Our primary aim was to investigate the incidence of non-cardioembolic minor acute ischemic stroke (AIS) and high-risk transient ischemic attack (TIA) and to identify predictors of stroke recurrence/death and severe bleeding. We also evaluated the rates of TIA, major vascular events, therapeutic management and predictors of poor functional outcome at 3 months in these patients.
MethodsWe retrospectively reviewed data from all stroke patients evaluated at the emergency department of 19 hospitals belonging to the NORDICTUS stroke network between July and December 2019. Consecutive patients with non-cardioembolic minor AIS (NIHSS ≤5) and high-risk TIA (ABCD2 ≥6 or ipsilateral stenosis ≥50%) were included. We recorded clinical, neuroimaging and therapeutic variables. Follow-up was performed at 30 and 90 days. Functional prognosis was assessed with the modified Rankin scale score (mRS).
ResultsOf 8275 patients, 1679 (20%) fulfilled IMMINENT criteria (1524 AIS/155 TIA), resulting in a global incidence of 48/100,000 inhabitants per-year. Recurrent stroke/death occurred in 73 (4.3%) patients. Extracranial ipsilateral stenosis (>50%): HR 1.999 (95% CI: 1.115–3.585, p=0.020) and lack of hyperacute cerebral arterial assessment: HR 1.631 (95% CI: 1.009–2.636, p=0.046) were associated with recurrent stroke/death at 90 days. Intracranial stenosis was associated with poor prognosis (p=0.044). Reperfusion therapy was given to 147 (9%) and urgent double antiplatelet therapy (DAPT) to 320 (21%) patients.
ConclusionTwenty percent of our stroke patients presented as non-cardioembolic high-risk TIA or minor AIS. Extracranial ipsilateral stenosis and lack of hyperacute cerebral arterial assessment were predictors of stroke recurrence/death; intracranial stenosis was associated with poor outcome. Despite current recommendations there was a low penetrance of DAPT.
Nuestro objetivo principal fue investigar la incidencia de ictus minor no cardioembólico y ataque isquémico transitorio (AIT) de alto riesgo, además de identificar predictores de recurrencia de ictus/muerte y sangrado grave. Evaluamos los porcentajes de AIT, eventos vasculares mayores, manejo terapéutico y predictores de mal pronóstico funcional.
MétodosEstudio retrospectivo de todos los pacientes con ictus evaluados en urgencias de 19 hospitales de la RED NORDICTUS entre julio-diciembre de 2019. Se incluyeron pacientes consecutivos con ictus minor no cardioembólico (National Institute of Health Stroke Scale [NIHSS] ≤ 5) y AIT de alto riesgo (ABCD2 ≥ 6 o estenosis ipsilateral ≥ 50%). Registramos variables clínicas, de neuroimagen y terapéuticas. Se realizó seguimiento a los 30 y 90 días. El pronóstico funcional se determinó mediante la escala de Rankin modificada (mRS).
ResultadosDe 8.275 pacientes, 1.679 (20%) cumplieron criterios del estudio IMMINENT (1.524 ictus/155 AIT), la incidencia global fue 48/100.000h habitantes-año. Hubo recurrencias de ictus/muerte en 73 (4,3%) pacientes. La estenosis extracraneal ipsilateral (>50%): HR 1.999 (IC 95%: 1.115-3.585); p=0,020 y la ausencia de estudio cerebrovascular hiperagudo: HR 1.631 (IC 95%: 1.009-2.636); p=0.046, fueron predictores de ictus/muerte a 90 días. La estenosis intracraneal se asoció a mal pronóstico (p=0,044). Se administró terapia de reperfusión a 147 (9%) y doble antiagregación a 320 (21%) pacientes.
ConclusiónUn 20% de los pacientes se presentó como ictus minor o AIT de alto riesgo. La estenosis extracraneal ipsilateral y la ausencia de estudio neurovascular hiperagudo fueron predictores de ictus/muerte; la estenosis intracraneal se asoció con mal pronóstico. A pesar de las recomendaciones actuales hay baja penetrancia de doble antiagregación.
Patients with non-cardioembolic minor acute ischemic stroke (AIS) and high-risk transient ischemic attack (HR-TIA) face a high risk of recurrent and disabling vascular events.1 Rates of subsequent stroke range from 5 to 10%2 in the following 3 months and this increases the burden of morbidity and mortality.3,4 Double antiplatelet therapy (DAPT) combining aspirin plus clopidogrel or ticagrelor,2 initiated early after symptom onset and continued for 21–90 days,5 has proven to be superior for the prevention of recurrent ischemic stroke in this population.2 In spite of both DAPT regimens increasing the risk of severe bleeding compared with single antiplatelet therapy (SAPT),1,6 the risk of ischemic stroke outweighs the risk of hemorrhage7–9 and current clinical guidelines7,8 now recommend short-term DAPT for preventing recurrent vascular events in this patients.10–12
According to previous studies, approximately 40% of stroke patients, present with TIA or minor AIS.13 Nevertheless, there are few data about the real incidence, the burden of illness for patients with non-cardioembolic minor AIS and HR-TIA, and about the impact of updates in clinical guidelines10–12 in real-world clinical practice.14
Our primary aim was to describe the incidence of non-cardioembolic minor AIS and HR-TIA as well as to identify predictors of stroke recurrence, all cause of death and severe bleeding. As secondary outcomes, we assessed the rates of recurrent TIA and other major vascular events, we described the therapeutic management, and identified predictors of poor functional outcome at 3 months in the same group of patients.
MethodsStudy design and populationIMMINENT study was a multicenter retrospective cohort study, of all consecutive stroke patients evaluated by a neurologist at the emergency room (ER) of hospitals from NORDICTUS (a research network in cerebrovascular diseases from 19 hospitals with stroke units in North-West Spain), between July 1st and December 31st, 2019.
