged 70 years and older.9 Mean age of onset is 53.9 years; women are more frequently affected (60%).10 No ethnic or geographical differences in incidence have been identified, although some diseases, such as multiple sclerosis (MS), do appear to increase the risk of TN.11
Familial cases are rare, accounting for 1%–2% of patients. Studies of familial cases report an autosomal dominant inheritance pattern with a genetic anticipation phenomenon.12 Due to the nature and the intensity of the pain, TN has a considerable impact on patients’ quality of life. These patients suffer as a result of diagnostic delay, fear of sudden onset of an attack, adverse reactions to treatment, and a lack of psychological support.13 The incidence of anxiety and depression among patients with TN is nearly 3 times higher than in the general population,14 as a result of the intensity of pain and long duration of the disease. The disease may also cause poor performance in activities of daily living, social isolation, sleep alterations, fatigue, and anorexia. In the light of all of the above, it is important for the management of these patients to take a multidisciplinary approach involving mental health professionals.
TN can also have a considerable economic impact, as disease onset is usually during working age, and more than half of patients present difficulties performing work duties.13
Aetiology and classification. Diagnostic criteria and differential diagnosisIn the third edition of the International Classification of Headache Disorders (ICHD-3), pain attributed to a lesion or disease of the trigeminal nerve is categorised into TN and painful trigeminal neuropathy. In turn, TN is divided into 3 main types (Table 1) according to pain aetiology: classical, idiopathic, or secondary. The classical form refers to cases of pain with no apparent cause, although it may also be attributed to neurovascular compression. In the idiopathic form, no neurophysiological or MRI alterations are detected, whereas an underlying cause can be identified in secondary TN. On the other hand, painful trigeminal neuropathy is classified into different types according to its aetiology.15 The diagnostic criteria for TN are presented in Table 2.
Classification of trigeminal neuralgia (ICHD-3).
| Classical trigeminal neuralgia |
| Classical trigeminal neuralgia, purely paroxysmal |
| Classical trigeminal neuralgia with concomitant continuous pain |
| Secondary trigeminal neuralgia |
| Trigeminal neuralgia attributed to multiple sclerosis |
| Trigeminal neuralgia attributed to space-occupying lesion |
| Trigeminal neuralgia attributed to other cause |
| Idiopathic trigeminal neuralgia |
| Idiopathic trigeminal neuralgia, purely paroxysmal |
| Idiopathic trigeminal neuralgia with concomitant continuous pain |
| Painful trigeminal neuropathy |
| Painful trigeminal neuropathy attributed to herpes zoster |
| Trigeminal post-herpetic neuralgia |
| Painful post-traumatic trigeminal neuropathy |
| Painful trigeminal neuropathy attributed to other disorder |
| Idiopathic painful trigeminal neuropathy |
ICHD-3: third edition of the International Classification of Headache Disorders.
Diagnostic criteria for trigeminal neuralgia (ICHD-3).
| A. Recurrent paroxysms of unilateral facial pain in the distribution(s) of one or more divisions of the trigeminal nerve, with no radiation beyond, and fulfilling criteria B and C |
| B. Pain has all of the following characteristics: |
| 1. lasting from a fraction of a second to 2 minutes |
| 2. severe intensity |
| 3. electric shock-like, shooting, stabbing, or sharp in quality |
| C. Precipitated by innocuous stimuli within the affected trigeminal distribution |
| D. Not better accounted for by another ICHD-3 diagnosis |
Classical TN (Table 3) refers to cases probably caused by compression of the nerve root by a tortuous blood vessel. In 58%–75% of cases, the vessel involved is the superior cerebellar artery.16 Venous compression is less frequent (10%). Simple contact is common. Up to 17.5% of autopsies of individuals who never had TN reveal vascular contact without significant neurovascular compression.16 Imaging techniques are able to identify significant neurovascular compression17 and differentiate it from simple contact, enabling better selection of surgical candidates, with classical series reporting no improvement after microvascular decompression in up to 30% of patients undergoing the procedure.18
Diagnostic criteria for classical trigeminal neuralgia (ICHD-3).
| A. Recurrent paroxysms of unilateral facial pain fulfilling criteria for trigeminal neuralgia |
| B. Demonstration on MRI or during surgery of neurovascular compression (not simply contact), with morphological changesa in the trigeminal nerve root |
| C. Not better accounted for by another ICHD-3 diagnosis |
ICHD-3: third edition of the International Classification of Headache Disorders; MRI: magnetic resonance imaging.
Secondary TN presents with “recurrent paroxysms of unilateral facial pain meeting the diagnostic criteria for TN, either purely paroxysmal or associated with concomitant continuous or near-continuous pain,” in patients with a documented underlying disease recognised as a cause of the neuralgia, which would explain the pain. Approximately 15% of cases of TN are secondary.19 Clinical characteristics suggesting secondary TN are a) onset before 50 years of age; b) bilateral involvement; c) V1 branch involvement; and d) signs and symptoms of sensory dysfunction (other than pain). In adults, the most frequent cause of secondary TN is extra-axial compression, usually secondary to tumours. In younger patients, intra-axial lesions due to MS are more frequent. Tumours account for 3%–9.4% of all cases of TN. Tumours are located on the trajectory of the nerve (meningioma, neuroma, meningeal carcinomatosis, epidermoid tumours) or in the posterior fossa (meningioma, neuroma).20 MS accounts for 2%–11% of all cases. Patients with MS are 20 times more likely to develop TN, which affects 2%–5% of patients.21 In 3.6% of cases, secondary TN is caused by a bone disease of the skull (osteomyelitis, Paget disease, osteoma), arteriovenous malformation, dural fistula, pontine infarction, tuberculoma, cholesteatoma, arachnoiditis, hydrocephalus, lipoma, syphilis, diabetes mellitus, malaria, etc.19
Trigeminal neuralgia with concomitant continuous painThe ICHD-3 recognises that, in addition to the characteristic paroxysms, patients with classical or idiopathic forms of TN may present continuous or near-continuous pain between attacks. This was previously referred to as TN type 2 (Burchiel type 2, as opposed to purely paroxysmal or type 1 TN) or atypical TN. This background pain may appear in up to 24%–49% of patients, and may be described as burning, stinging, or pulsatile.22,23 The most recent data do not fully support the theory that continuous pain is the result of longstanding paroxysmal TN.23 While the underlying pathophysiological mechanism is not fully understood, it is believed to involve central sensitisation and damage to C fibres of the nerve root. The loss of unmyelinated fibres may cause abnormal hyperactivity of second-order neurons of the brainstem.24 Given the suggestion that the continuous pain is a form of neuropathic pain, it may be possible to treat these patients with such pain modulators as antidepressants (amitriptyline, duloxetine) or antiepileptic drugs, among others, although most studies present low levels of evidence.25
Painful trigeminal neuropathyThe ICHD-3 defines painful trigeminal neuropathy as pain in the territory of one or more branches of the trigeminal nerve with clinically detectable sensory deficits (hyperalgesia, allodynia, hypoaesthesia, hypoalgesia) presumably indicative of neural damage (Table 4).15 Trigeminal neuropathy is classified as being idiopathic or secondary to herpes zoster infection, trauma, or other disorders (Table 5). This classification includes terminal branch neuralgia of the trigeminal nerve within the broader concept of painful trigeminal neuropathy.
Diagnostic criteria for idiopathic painful trigeminal neuropathy (ICHD-3).
| A. Unilateral or bilateral facial pain in the distribution(s) of one or both trigeminal nerve(s) and fulfilling criterion C |
| B. Clinically evident positive (hyperalgesia, allodynia) and/or negative (hypoaesthesia, hypoalgesia) signs of trigeminal nerve dysfunction |
| C. No cause has been identified |
| D. Not better accounted for by another ICHD-3 diagnosis |
ICHD-3: third edition of the International Classification of Headache Disorders.
Diagnostic criteria for painful trigeminal neuropathy attributed to other disorder (ICHD-3).
| A. Unilateral or bilateral facial pain in the distribution(s) of one or both trigeminal nerve(s) and fulfilling criterion C |
| B. A disorder, other than those described above but known to be able to cause painful trigeminal neuropathy with clinically evident positive (hyperalgesia, allodynia) and/or negative (hypoaesthesia, hypoalgesia) signs of trigeminal nerve dysfunction, and affecting one or both trigeminal nerves, has been diagnosed. |
| C. Evidence of causation demonstrated by both of the following: |
| 1. pain is localised to the distribution(s) of the trigeminal nerve(s) affected by the disorder |
| 2. pain developed after onset of the disorder, or led to its discovery |
| D. Not better accounted for by another ICHD-3 diagnosis |
ICHD-3: third edition of the International Classification of Headache Disorders.
