Excluding cases of vasospasm following rupture of saccular aneurysms in the anterior cerebral artery (ACA) and/or the anterior communicating artery, ACA infarcts account for 0.6%-3% of all acute ischaemic strokes.1 Bilateral ACA infarct is extremely rare in the absence of such angioarchitectural abnormalities as A1 segment hypoplasia, a bihemispheric ACA (a single A2 segment supplying both ACA territories), or an azygos ACA (both A1 segments joining to form a single A2 segment).2 The main cause of ACA infarct is embolism, usually accompanied by contralateral ACA hypoplasia.1–3 Recent studies suggest an association between atrial fibrillation (AF) and acute myocardial infarction (AMI); this is a bidirectional process induced by a proinflammatory and prothrombotic state.4–6 We present an exceptional case of AF associated with AMI and subacute ischaemic stroke of the bilateral ACA with haemorrhagic transformation and a double emboligenic source.
Clinical caseOur patient was a 67-year-old right-handed woman with a history of arterial hypertension, type 2 diabetes mellitus, dyslipidaemia, and obesity, but no history of heart disease or arrhythmia (mRS score: 1). She came to the emergency department due to a 26-hour history of palpitations, dyspnoea, oppressive retrosternal pain, difficulty articulating speech, and right lower limb weakness. Symptoms had started abruptly upon waking. She had a blood pressure of 96/70mmHg in both arms, a temperature of 36.9°C, and an irregular heart rate of 160bpm; all other results from the physical examination were normal. The neurological examination revealed drowsiness, left-sided deviation of the head and eyes, right-sided central facial palsy upon licking and sucking, right-sided hemiplegia (especially at the level of the thigh), right-sided hyperreflexia (including marked grasp reflex and Babinski sign), right lower limb anaesthesia, transcortical motor aphasia, dysarthria, and right hemispatial neglect (NIHSS score: 23). An initial blood analysis showed increased levels of cardiac biomarkers: CPK-MB 261U/L (normal range, 0-16U/L) and troponin T 1.5ng/mL (0-0.1ng/mL). A further blood analysis performed 6 hours later revealed a troponin T level of 2.3ng/mL; levels decreased during the following 8 days. An ECG revealed AF with rapid ventricular response and pathological Q waves in leads V1-V4 (Fig. 1A). An emergency head CT scan revealed ischaemic stroke at the level of the left ACA (Fig. 1B); a transthoracic echocardiogram (TTE) detected akinesis of the left ventricle and a mural thrombus (Fig. 1D). Reperfusion therapy was ruled out due to the diagnostic delay. Imaging results (particularly those of the TTE, which revealed a mural thrombus) led us to start anticoagulation therapy with intravenous sodium heparin (we aimed to maintain partial thromboplastin time at 1.5-2 times the reference time). Following assessment by the cardiology department, the patient also received antiplatelet therapy with acetylsalicylic acid 100mg/day plus clopidogrel 75mg/day as a secondary preventive measure for acute coronary syndrome (ACS). The patient was admitted to the stroke unit for further medical assessment and treatment. On post-stroke day 8, however, the patient developed akinetic mutism. A head CT scan revealed several subacute strokes, with areas displaying haemorrhagic transformation of ischaemic tissue in the territory of both ACAs (Fig. 1C); MRI-angiography of the head and neck revealed no vascular alterations. A month later, the patient displayed haematochezia, causing haemoglobin concentration to fall by 5.3g/dL. Triple antithrombotic therapy (anticoagulation plus dual antiplatelet therapy) was replaced by enoxaparin 40mg/day plus clopidogrel 75mg/day. The results of a complete blood count (including autoimmunity and hypo- and hypercoagulability tests), a full-body CT scan, and a colonoscopy were all normal. Haematochezia resolved progressively and analysis results improved. The neurological examination performed at discharge (6 months after stroke) revealed aboulia, apathy, motor aphasia, and moderate quadriparesis mainly affecting the lower limbs (NIHSS: 9; mRS: 5). A follow-up TTE found no thrombi (Fig. 1E). The patient died 9 months after stroke due to respiratory insufficiency secondary to aspiration pneumonia.