Inclusion criteria were pre-specified and accorded to be similar to the THALES clinical trial population: age ≥40 years, diagnosis of non-cardioembolic acute minor stroke [baseline National Institute of Health Stroke Scale (NHISS ≤5)] and HR-TIA (ABCD2 ≥6),15 or symptomatic ipsilateral extracranial or intracranial arterial stenosis ≥50%.
Data collectionParticipant hospitals have electronic medical records of all clinical episodes leading to patient's ER consultation. We recorded all consecutive patients who received the following diagnostic codes: “TIA”, “stroke”, “ischemic stroke”, “hemorrhagic stroke”, “cerebrovascular accident” and “intracerebral hemorrhage”
Anonymized data was entered in an electronic case report form (eCRF) by each site. It included demographic, medical history, laboratory, urgent neuroimaging, cerebral artery imaging, modality of urgent attention, antithrombotic and reperfusion treatment. Follow-up data were obtained for day 30 and 90. The eCRF was hosted in a private server at the University of Valladolid in accordance with the Spanish Personal Data Protection law.
Local institutional ethics committee approval was obtained from all sites. Written informed consent was waived because of the retrospective nature of this study. This study complies with Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Outcome variablesThe primary endpoint was the incidence of non-cardioembolic high-risk TIA and minor AIS according to TOAST criteria (trial of ORG 10172 in acute stroke treatment) between July 1, 2019 and December 31, 2019. Primary efficacy outcome were rates of recurrent stroke and all cause of death within 30 and 90 days after the index event. Primary safety outcome was incidence of severe bleeding events based on criteria from the Global Utilization of Streptokinase and tissue-type plasminogen activator for Occluded Coronary Arteries (GUSTO) trial. As secondary outcomes we also evaluated: (1) the proportion of TIA and major cardiovascular events during follow-up, (2) the therapeutic management in the hyperacute and acute phase and, (3) the proportion and predictors of patients with poor functional prognosis at 90 days, which was defined as a modified Rankin scale (mRS) >2.
Statistical analysisBaseline quantitative continuous variables were expressed using mean, standard deviation (SD) or median (interquartile range – IQR). Categorical variables were presented as number of cases and their percentage (%). Normality distribution of the data was assessed by using Kolmogorov–Smirnov test. Baseline characteristics of the study groups were compared using the Chi-square or Fisher test for discrete variables, t-Student and Mann–Whitney U test for quantitative variables depending on the normal distribution.
Incidence was calculated using as a denominator the catchment area covered by the participating hospitals (6,972,170 inhabitants). We also calculated the incidence relative to the total number of patients with TIA and stroke.