Although it is not specifically included in the current edition of the ICHD, terminal branch neuralgia of the trigeminal nerve is a relatively frequent condition, characterised by pain in the territory of one or more terminal branches of a division of the trigeminal nerve. It has been described in V1 (supraorbital, supratrochlear, infratrochlear, and lacrimal neuralgia), V2 (infraorbital neuralgia), and V3 (auriculotemporal, inferior alveolar, and mental neuralgia); aetiology may be primary or secondary to another, typically local process (trauma, surgery, tumour, etc). The most important characteristics of these neuralgias include the fact that pain is circumscribed to the area innervated by the affected nerve and is usually continuous (except in infratrochlear neuralgia, which tends to be paroxysmal), the presence of hypersensitivity at the emergence or along the trajectory of the nerve, the appearance of clinical or subclinical signs of sensory dysfunction in the affected territory, and the fact that pain is completely and transiently relieved by blocking the specific nerve.26–30
NeuroimagingWith a view to ruling out secondary causes, brain MRI studies are advisable in all patients with a clinical diagnosis of TN.31 The use of 3D reconstruction techniques, rather than the classical 2D sequences, enables optimal anatomical assessment of the nerve. Head CT is not informative in the work-up of patients with TN. The most recommended MRI techniques for evaluating the presence of neurovascular compression at the REZ are FIESTA, DRIVE, or CISS sequences, including 3D T2-weighted sequences, and MRI angiography with TOF and gadolinium-enhanced T1-weighted sequences and 3D reconstruction; compared against surgical detection of neurovascular contact, MRI evaluation has a sensitivity of 98%, specificity of 100%, positive predictive value of 93%, and negative predictive value of 97%.20 False negative results may be explained by small arteries (diameter < 1 mm), thickening of the arachnoid, and less common causes not detectable with MRI.32 Diffusion tensor imaging and tractography studies detect anomalies at the trigeminal nerve root, which are normalised after decompression or radiosurgery.33–36
Differential diagnosis of trigeminal neuralgiaTN is diagnosed clinically. The most frequent diseases considered in differential diagnosis are listed in Table 6.5
Differential diagnosis of trigeminal neuralgia.
| Trigeminal autonomic cephalalgias | Other craniofacial neuralgias | Other craniofacial pains |
|---|---|---|
| SUNCT, SUNA | Glossopharyngeal neuralgia | Pain secondary to structural craniofacial disorders (odontological disease, temporomandibular joint disorders) |
|
|
|
| Cluster headache | Terminal branch neuralgia of the trigeminal nerve | Persistent idiopathic facial pain |
|
|
|
| Paroxysmal hemicrania | Nervus intermedius neuralgia | Painful trigeminal neuropathy |
|
|
|
The table shows the most significant clinical characteristics that most frequently differentiate trigeminal neuralgia from the other entities listed.
CBZ: carbamazepine; SUNA: short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms; SUNCT: short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing; TN: trigeminal neuralgia.
We must seek to rule out facial pain originating in the teeth, caused by trigeminal autonomic cephalalgia (inquiring about autonomic signs), or secondary to facial herpes zoster infection or ipsilateral facial trauma.37,38 We must also rule out temporomandibular joint disorder and consider the possibility of persistent idiopathic facial pain. It is also important to consider such other neuralgias as glossopharyngeal, nervus intermedius, or terminal branch neuralgias.
Semiology and examinationExamination of patients with TN should seek to identify trigger points whose stimulation generates pain with similar characteristics to that described by the patient. Trigger points may be located either in the painful area or in adjacent regions, and may be intraoral (speaking, chewing, brushing teeth) or extraoral (softly touching the face; cool breeze).10 Inducing these episodes of pain is highly informative. It enables us to localise pain, to measure its duration, and to verify the existence of the characteristic refractory period observed in TN.5 We must also assess any accompanying symptoms, including autonomic signs (conjunctival injection, tearing, rhinorrhoea), which are less pronounced and briefer than those observed in short-duration trigeminal autonomic cephalalgias, such as short-lasting unilateral neuralgiform headache attacks (SUNCT) and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA).39,40
In the medical history interview, painful trigeminal neuropathy is described as continuous or near-continuous pain, without the predominance of painful paroxysms observed in TN. Examination may identify numerous signs of deficits (hypoaesthesia) and irritation (dysaesthesia), as well as corneal reflex alterations.41 In painful post-herpetic and post-traumatic trigeminal neuropathy, this relevant history may be gathered in the clinical interview. Remnants of skin lesions may also be observed in post-herpetic cases.
Patients with these secondary forms may also present symptoms of involvement of other cranial nerves and nervous system structures, and signs suggestive of systemic disease in the physical examination. The face, neck, and mouth should be examined in detail.42,43 A proposed system for this examination may be as follows: beginning with an inspection of the skin and anatomy of the ears, nose, mouth, teeth, gums, and pharynx, evaluating colour, inflammation, and potential asymmetry, then palpating the paranasal sinuses, temporomandibular joint, and muscles of mastication, exploring strength and range of motion in jaw opening. It is also advisable to palpate the soft tissues of the face, neck, and mouth, including potential adenopathies; finally, we should assess neck mobility, the corneal reflex,44,45 and the masseter reflex.46,47
It is important to differentiate between TN and terminal branch neuralgias of the trigeminal nerve. To that end, it is advisable to delimit the painful area as exactly as possible and to establish the extent of sensory alterations. This system, together with palpation of the emergence sites of the pericranial nerves, may assist in the diagnosis of the corresponding neuralgias, which may be treated with anaesthetic block.30,48 Similarly, cervical nerve branch involvement should be considered if pain extends to the back of the skull, back of the ear, or mandibular angle.49,50
Pharmacological treatment of trigeminal neuralgiaCarbamazepine is the only drug with class I evidence (grade of recommendation A); therefore, this continues to be the treatment of choice and the only approved treatment for this indication. Though with a relatively low degree of certainty, 2 main lines of treatment have been established (Table 7).
Pharmacological treatment of trigeminal neuralgia, with levels of evidence.
| Treatment line | Drugs |
|---|---|
| First line | Carbamazepine (I, A)Oxcarbazepine (IV, C)Eslicarbazepine acetate (IV, C) |
| Second line | Lamotrigine (II, B)Baclofen (II, B)Gabapentin (IV, C)Pregabalin (IV, C) |
| Others | Phenytoin (IV, C) |
| Lidocaine (IV, C) | |
| Miscellaneous (IV, C) | |
| Pimozide | |
| Tocainide | |
| Clonazepam | |
| Topiramate | |
| Valproic acid | |
| Levetiracetam | |
| Lacosamide |
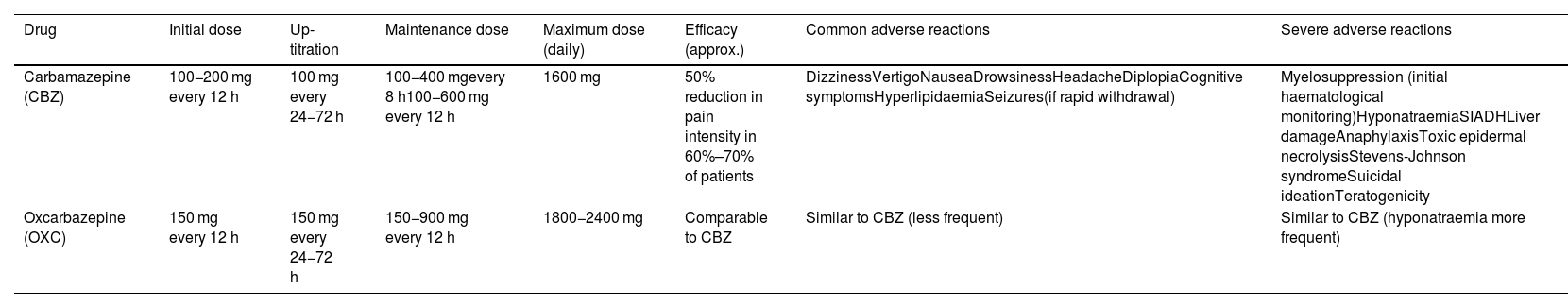
This group of drugs includes carbamazepine and oxcarbazepine (Table 8). The former drug achieves a response in over 60% of patients, although 30% present adverse reactions, which are severe in one in every 24 patients treated. Regarding oxcarbazepine, open-label studies comparing the drug against carbamazepine report similar efficacy, better tolerance, and a lower risk of drug-drug interactions (class IV, grade of recommendation C).3,7,41,51 A study is underway with an extended-release form of oxcarbazepine (ClinicalTrials.gov identifier: NCT03374709).
First-line drugs.
| Drug | Initial dose | Up-titration | Maintenance dose | Maximum dose (daily) | Efficacy (approx.) | Common adverse reactions | Severe adverse reactions |
|---|---|---|---|---|---|---|---|
| Carbamazepine (CBZ) | 100−200 mg every 12 h | 100 mg every 24−72 h | 100−400 mgevery 8 h100−600 mg every 12 h | 1600 mg | 50% reduction in pain intensity in 60%–70% of patients | DizzinessVertigoNauseaDrowsinessHeadacheDiplopiaCognitive symptomsHyperlipidaemiaSeizures(if rapid withdrawal) | Myelosuppression (initial haematological monitoring)HyponatraemiaSIADHLiver damageAnaphylaxisToxic epidermal necrolysisStevens-Johnson syndromeSuicidal ideationTeratogenicity |
| Oxcarbazepine (OXC) | 150 mg every 12 h | 150 mg every 24−72 h | 150−900 mg every 12 h | 1800−2400 mg | Comparable to CBZ | Similar to CBZ (less frequent) | Similar to CBZ (hyponatraemia more frequent) |
Regarding eslicarbazepine acetate, see text.