We performed bivariate analyses to detect baseline variables associated with the occurrence of the primary and secondary endpoints. The rates of RIS/mortality at 30 and 90 days, and composite of severe bleeding and ICH were estimated by Kaplan–Meier analyses and Cox regressions. Adjustment was performed for all variables showing a p<0.1 on the respective bivariate analyses.
A logistic regression (LR) analysis was performed to identify independent predictors of poor functional outcome at 90 days. To assess functional prognosis, we performed a subgroup analysis including only the patients functionally independent prior to the index event (mRS: 0–2). The LR data are presented as adjusted odds ratio (OR) and 95% confidence intervals (CI) and statistical significance level was defined as a p value <0.05. OR for NIHSS was expressed as per 1-point increased in LR model. Statistical analysis was performed by using the SPSS statistics version 26.
Sample size was determined by the pre-specified duration of recruitment period of 6 months, all consecutive patients who met the inclusion criteria in each site were included.
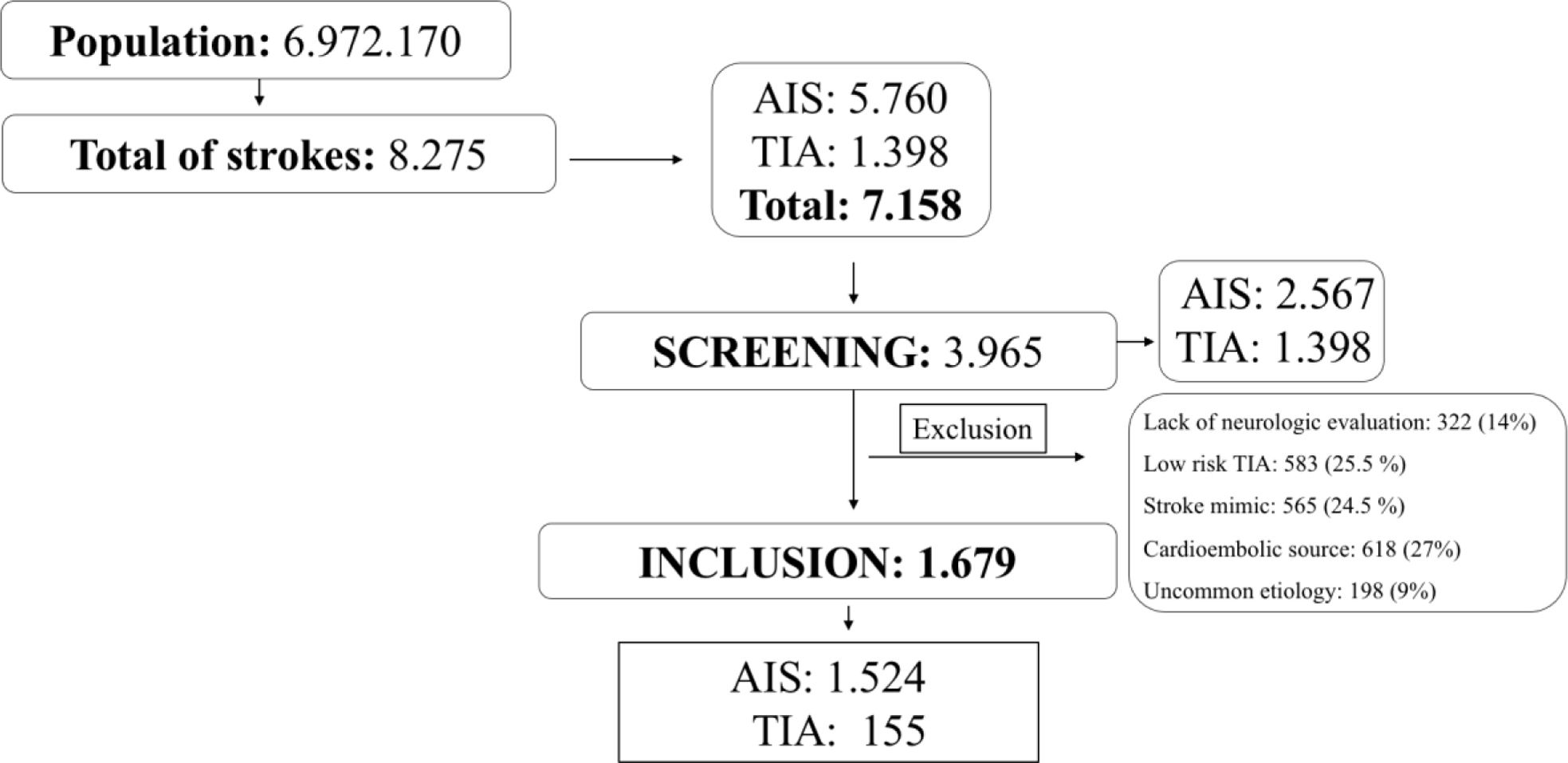
ResultsFrom July 2019 to December 2019, 8275 consecutive stroke patients were attended in the 19 hospitals belonging to NORDICTUS stroke network. Of these, 1117 patients had intracerebral hemorrhage, 5760 had ischemic stroke and 1398, TIA. We included 3965 (48%) patients with minor ischemic stroke and TIA into the screening process; of them, we excluded patients with low-risk TIA (n=583), lack of neurologic evaluation in ER (n=322), stroke mimic (n=565), cardioembolic source (n=618) and uncommon etiology (n=198). Finally, 1679 (20%) of patients fulfilled IMMINENT inclusion criteria as shown in Fig. 1. Of the IMMINENT patients 47 (2.8%) were lost to follow-up at 90 days and 37 (2.2%) had incomplete data for primary outcome in the first month. Most patients 1526 (91%) presented with ischemic stroke and 153 (9%) with high-risk TIA.
Considering the whole NORDICTUS population, the accumulated incidence of minor stroke and TIA for a covered population of 6,972,170 inhabitants was 74 per 100,000 inhabitants per-year and 44 per 100,000 inhabitants per-year, respectively. For the IMMINENT cohort, global incidence of non-cardioembolic minor stroke and high-risk was 48 per 100,000 inhabitants per-year.
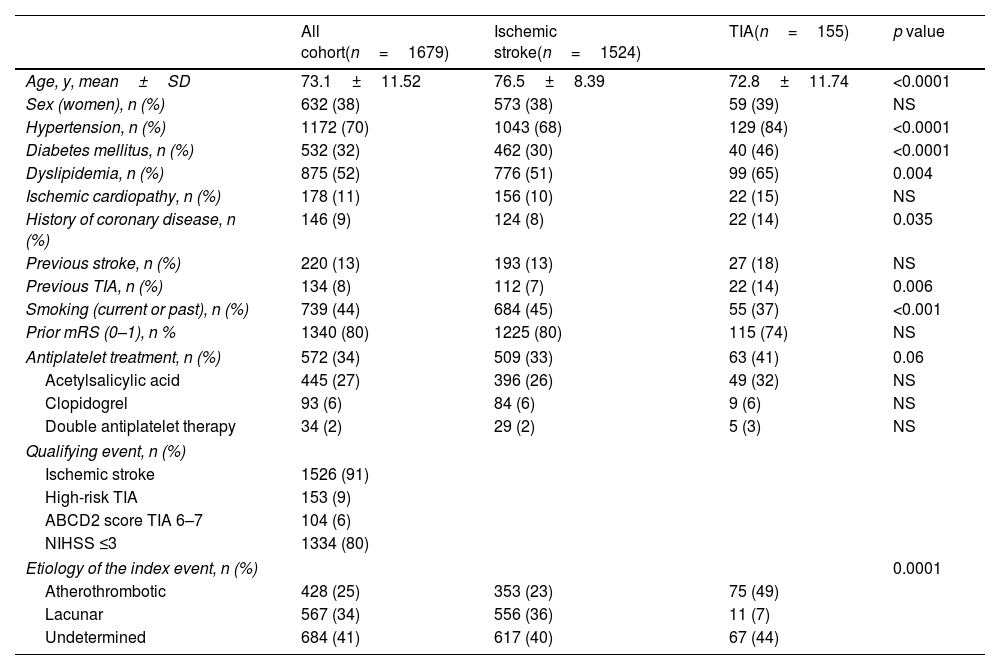
Demographic characteristics of the IMMINENT patients are shown in Table 1. Mean (SD) age of patients was 73.1 (11.51) years, and 632 (38%) were women. Regarding vascular risk factors, TIA patients had higher rates of arterial hypertension, type 2 diabetes mellitus, dyslipidemia, and previous history of coronary artery disease compared to stroke patients, Also, previous TIA was more frequent in this group. Premorbid functional status according to mRS was 0–1 for 1340 (80%) of the patients and was not statistically different between AIS and TIA patients. Prior to the index event 572 (34%) of all patients were on antiplatelet treatment, 445 (27%) on aspirin, 93 (6%) on clopidogrel and 34 (2%) were on DAPT. Undetermined etiology was the most frequent in the whole cohort, whereas atherothrombotic etiology was the predominant in patients with TIA 75 (49%).
Demographic characteristics of IMMINENT cohort.
| All cohort(n=1679) | Ischemic stroke(n=1524) | TIA(n=155) | p value | |
|---|---|---|---|---|
| Age, y, mean±SD | 73.1±11.52 | 76.5±8.39 | 72.8±11.74 | <0.0001 |
| Sex (women), n (%) | 632 (38) | 573 (38) | 59 (39) | NS |
| Hypertension, n (%) | 1172 (70) | 1043 (68) | 129 (84) | <0.0001 |
| Diabetes mellitus, n (%) | 532 (32) | 462 (30) | 40 (46) | <0.0001 |
| Dyslipidemia, n (%) | 875 (52) | 776 (51) | 99 (65) | 0.004 |
| Ischemic cardiopathy, n (%) | 178 (11) | 156 (10) | 22 (15) | NS |
| History of coronary disease, n (%) | 146 (9) | 124 (8) | 22 (14) | 0.035 |
| Previous stroke, n (%) | 220 (13) | 193 (13) | 27 (18) | NS |
| Previous TIA, n (%) | 134 (8) | 112 (7) | 22 (14) | 0.006 |
| Smoking (current or past), n (%) | 739 (44) | 684 (45) | 55 (37) | <0.001 |
| Prior mRS (0–1), n % | 1340 (80) | 1225 (80) | 115 (74) | NS |
| Antiplatelet treatment, n (%) | 572 (34) | 509 (33) | 63 (41) | 0.06 |
| Acetylsalicylic acid | 445 (27) | 396 (26) | 49 (32) | NS |
| Clopidogrel | 93 (6) | 84 (6) | 9 (6) | NS |
| Double antiplatelet therapy | 34 (2) | 29 (2) | 5 (3) | NS |
| Qualifying event, n (%) | ||||
| Ischemic stroke | 1526 (91) | |||
| High-risk TIA | 153 (9) | |||
| ABCD2 score TIA 6–7 | 104 (6) | |||
| NIHSS ≤3 | 1334 (80) | |||
| Etiology of the index event, n (%) | 0.0001 | |||
| Atherothrombotic | 428 (25) | 353 (23) | 75 (49) | |