SIADH: syndrome of inappropriate antidiuretic hormone secretion.
Regarding the other currently available member of this group of drugs, eslicarbazepine acetate, insufficient data have been published to recommend its use to treat TN or neuropathic pain in general (class IV, grade of recommendation C). In observational, open-label studies with small patient samples, eslicarbazepine acetate has been shown to be effective both in TN and in post-herpetic neuralgia; it is increasingly widely used in clinical practice due to its good tolerability and dosage.52,53 However, the drug can have adverse effects, which are similar to those observed with other drugs in this group. If one of these drugs is ineffective or poorly tolerated, another member of the same group can be tried. The recommendations for switching treatments are shown in Table 9.54,55
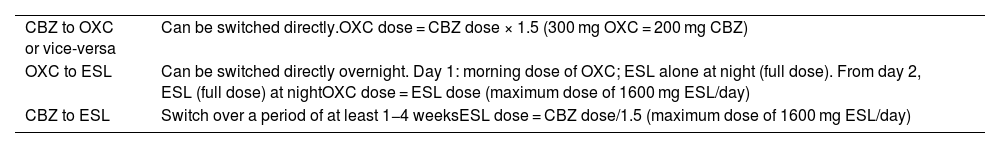
Switches between sodium channel blockers.
| CBZ to OXC or vice-versa | Can be switched directly.OXC dose = CBZ dose × 1.5 (300 mg OXC = 200 mg CBZ) |
| OXC to ESL | Can be switched directly overnight. Day 1: morning dose of OXC; ESL alone at night (full dose). From day 2, ESL (full dose) at nightOXC dose = ESL dose (maximum dose of 1600 mg ESL/day) |
| CBZ to ESL | Switch over a period of at least 1−4 weeksESL dose = CBZ dose/1.5 (maximum dose of 1600 mg ESL/day) |
CBZ: carbamazepine; ESL: eslicarbazepine acetate; OXC: oxcarbazepine.
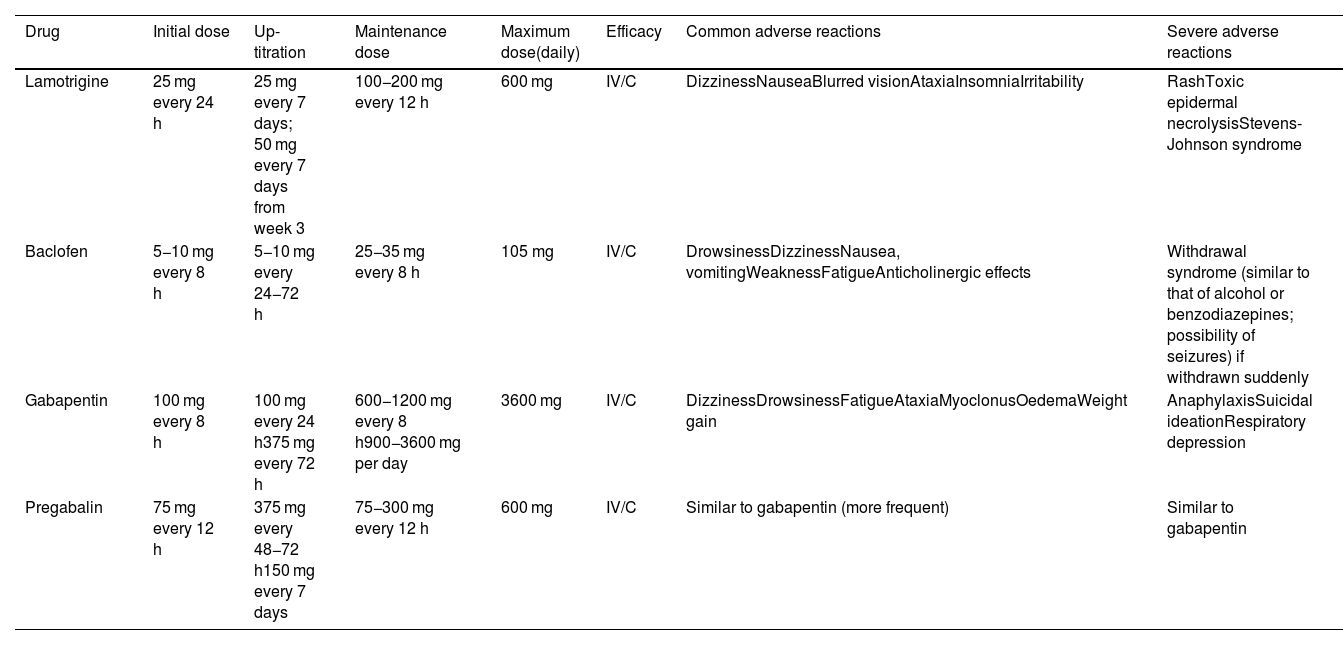
Lamotrigine and baclofen are second-line treatments for TN (class II, grade of recommendation B). Monotherapy with lamotrigine is recommended if the first-line drugs are contraindicated or are not tolerated. Baclofen is typically used in association with other drugs, although it may also be useful in monotherapy (Table 10).
Second-line treatments.
| Drug | Initial dose | Up-titration | Maintenance dose | Maximum dose(daily) | Efficacy | Common adverse reactions | Severe adverse reactions |
|---|---|---|---|---|---|---|---|
| Lamotrigine | 25 mg every 24 h | 25 mg every 7 days; 50 mg every 7 days from week 3 | 100−200 mg every 12 h | 600 mg | IV/C | DizzinessNauseaBlurred visionAtaxiaInsomniaIrritability | RashToxic epidermal necrolysisStevens-Johnson syndrome |
| Baclofen | 5−10 mg every 8 h | 5−10 mg every 24−72 h | 25−35 mg every 8 h | 105 mg | IV/C | DrowsinessDizzinessNausea, vomitingWeaknessFatigueAnticholinergic effects | Withdrawal syndrome (similar to that of alcohol or benzodiazepines; possibility of seizures) if withdrawn suddenly |
| Gabapentin | 100 mg every 8 h | 100 mg every 24 h375 mg every 72 h | 600−1200 mg every 8 h900−3600 mg per day | 3600 mg | IV/C | DizzinessDrowsinessFatigueAtaxiaMyoclonusOedemaWeight gain | AnaphylaxisSuicidal ideationRespiratory depression |
| Pregabalin | 75 mg every 12 h | 375 mg every 48−72 h150 mg every 7 days | 75−300 mg every 12 h | 600 mg | IV/C | Similar to gabapentin (more frequent) | Similar to gabapentin |
Doses of pregabalin and gabapentin must be adjusted in patients with chronic kidney disease.
The main disadvantages of lamotrigine are the need to increase the dose very slowly in order to minimise the risk of rash, and the fact that high doses are usually needed to achieve a benefit, increasing the likelihood of adverse reactions.
Gabapentin and pregabalin can be used in association with one of the first- or second-line drugs, or in monotherapy if those drugs cannot be used (class IV, grade of recommendation C).
Due to its more favourable tolerability, gabapentin can be particularly useful in elderly patients and in those with TN secondary to MS; these groups are more frequently intolerant to the effects of the first- and second-line treatments on the central nervous system. Pregabalin seems to be similarly effective, but with poorer tolerability.41,56
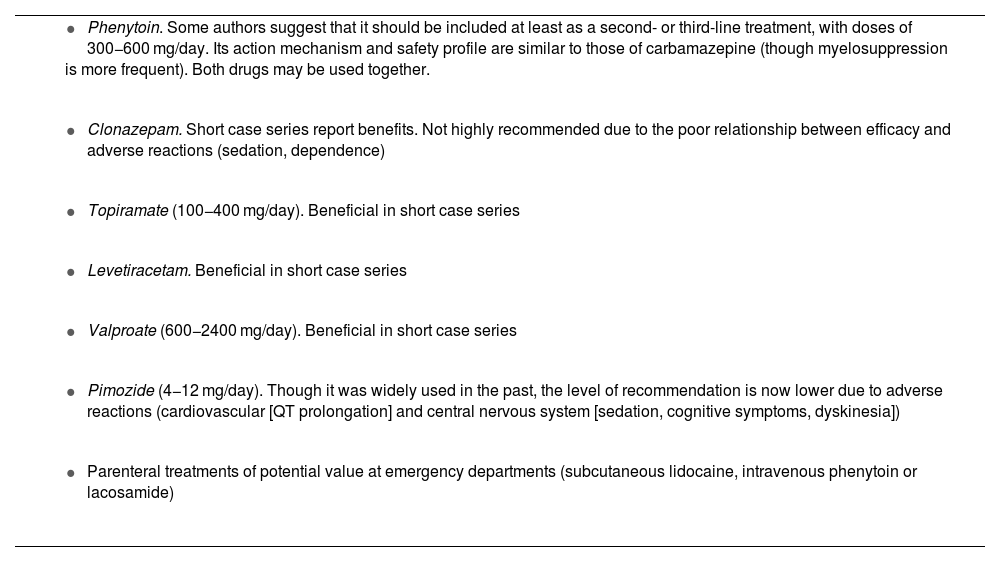
Other drugsTable 11 lists other drugs for which the literature reports favourable though inconsistent results, whose generalised use cannot be recommended (class IV, grade of recommendation C). These include phenytoin, which warrants separate consideration (Table 11).57 Another neuromodulator that is increasingly used in daily practice is lacosamide, both orally and intravenously.58
Other drugs potentially useful in the treatment of trigeminal neuralgia.