| Lacunar | 567 (34) | 556 (36) | 11 (7) | |
| Undetermined | 684 (41) | 617 (40) | 67 (44) | |
TIA: transient ischemic attack; mRS: modified Rankin scale; NHISS: National Institute of Health Stroke Scale.
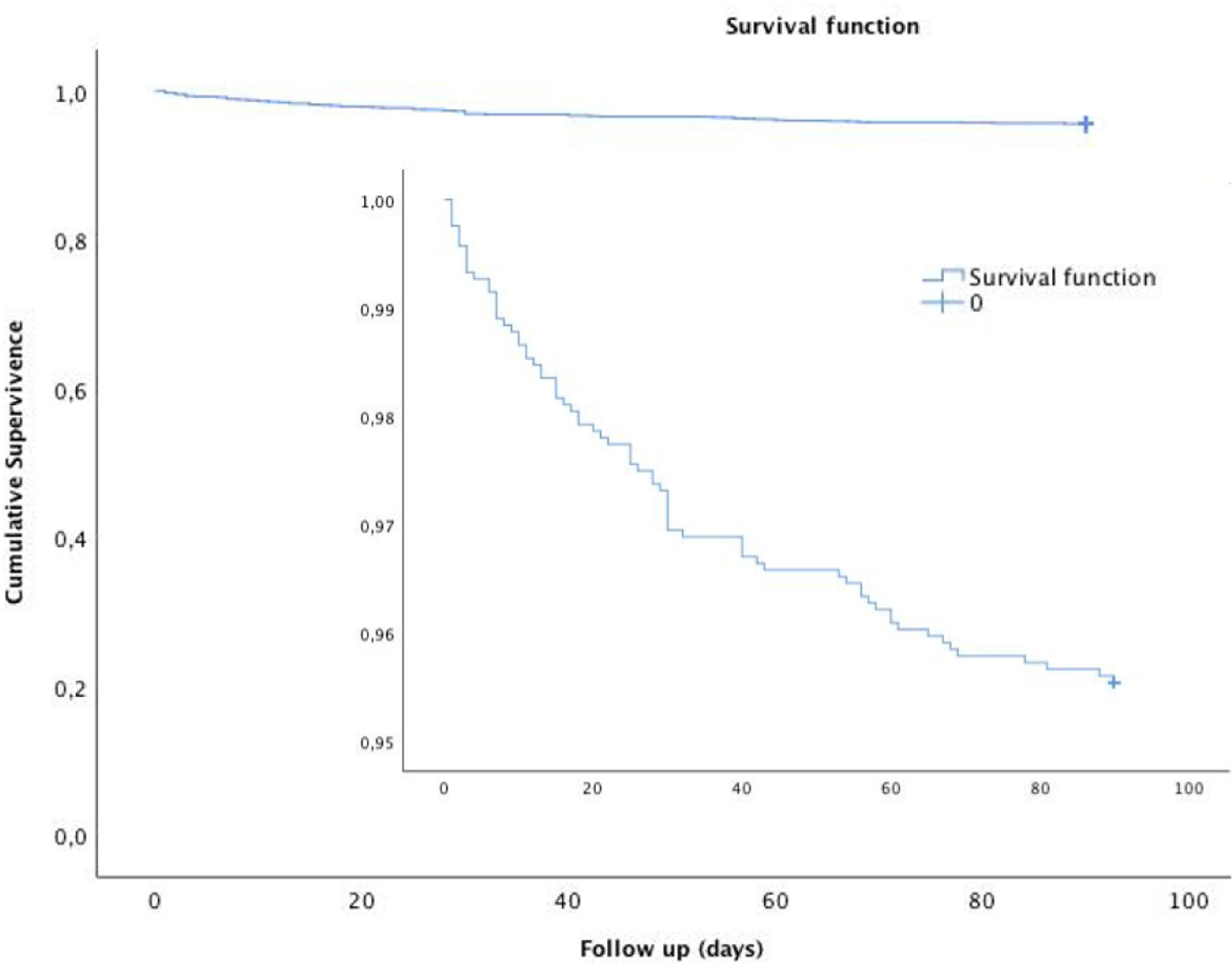
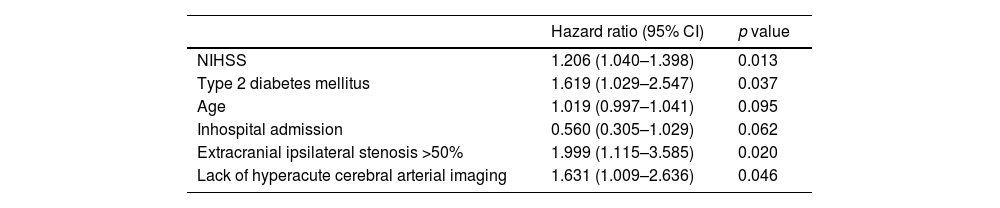
Fig. 2 shows that the Kaplan–Meier estimate of the probability of primary-outcome events decreased after the first month. A primary-outcome event occurred in 73 patients (4.3%) at 90 days of follow-up (Suppl. Table 1). Recurrent ischemic stroke occurred in 39 patients (2.3%), of whom 29 (74%) occurred during the first month after the index event. Etiology of the recurrences were: 22 (56%) atherothrombotic, undetermined 8 (20%), lacunar 5 (12%), cardioembolic 3 (8%) and uncommon 1 (2%), according to TOAST classification. Death from any cause occurred in 34 (2%) patients, of which 6 (0.4%) were vascular deaths. Extracranial ipsilateral stenosis ≥50% HR 2.386 (95% CI: 1.220–4.667, p=0.011) and lack of cerebral arterial assessment on admission HR: 2.732 (95% CI: 1.122–6.654, p=0.027) independently predicted recurrent stroke or death at 30 days. As shown in Table 2, at 90 days predictors of stroke or death were: basal NIHSS: HR: 1.206 (95% CI: 1.040–1.398, p=0.013), type 2 diabetes mellitus: HR 1.619 (95% CI: 1.029–2.547, p=0.037), extracranial ipsilateral stenosis: HR 1.999 (95% CI: 1.115–3.585, p=0.020); and lack of hyperacute cerebral arterial assessment: HR 1.631 (95% CI: 1.009–2.636, p=0.046).
Predictors of stroke or death at 90 days in all cohort.
| Hazard ratio (95% CI) | p value | |
|---|---|---|
| NIHSS | 1.206 (1.040–1.398) | 0.013 |
| Type 2 diabetes mellitus | 1.619 (1.029–2.547) | 0.037 |
| Age | 1.019 (0.997–1.041) | 0.095 |
| Inhospital admission | 0.560 (0.305–1.029) | 0.062 |
| Extracranial ipsilateral stenosis >50% | 1.999 (1.115–3.585) | 0.020 |
| Lack of hyperacute cerebral arterial imaging | 1.631 (1.009–2.636) | 0.046 |
NIHSS: National Institute of Health Stroke Scale.
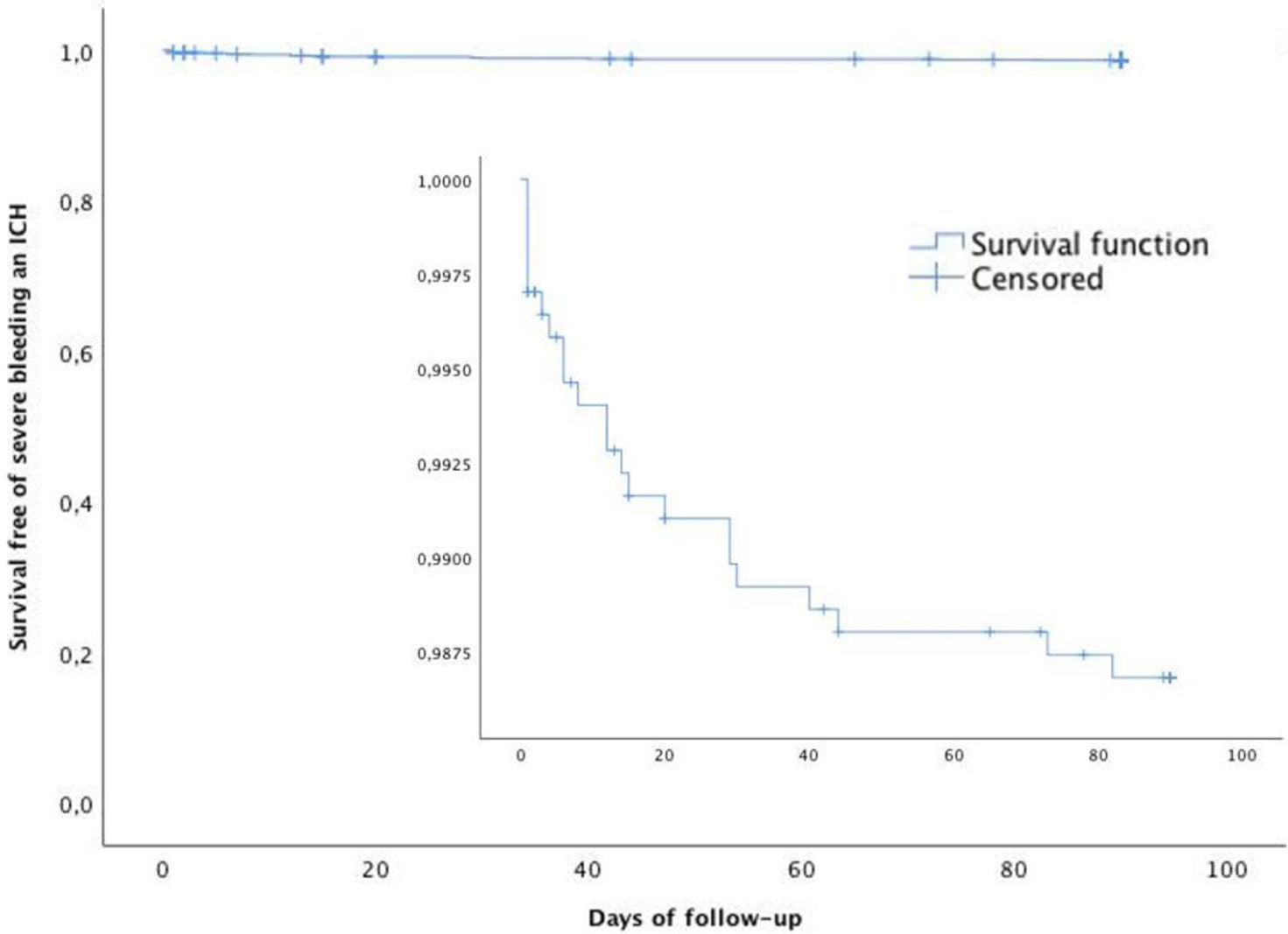
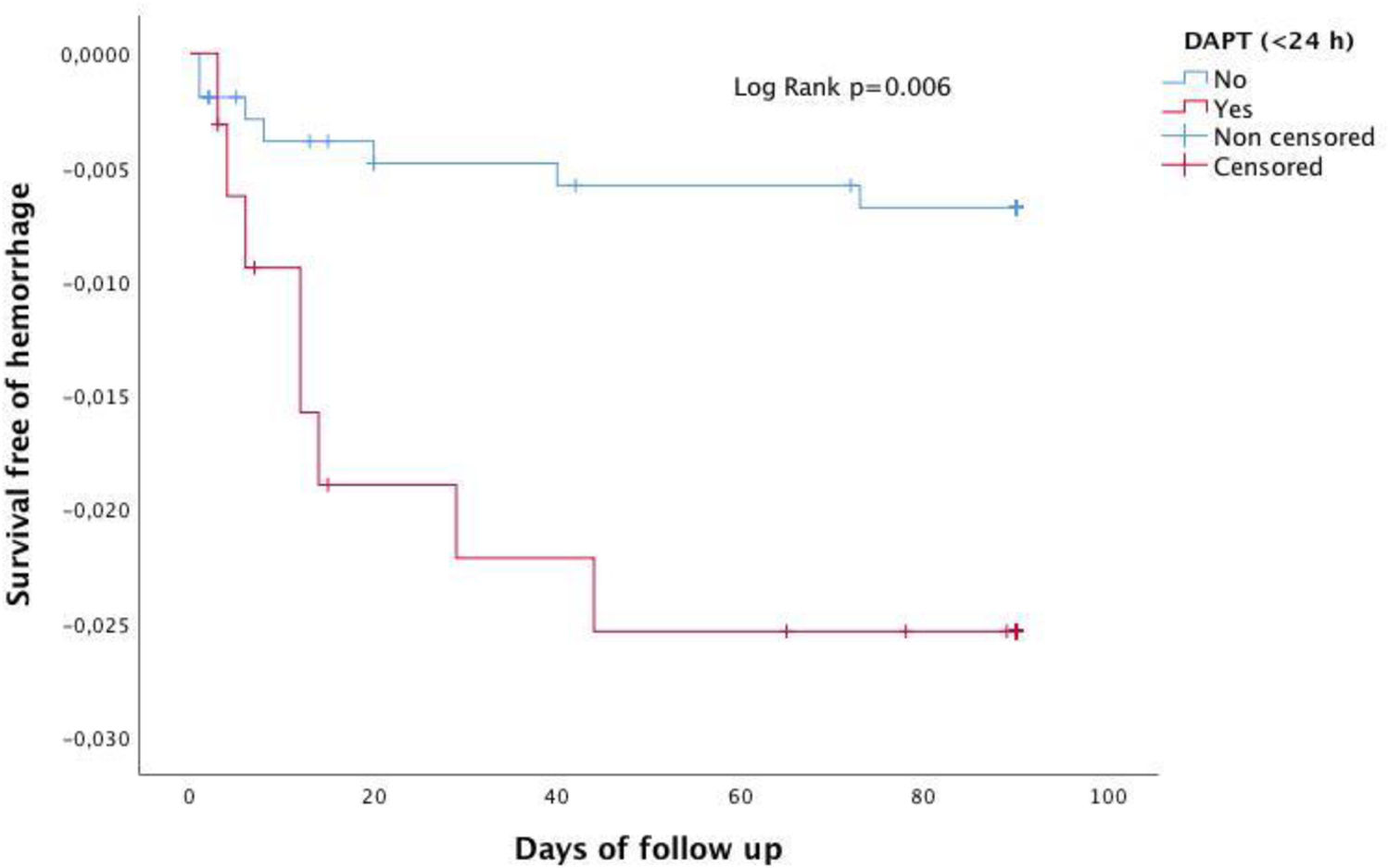
Fig. 3 shows that the Kaplan–Meier estimate of the probability of severe bleeding or ICH decreased after the first month. Severe extracranial bleeding occurred in 14 patients (0.8%) and intracranial hemorrhage in 20 (1.1%). The composite of ICH or severe bleeding occurred in 22 (1.3%) patients (Suppl. Table 1). Predictors of ICH and severe bleeding were previous antiplatelet therapy HR 3.545 (95% CI: 1.474–8.524, p=0.005) and treatment with DAPT initiated within the first 24h of index event HR 4.03 (95% CI: 1.493–12.401, p=0.007). Fig. 4 shows that the Kaplan–Meier estimate of the probability of severe bleeding or ICH during follow-up (90 days) was associated with DAPT initiated within the first 24h of index event Log Rank: p=0.006.