|
|
|
|
|
|
|
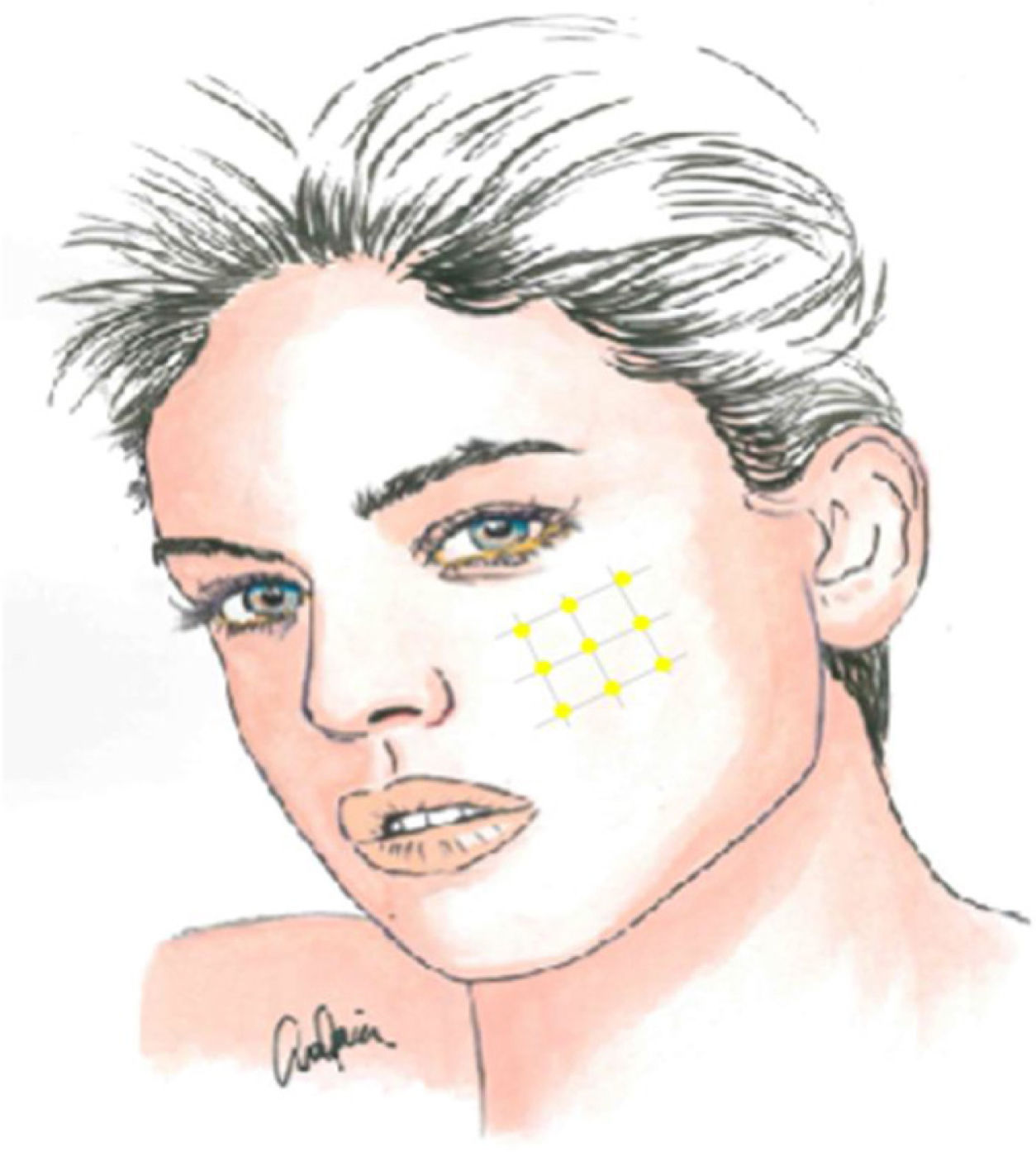
Local infiltration of botulinum toxin A in the painful area may be an interesting therapeutic option, given the pathophysiology of TN and the drug’s results in models of neuropathic pain, with the potential to reduce the transmission of ephaptic impulses and to desensitise trigger points. In addition to various case reports, series, and open-label studies, several randomised clinical trials have been conducted since 2011, though they vary in terms of methodology and results. Botulinum toxin A infiltration is currently recommended in patients with treatment-resistant TN, at doses of 25–75 IU (2.5–5 IU per point), with a space of 15 mm between infiltration points, which may be located on the oral mucosa (class II, grade of recommendation B) (Fig. 2).59–63
Acute treatmentSevere exacerbations of TN are characterised by a marked increase in the frequency, intensity, or duration of pain. In addition to treating the exacerbation, we must also reconsider treatment in the medium term. Exacerbations require intravenous drug treatment to rapidly alleviate pain. The use of opioids is completely inadvisable. The most useful drugs are phenytoin (or fosphenytoin, which is not available in Spain) and lidocaine (class IV, grade of recommendation C for both drugs).64,65
Treatment of trigeminal neuralgia with concomitant continuous painAs mentioned previously, some patients may present a dull background pain between paroxysms, potentially due to a combination of structural nerve damage secondary to prolonged neurovascular compression on the one hand and central sensitisation phenomena on the other. This continuous pain usually has a negative effect on treatment response, and may be predictive of poorer surgical outcomes, particularly in longstanding cases. For this persistent pain, it may be reasonable to add amitriptyline, another tricyclic antidepressant, or duloxetine (class IV, grade of recommendation C, for all these drugs).66 Given the high prevalence of depression in this patient group, these drugs may be useful as an adjuvant therapy.67
Treatment durationA considerable proportion of patients present complete remission, usually early; this requires us to consider withdrawing medication when the patient has been completely pain-free for a sufficiently long period, estimated at approximately 6 months (grade of recommendation GECSEN). In any case, treatment withdrawal must be gradual.43 Surgical treatment is recommended in the event that appropriate pharmacological treatment is ineffective or poorly tolerated, or when its effectiveness decreases over time. Around 23% of patients with TN do not respond to pharmacological treatment, and are considered eligible for surgery.68 In specialised units, up to 35% of patients are non-responders.69 The best time for surgical intervention is not well established, although it is reasonable to avoid excessive delay: surgery should ideally be considered after the first year of lack of response or intolerance to pharmacological treatment. Generally, we should not expect to achieve significant benefits with pharmacological treatment if drugs from 3 different groups with different action mechanisms have already been tried, whether alone or in combination, at suitable doses and for a maximum period of 3 months per drug to establish their ineffectiveness. Table 12 presents the fundamental premises of the surgical treatment of TN.
Premises for surgical management of refractory trigeminal neuralgia.
| 1. Evidence on the efficacy of surgery for trigeminal neuralgia is based on observational studies. No clinical trials have been conducted. |
| 2. Indication of surgery should be clinical, except in the case of microvascular decompression, for which there are radiological criteria. |
| 3. Surgery is the best regarded treatment option by patients. |
| 4. Surgery should be performed by excellent teams that have surpassed the learning curve and have acceptable morbidity/mortality rates. |
| 5. It is advisable to create a multidisciplinary craniofacial pain committee to evaluate eligibility for surgery on an individual basis. |
What technique should be the first choice in the treatment of refractory classical TN?
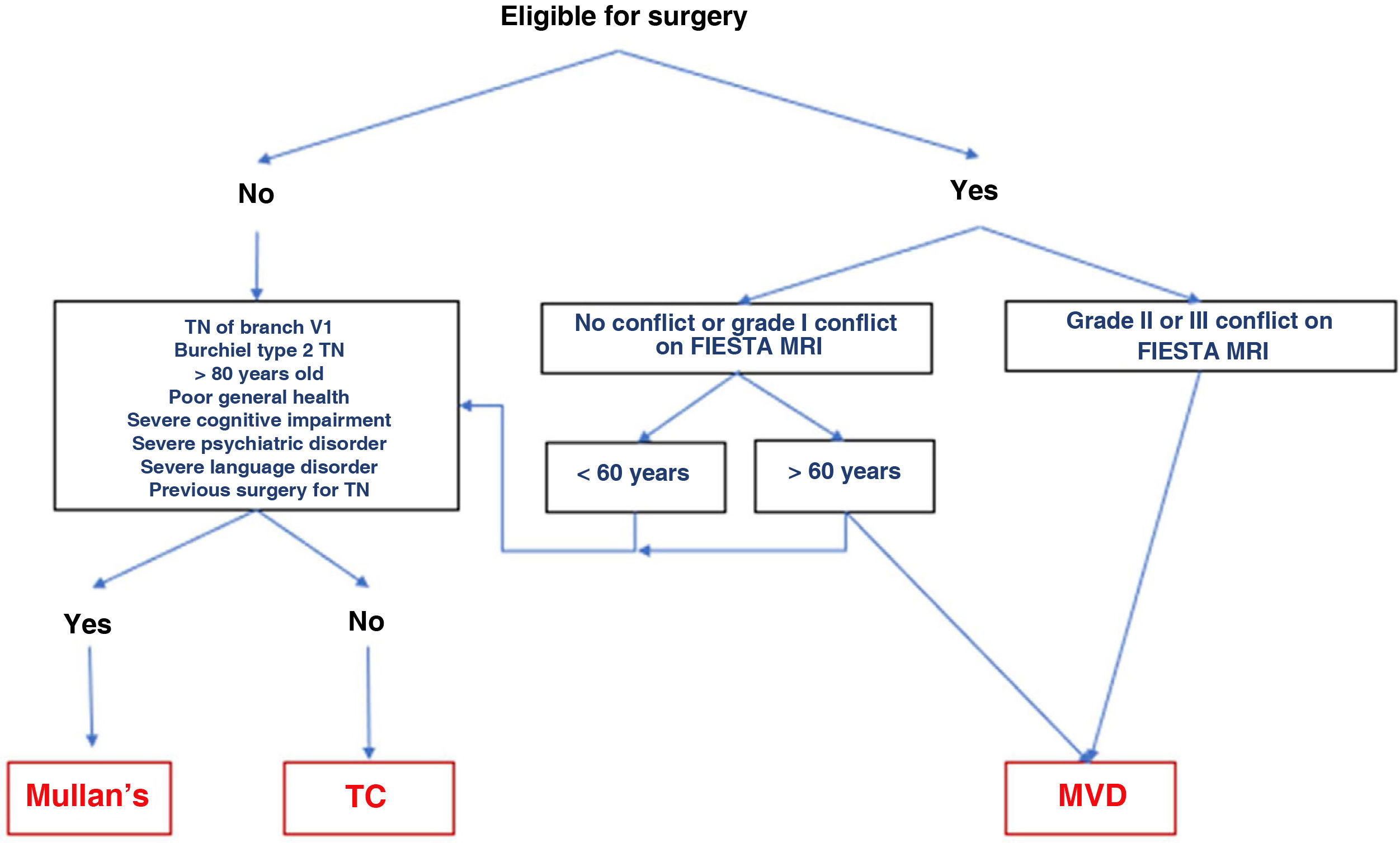
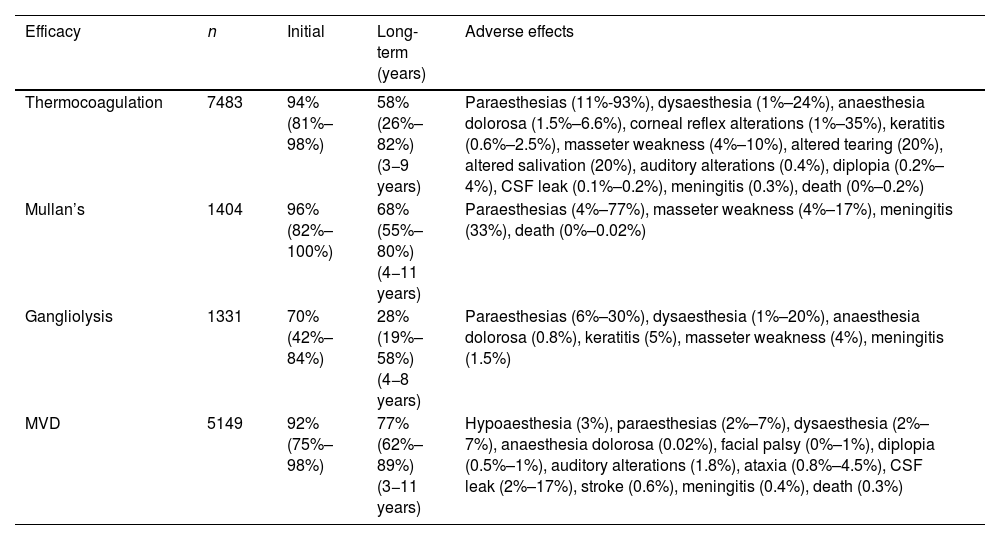
The different surgical techniques present very similar short-term efficacy (Table 13). Therefore, microvascular decompression (MVD) is the surgical treatment of choice, particularly in patients in whom neurovascular contact is detected. Fig. 3 shows a proposed algorithm for surgical decision-making in classical and idiopathic TN.
Surgical decision-making algorithm for refractory classical or idiopathic trigeminal neuralgia. Note that patients aged under 60 years and without neurovascular conflict or with grade I conflict may be treated either with percutaneous procedures or with MVD. Proponents of MVD to treat patients without MRI evidence of neurovascular conflict argue that there may be small conflicts that are undetectable on MRI, or arachnoiditis.
MRI: magnetic resonance imaging; Mullan’s: percutaneous balloon compression; MVD: microvascular decompression; TC: thermocoagulation; TN: trigeminal neuralgia.
All these procedures follow a similar approach, and are performed under an operating microscope, with sedation and monitoring of vital signs due to the risk of arterial hypertension. In these procedures, a needle is inserted through the foramen ovale in order to place an electrode for thermocoagulation, a probe to inflate a balloon (Mullan’s technique), or a cannula for the injection of substances (gangliolysis).25,70,71
- •
Thermocoagulation of the Gasserian ganglion. The needle inserted into the foramen ovale carries an electrode used to apply a thermal radiofrequency to the Gasserian ganglion (Fig. 4A). The patient is awoken before application of the radiofrequency, as their collaboration is needed for a sensory test that uses electric stimulation to provoke paraesthesias in order to localise the target branch. Electric stimulation may also be performed with the patient under sedation, with intraoperative neurophysiological monitoring (antidromic nerve stimulation) to locate the 3 branches of the trigeminal nerve. This procedure is not generally recommended for TN involving branch V1 due to the risk of sensory deficits affecting the cornea.