Regarding recurrent events, 17 (1%) patients experienced a TIA, 4 (0.7%) had an acute coronary syndrome and 6 (0.4%) had other vascular events (Suppl. Table 1).
Models of hyperacute and acute patient managementAs shown in Suppl. Table 2, most patients were attended by neurologists during the first 24h of symptoms onset 1509 (90%). Stroke code was activated in 725 (43%). Cerebrovascular (extracranial and intracranial) imaging was evaluated in the first 24h in 1226 (73%) patients and during hospital admission in 1584 (94%) patients. Most hospitalized patients were admitted in a stroke unit 1123 (66%).
Reperfusion therapy was given to 147 patients (9%). One hundred and sixteen patients (7%) received intravenous thrombolysis, 23 (1.4%) endovascular treatment, and 7 (0.6%) patients underwent both intravenous thrombolysis and mechanical thrombectomy.
In the first 24h, 1024 (61%) patients received SAPT and 320 (21%) were started on DAPT. At patient discharge, 1235 (75%) of the total cohort were taking SAPT and only 410 (24%) were on DAPT.
Functional outcome at three monthsOf the 1489 functionally independent patients prior to the index event (mRS 0–2), 1229 (84%) patients had good functional outcome at day 30 and 230 (16%) had poor prognosis. At three months, 1250 (86%) had a mRS 0–2, and 201 (13%) had poor outcome (Suppl. Table 2). In patients previously fully independent (mRS 0–1) the rate of (mRS 2–6) at 3 months was 26%.
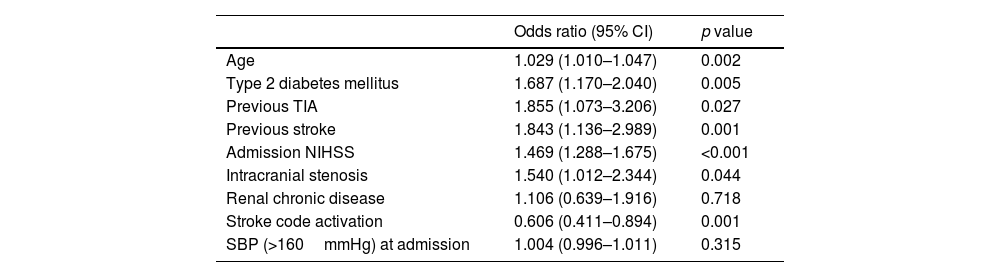
Independent predictors of poor functional outcome at 90 days were age (p=0.002), prior type 2 diabetes mellitus (p=0.005), previous TIA (p=0.027), previous stroke (p=0.001), NIHSS at admission (p<0.001), no activation of stroke code (p<0.001) and diagnosis of intracranial stenosis (p=0.044) (Table 3).
Predictors of poor functional outcome at 90 days.
| Odds ratio (95% CI) | p value | |
|---|---|---|
| Age | 1.029 (1.010–1.047) | 0.002 |
| Type 2 diabetes mellitus | 1.687 (1.170–2.040) | 0.005 |
| Previous TIA | 1.855 (1.073–3.206) | 0.027 |
| Previous stroke | 1.843 (1.136–2.989) | 0.001 |
| Admission NIHSS | 1.469 (1.288–1.675) | <0.001 |
| Intracranial stenosis | 1.540 (1.012–2.344) | 0.044 |
| Renal chronic disease | 1.106 (0.639–1.916) | 0.718 |
| Stroke code activation | 0.606 (0.411–0.894) | 0.001 |
| SBP (>160mmHg) at admission | 1.004 (0.996–1.011) | 0.315 |
NHISS: National Institute of Health Stroke Scale; SBP: systolic blood pressure.
Our data provides epidemiological data of the Spanish population with non-cardioembolic minor AIS and HR-TIA, which is currently lacking. In our study, 20% of stroke patients met the IMMINENT criteria, which yielded a global incidence of 48/100,000 inhabitants in our IMMINENT population. Recurrent ischemic stroke/death occurred in 3% during the first month and increased to 4.3% at 90 days. At three months, 13% of our patients had poor functional outcome. Despite current recommendations, only one out of four patients were on DAPT at discharge.
Our data confirm that this population has a high risk of recurrent ischemic stroke/death especially during the first month after the index event emphasizing the importance of initiating secondary stroke prevention as soon as possible. In two observational studies including patients with non-cardioembolic minor AIS and HR-TIA, the risk of a subsequent stroke or death was 2.8% and 3.7% at 30 days and increased to 3.7% and 7.6% at 90 days.3,16 Our recurrence rate was lower that observed in other historical cohorts.17 This could reflect better and faster implementation of secondary stroke prevention strategies.
In this study, extracranial ipsilateral stenosis was the most important independent predictor of RIS/death during follow-up, doubling the risk of occurrence of these events, and the highest percentage of RIS were of atherothrombotic etiology. These results are in line with other studies reporting that atherosclerotic stenosis ipsilateral to the index stroke confers the highest risk of recurrence.17–20 Lack of hyperacute vascular study imaging was another predictor of the primary endpoint at 30 and at 90 days. This reinforces the importance of urgent vascular assessment, as added value to current prediction scores like ABCD2, for early risk stratification and initiation of appropriate treatment for preventing recurrent vascular events, as described previously.20,21 Type 2 diabetes mellitus and each point increase in admission NIHSS were also predictors of RIS/death. Both variables were associated with disability in a previous cohort,22 probable reflecting poor prognosis caused by the recurrent vascular events.