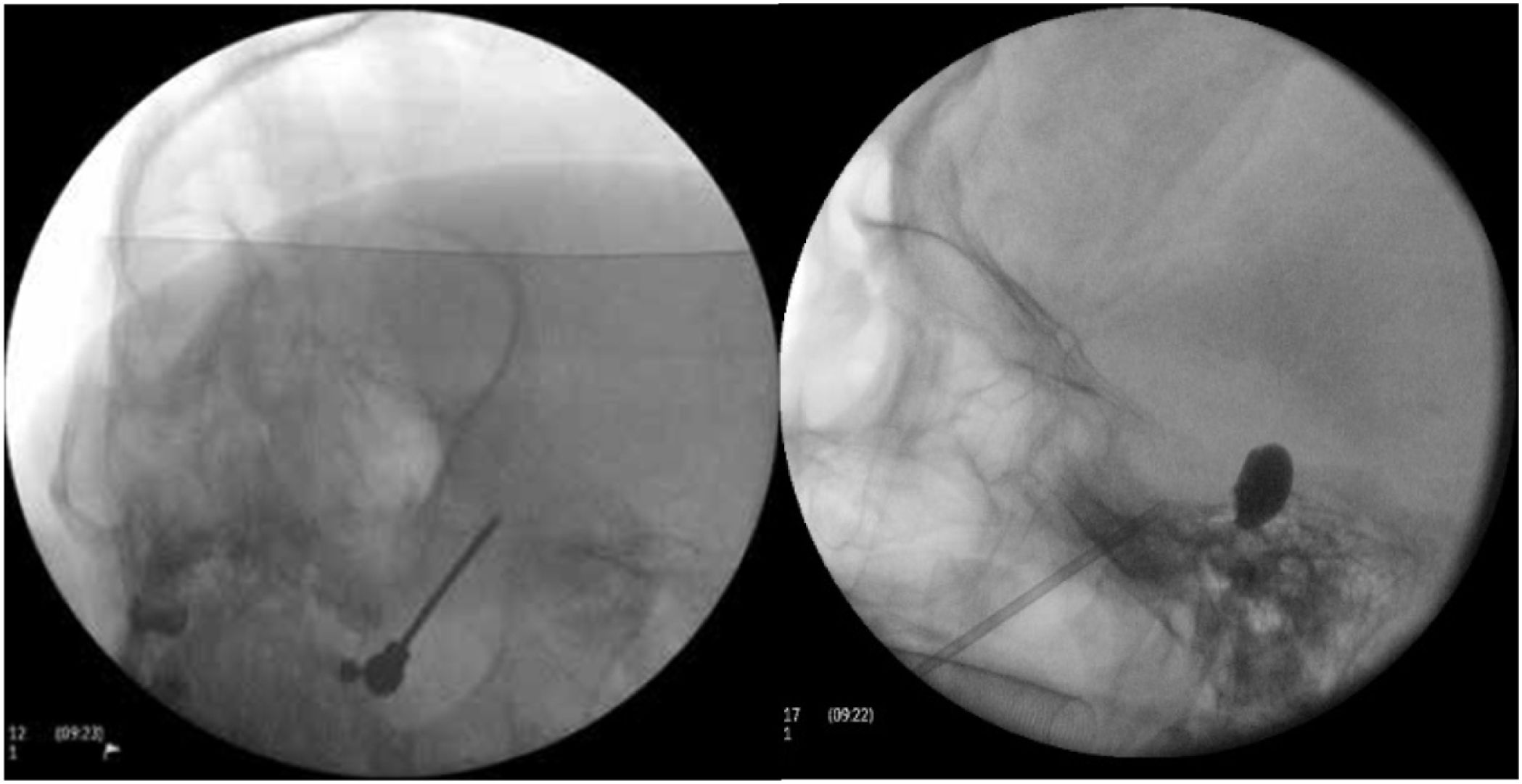
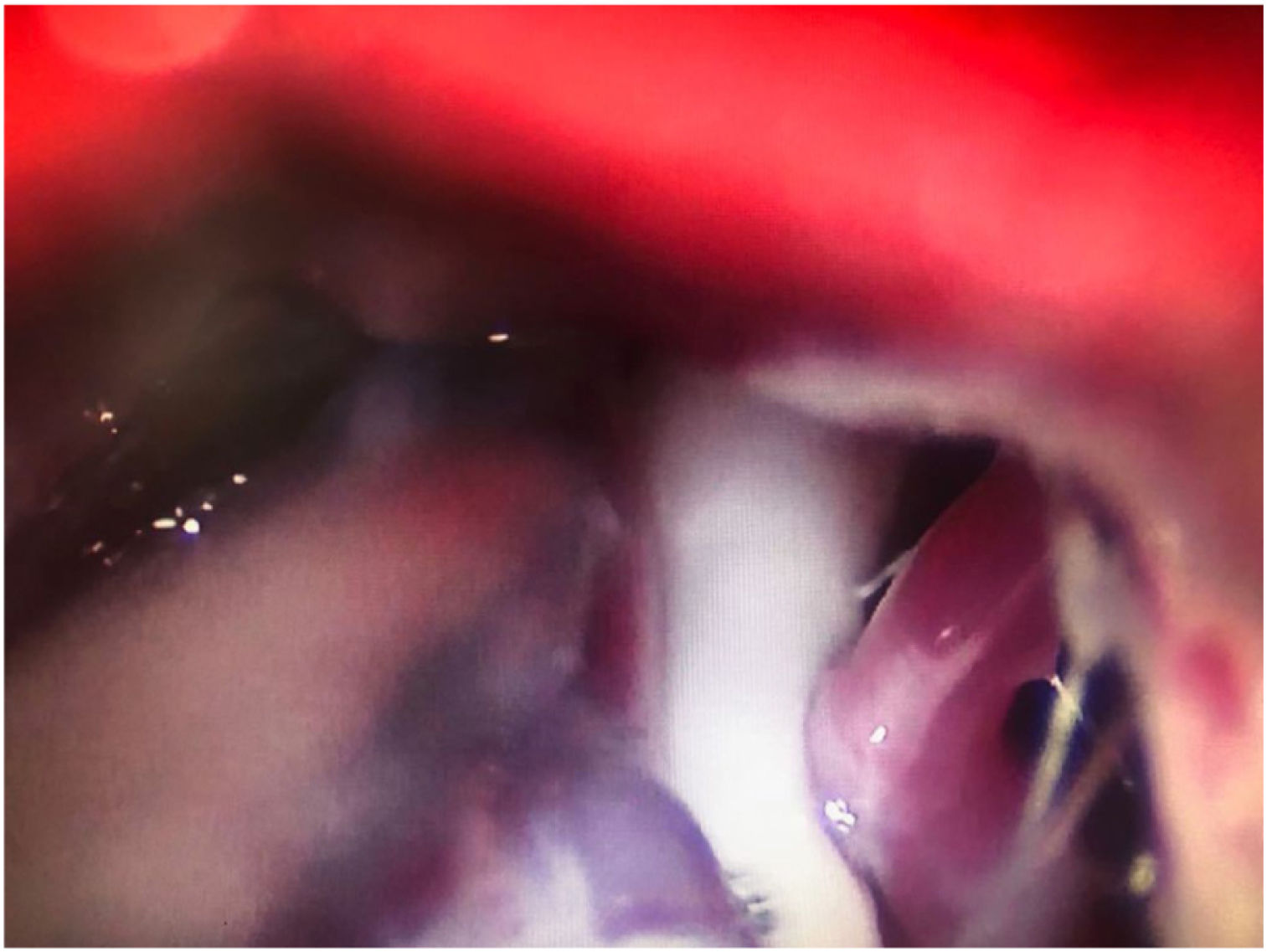
Figure 4.A) A thermal radiofrequency (thermocoagulation) electrode inserted via the foramen ovale in a patient with refractory trigeminal neuralgia of the V3 branch (Waters view). B) Cannula carrying a size 4 Fogarty balloon (shown inflated), inserted through the foramen ovale in an 84-year-old female patient with trigeminal neuralgia affecting the V2 branch (Hospital de la Santa Creu i Sant Pau).
(0.11MB). - •
Percutaneous balloon compression of the Gasserian ganglion. This procedure, known as Mullan’s technique, consists in the insertion through the foramen ovale of a 15 G needle carrying a size 4 Fogarty balloon; the procedure is guided by radioscopy with the patient under general anaesthesia, as their collaboration is not needed in the procedure. After placement, the balloon is inflated with contrast to a volume of 0.7−0.75 cc and pressure of 650−950 mm Hg; radioscopy shows an inverted pear or hourglass shape in Meckel’s cave (Fig. 4B). Compression is maintained for 60–120 seconds, then the balloon is deflated. Inflation of the balloon may cause bradycardia or a hypertensive crisis/emergency.
- •
Percutaneous retrogasserian glycerol rhizotomy. This procedure consists in the injection of 0.2−0.5 cc of 99.9% glycerol anhydrous into the Gasserian ganglion via a 20 G spinal needle inserted through the foramen ovale, after a preliminary contrast cisternography study; the procedure is performed with the patient under local anaesthesia. The main disadvantages of this approach are the fact that pain may take 7–19 days to improve, the high rate of early failures, and the high risk of recurrence. This technique may be indicated for TN affecting branch V1 and bilateral TN attributed to MS.72
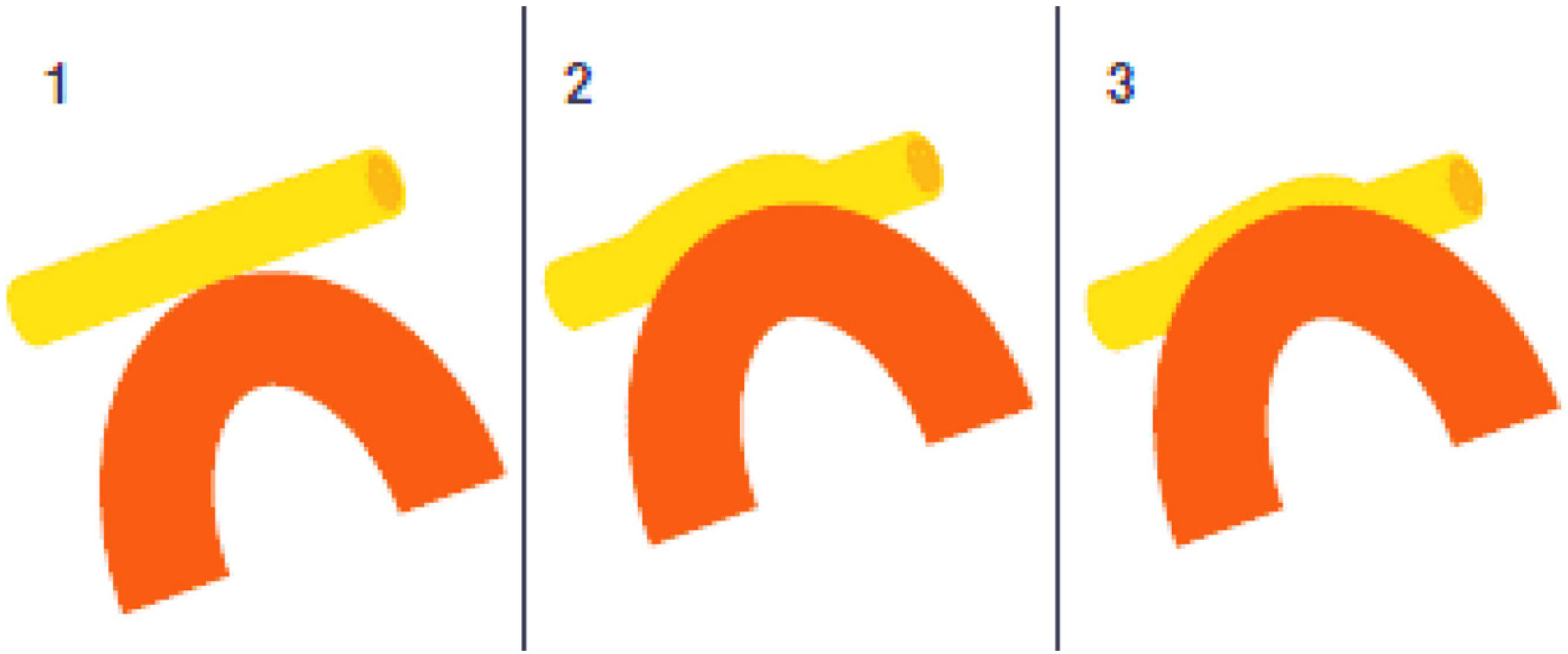
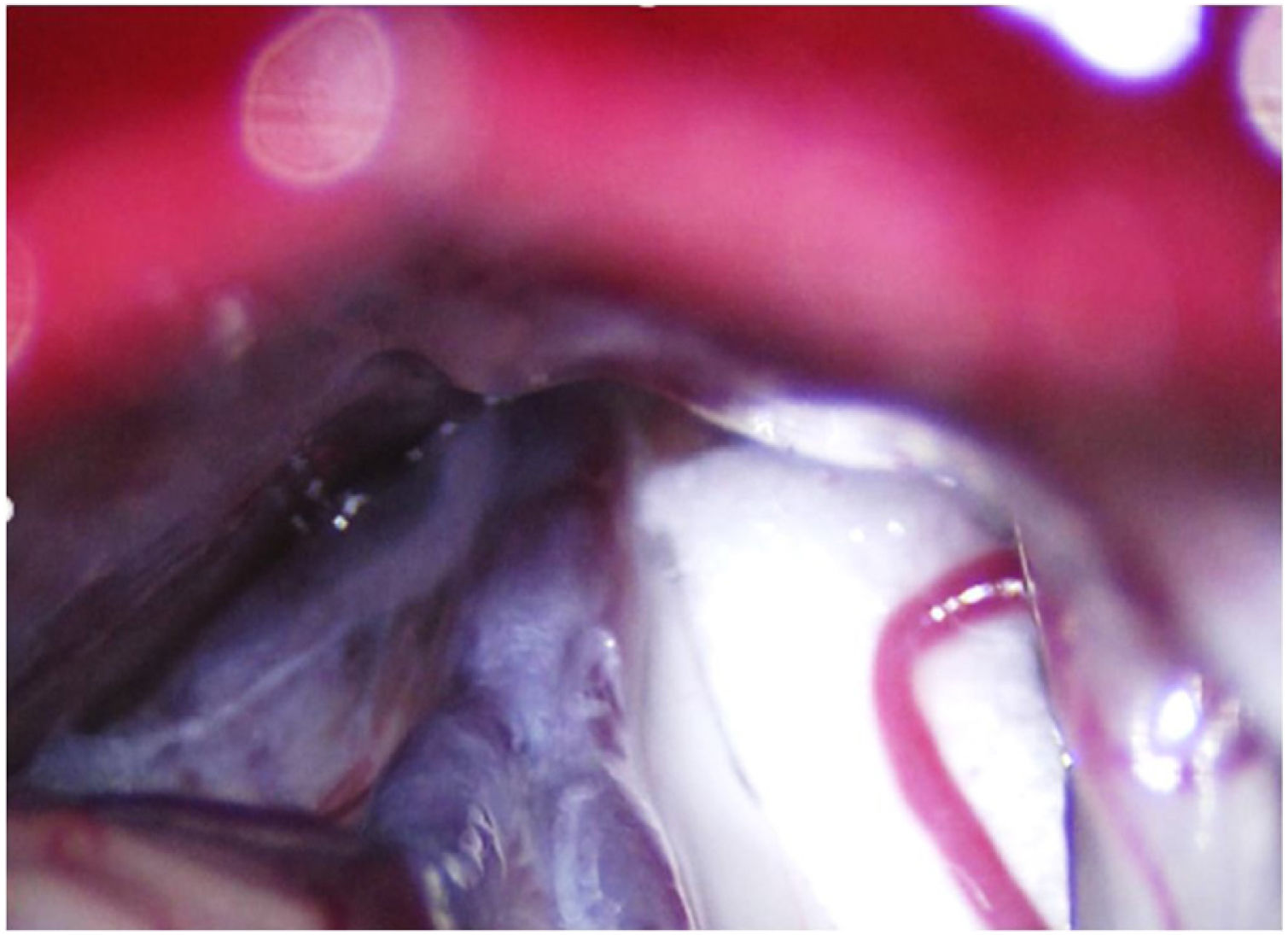
Leal et al.73 proposed a classification of neurovascular compression in classical TN (Fig. 5): grade I, simple contact; grade II, nerve distortion or displacement; grade III, vessel causing a large groove on the nerve. Only grades II and III are considered to constitute true compression (Fig. 6). The MVD procedure, which was proposed by Gardner and developed by Jannetta, begins with the performance of a craniotomy of 2−3 cm in the posterior fossa, accessing the cerebellopontine angle cistern via a retromastoid approach. Cranial nerves are identified with an operating microscope, and neurophysiological stimulation can be used to identify the facial nerve. Once the neurovascular conflict is located, microdissectors are used to separate the vascular structure from the nerve. To avoid further contact and ensure continued prevention or damping of the arterial pulsation, microsurgical Teflon pieces are placed (Fig. 7) and fixed with fibrin. The global rate of complications in MVD procedures ranges from 10% to 23%, although some studies report that complications are extremely rare in centres with experience performing surgery for TN.74,75Table 14 summarises safety and effectiveness data for some of the most frequently used surgical techniques for TN.7,41,74–76