In our study, 22 (1.3%) patients had severe bleeding and ICH, a proportion which is similar to that reported in other observational cohorts.17 Predictors of severe bleeding were previous antiplatelet treatment and DAPT administered in the first 24h, these events were more frequent in non-cardioembolic minor AIS patients (n=20.91%).
The majority of patients (90%) were attended by a neurologist within 24h after symptom onset, ensuring detection of early recurrences. However, the stroke code was activated only in 43% of them, indicating that the recognition of these stroke subtypes is still suboptimal, maybe related to mild symptoms or prompt recovery, and there is a delay in urgent neurological care. Regarding urgent management, 9% of patients were treated with reperfusion therapies. This could represent a subgroup with disabling symptoms or a large vessel occlusion despite minor symptoms. However, there is still uncertainty about which is the best approach for these patients since they were excluded from the pivotal clinical trials of mechanical thrombectomy.23
However, our results (only 21% of patients received DAPT in the first 24h and only 24% at discharge) reflect current low adherence to the guidelines recommendations favoring the use of DAPT7,8,10 over monotherapy and taking in account that 34% of the patients were under antithrombotic therapy before the index event. In agreement with our results, a recent work24 reported that 38.2% of patients were under aspirin monotherapy before stroke; among them, nearly half of patients remained on aspirin at discharge and just 24.6% received DAPT. A recent post hoc analysis of the POINT trial25 showed that DAPT reduces the risk of ischemic stroke regardless of premorbid antiplatelet use in this population.
We did not find significant differences in prognosis or recurrences regarding the antithrombotic treatment (DAPT versus SAPT). However, this is a real-world clinical practice study with an imbalance between both treatments that precludes any conclusion.
In our study, in patients previously fully independent (mRS 0–1), the rate of (mRS 2–6) at 3 months was 26%, in line with the results of the MaRISS study26 (37% with mRS 2–6 at day 90). This highlight that, in fact, the prognosis is not so benign as could be expected in this population. Our results indicate that age, diabetes mellitus, previous TIA/stroke, higher basal NIHSS and intracranial stenosis were independently associated to bad prognosis. These findings are consistent with data from other studies4,22,26,27 all of which included patients with minor AIS/TIA.
Our study has some limitations due to its retrospective design, which implies a risk of an underestimation of the incidence; although we analyzed data from all hospitals with stroke units from the North of Spain, some patients may have gone to other centers. We included patients evaluated in centers staffed by stroke specialists, which limits generalization of the findings to the population that might not have ready access to specialized services or to other sites with different protocols of attention to minor AIS/TIA.
In conclusion, in our study 20% of stroke patients presented as non-cardioembolic minor AIS or HR-TIA. This population has a high risk of stroke recurrence or death especially during the first month after the index event. Extracranial ipsilateral stenosis and lack of urgent cerebral arterial imaging were powerful predictors of stroke recurrence or death, whereas intracranial stenosis was associated with poor outcome. Despite current recommendations only one out of four patients are on DAPT at discharge, which demonstrates the need to insist on the proper implementation of current management guidelines of secondary stroke prevention.
Ethical approvalWritten informed consent was waived by ethics committee CEIm Área de Salud Valladolid Este (EPA OD: 20347), due to the study being a review of anonymized medical records.
FundingThis study was sponsored by AstraZeneca, funder had no involvement in the analysis or interpretation of the data, or the writing of the manuscript. MER-A was funded by the Instituto de Salud Carlos III (ISCIII)JR19/00020, co-funded by ERDF/ESF, “A way to make Europe”/“Investing in your future”). Investigators of this study belong to the RETICS-RICORS ICTUS financed by ISCIII (RD21/0006/0005-RD21/0006/0016-RD21/0006/0017-RD21/0006/0020-RD21/0006/0022).
Conflicts of interestDr. Arenillas reports having received honoraria as speaker/consultant for the following companies: BI, Pfizer, Daiichi, Bayer, Amgen, and Medtronic. The other authors report no conflicts.
Data availabilityAnonymized data will be shared by request to the corresponding author from any qualified investigator.
Hospital Clínico Universitario Valladolid: Alba Chavarría-Miranda, Isabel Hernández-Pérez, Mercedes De Lera. Complejo Hospitalario Universitario A Coruña: Lucía García Roca. Hospital Central Universitario de Asturias: María Castañón Apilanez, Begoña López López. Hospital Universitario Miguel Servet: Daniel Segarra Mur, Marta Serrano Ponz, Beatriz Pardiñas Barón. Hospital de Cabueñes: Aida García Rúa, Ignacio Casado Menéndez. Complejo Hospitalario Universitario de Vigo: Carmen Labandeira Guerra. Hospital Universitario de Basurto: Iván Caballero Romero. Hospital Universitario de Donostia: Inés Albájar Gómez. Hospital Universitario Marqués de Valdecilla: Daniel Gallo Valentín. Hospital Universitario de Santiago de Compostela: Susana Arias Rivas, Iria López Dequiat, Emilio Rodríguez Castro. Hospital de Txagorritxu: Eva Porqueres Bosch. Hospital Lozano Blesa: Cristina Pérez, Sara Ballesta. Hospital Universitario de Salamanca: Sergio Borja Andrés, Octavio López Agudelo, Guillherme Carvalho Monteiro. Hospital Universitario de Navarra: Mikel San Miguel Oroz, Dagoberto Aspra Martínez.