Intraoperative microscopy image of a grade III neurovascular conflict (groove on nerve) between the superior cerebellar artery and the root entry zone; image taken during a microvascular decompression procedure in a patient with refractory trigeminal neuralgia affecting the V2 branch (Hospital de la Santa Creu i Sant Pau).
Safety and efficacy of the most widely used surgical techniques in the treatment of refractory trigeminal neuralgia.
| Efficacy | n | Initial | Long-term (years) | Adverse effects |
|---|---|---|---|---|
| Thermocoagulation | 7483 | 94% (81%–98%) | 58% (26%–82%) (3−9 years) | Paraesthesias (11%-93%), dysaesthesia (1%–24%), anaesthesia dolorosa (1.5%–6.6%), corneal reflex alterations (1%–35%), keratitis (0.6%–2.5%), masseter weakness (4%–10%), altered tearing (20%), altered salivation (20%), auditory alterations (0.4%), diplopia (0.2%–4%), CSF leak (0.1%–0.2%), meningitis (0.3%), death (0%–0.2%) |
| Mullan’s | 1404 | 96% (82%–100%) | 68% (55%–80%) (4−11 years) | Paraesthesias (4%–77%), masseter weakness (4%–17%), meningitis (33%), death (0%–0.02%) |
| Gangliolysis | 1331 | 70% (42%–84%) | 28% (19%–58%) (4−8 years) | Paraesthesias (6%–30%), dysaesthesia (1%–20%), anaesthesia dolorosa (0.8%), keratitis (5%), masseter weakness (4%), meningitis (1.5%) |
| MVD | 5149 | 92% (75%–98%) | 77% (62%–89%) (3−11 years) | Hypoaesthesia (3%), paraesthesias (2%–7%), dysaesthesia (2%–7%), anaesthesia dolorosa (0.02%), facial palsy (0%–1%), diplopia (0.5%–1%), auditory alterations (1.8%), ataxia (0.8%–4.5%), CSF leak (2%–17%), stroke (0.6%), meningitis (0.4%), death (0.3%) |
CSF: cerebrospinal fluid; Mullan’s: percutaneous balloon compression; MVD: microvascular decompression.
- •
Stereotactic surgery. A recent review of 65 studies, including a total of 6461 patients treated with different stereotactic radiosurgery procedures reported similar efficacy for each technique: 53% for Gamma Knife, 49% for linear accelerator, and 56% for CyberKnife. Recurrence rates ranged from 24% to 32%. According to 2 studies, 30%–45% of patients remained pain-free without pharmacological treatment at 10 years of follow-up. The most frequent adverse effect was trigeminal hypoaesthesia (0%–68%). Other secondary effects included dysaesthesia, paraesthesias, dry eyes, deafferentation pain, and keratitis.77
- •
However, 2 meta-analyses, including a total of 13 studies (1353 patients), found that, compared with stereotactic radiosurgery, MVD is more effective in short- and long-term pain control (96% vs 71%), presents lower rates of complications and reinterventions, and is less costly. Therefore, stereotactic radiosurgery should only be considered in patients with contraindications for MVD or in patients with MS.72
- •
High-intensity focused ultrasound (HIFU). HIFU is a non-invasive treatment option that has begun to be used to treat pain in different diseases. To treat TN, ultrasound is applied to the Gasserian ganglion via the foramen ovale, in an MRI-guided procedure. Though promising, the available efficacy data are from isolated case reports.78
- •
Neuromodulation.
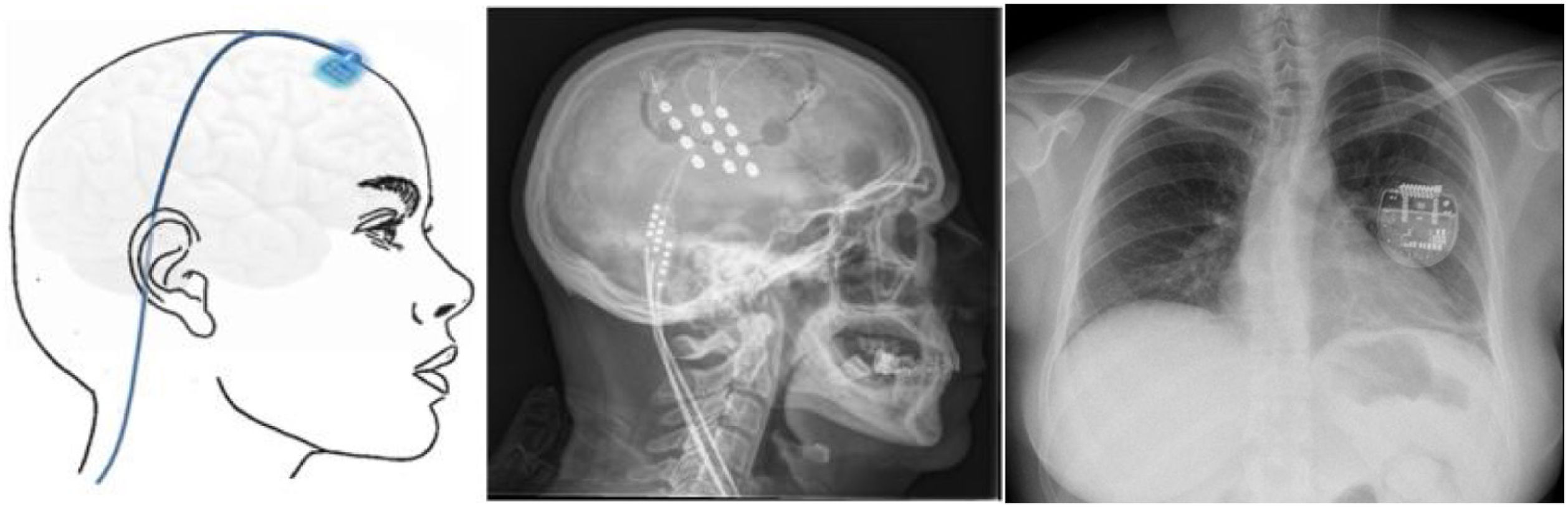
▪Motor cortex stimulation. After localisation of the painful area using MRI, neuronavigation-guided craniotomy is performed with the patient under anaesthesia, and the hand area is located with intraoperative somatosensory evoked potentials. Electrodes (generally a plate with 4 electrodes) may be placed epidurally or subdurally, and are connected to a subclavicular generator with a lead placed via subcutaneous tunnelling (Fig. 8). Efficacy data are based on small series. Of a total of 50 patients, with follow-up times shorter than 40 months, 45%–75% achieved a 50% reduction in pain. Reported complications include infection, epidural haematoma, seizures, and cognitive adverse effects.79
Figure 8.Diagram and radiography images of the skull (sagittal plane) showing the location of the motor cortex stimulation electrode plate and the subclavicular generator in a patient with trigeminal neuralgia refractory to pharmacological treatment, thermocoagulation of the Gasserian ganglion, percutaneous balloon compression, and microvascular decompression (Hospital de la Santa Creu i Sant Pau).
(0.15MB).
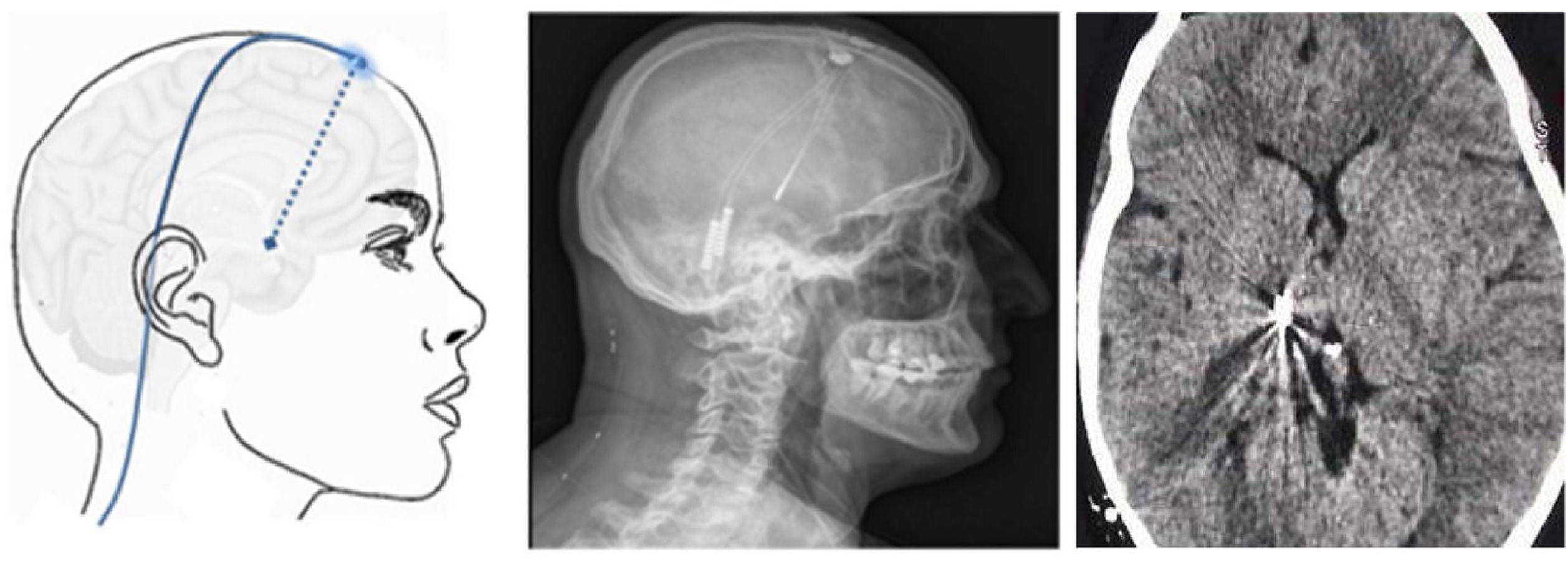
▪Thalamic stimulation. Deep brain stimulation is rarely used in TN, but has been performed in cases secondary to MS or herpes zoster infection and to treat facial deafferentation pain. Other case reports simply refer to untreatable TN. The typical target is the ventral posteromedial nucleus of the thalamus (Fig. 9), the periventricular/periaqueductal grey matter, or both. Pain decreased in 37%–75% of the 15 reported cases, with follow-up times shorter than 30 months.80
Figure 9.Diagram, sagittal head radiography, and axial head CT scan showing the trajectory of the stimulation electrode through the craniotomy to its insertion in the thalamus, in a patient with refractory trigeminal neuralgia secondary to multiple sclerosis (Hospital de la Santa Creu i Sant Pau).
(0.2MB).
TN is diagnosed clinically. Patients consulting due to facial pain (whether paroxysmal or continuous) should be assessed thoroughly (history-taking and physical examination). In the ICHD-3, pain attributed to a lesion or disease of the trigeminal nerve is categorised into TN and painful trigeminal neuropathy. In turn, TN is divided into 3 main types according to pain aetiology: classical, idiopathic, or secondary. The most relevant conclusions of this review are that:
- 1
In patients with TN (and in the absence of contraindications), an MRI study is essential in the proper assessment of the brainstem and posterior fossa and to rule out secondary causes. To establish the presence of neurovascular compression, FIESTA, DRIVE, and CISS protocols are recommended.
- 2
After diagnosis of TN, the pharmacological treatment of choice should be carbamazepine, unless contraindicated. Other sodium channel blockers, such as oxcarbazepine, may be better tolerated.
- 3
In patients unresponsive/intolerant to a first-line drug, second-line treatment with other neuromodulators or polytherapy should be considered. In patients presenting concomitant continuous pain, such antidepressants as amitriptyline or duloxetine may be indicated. Local infiltration of botulinum toxin may be an effective alternative in non-responders or in polytherapy.
- 4
Surgical treatment should be considered in refractory cases. The specific procedure should be selected based on the presence or absence of neurovascular compression, patient age, and other factors. The technique of first choice is MVD, especially in patients in whom neurovascular compression is identified.
The authors have no conflicts of interest to declare.