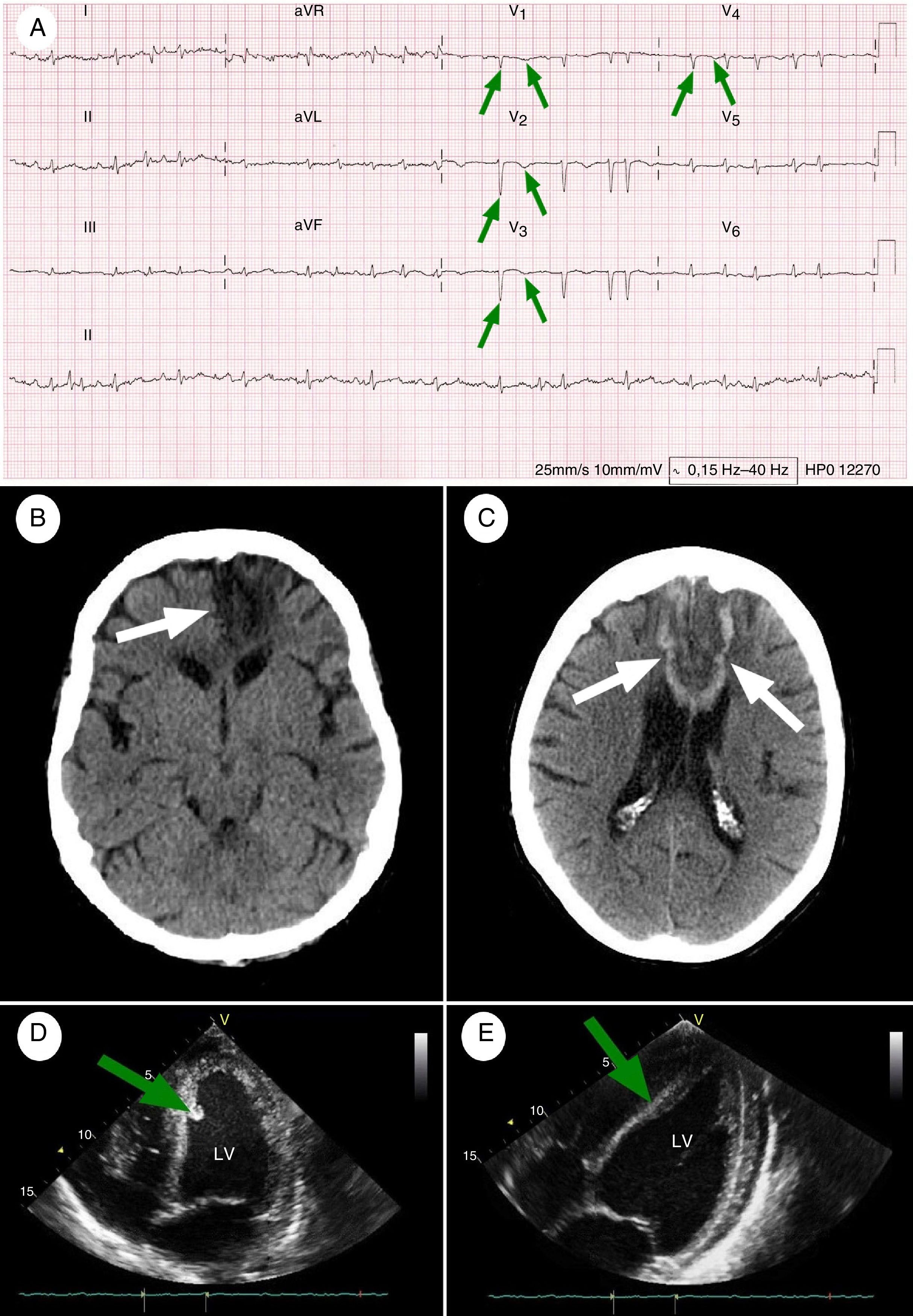
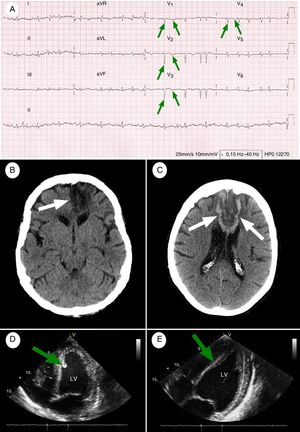
Baseline and follow-up complementary tests. (A) 12-Lead ECG performed at admission (26 hours after symptom onset): AF at a rate of 114bpm, with pathological Q waves and inverted T waves in precordial leads V1-V4 (green arrows), indicating necrosis in the anterior wall of the left ventricle and interventricular septum (paper speed, 25mm/s). (B) Simple head CT scan performed at baseline: wedge-shaped hypodense area in the territory of the left ACA, affecting the corpus callosum (white arrow), the territory of the recurrent artery of Heubner, and the orbitofrontal, medial, and superior regions of the left frontal lobe. (C) Simple head CT scan (8 days after symptom onset) showing hypodense areas with haemorrhagic transformation, associated with subacute strokes in the territory of both ACAs (white arrows). Dark areas: ischaemia; white areas: haemorrhage. (D) TTE at admission: akinetic area at the apex of the heart during diastole, and hyperechogenic area around the interventricular septum, compatible with a mural thrombus (green arrow). These findings were associated with a left ventricular ejection fraction (LVEF) of up to 45% during systole. (E) A follow-up TTE performed 6 months after admission showed considerable improvements in the motility of the anteroapical wall of the left ventricle, near-complete disappearance of the mural thrombus (green arrow), and an LVEF of 60%.
LV: left ventricle.
In case reports, ACA infarcts are more frequently associated with internal carotid artery (ICA) atherosclerosis than with primary ACA thrombosis.1 In contrast, a retrospective study conducted in South Korea found local ACA atherosclerosis in 68 of a series of 100 patients with ACA infarct.7 In the Lausanne Stroke Registry, a prospective study conducted in Switzerland, 17 of the 27 patients included (63%) had emboli originating in the ICA or the heart.8 A recent retrospective study conducted in Spain reports that 23 of the 51 cases of ACA infarct (45.1%) were of cardioembolic origin.9 ACA infarct is highly variable in terms of symptoms; a deep understanding of the neuroanatomy of the ACA territory is necessary for diagnosis.1,8 AMI and coronary artery disease are well-known risk factors for AF, mainly due to atrial remodelling and/or transient ventricular ischaemia, which results in atrial pressure overload. According to several recent studies, however, this relationship works in the opposite direction: AF may lead to AMI due to increased heart rate, which results in increased oxygen demand, sympathetic hyperactivation, endothelial dysfunction, and proinflammatory and prothrombotic effects.4–6 Insufficient evidence is available to suggest that triple antithrombotic therapy is superior to anticoagulation alone for reducing the risk of stroke and/or AMI in patients with FA; however, an exception may be the case of patients with clinically evident coronary artery disease, particularly ACS, since nearly 20% of patients with ischaemic stroke associated with AF also present this heart disease.10 No randomised clinical trials have evaluated the ideal antithrombotic therapy for preventing mural thrombi and stroke in patients with ACS. However, an open randomised clinical trial comparing the effects of warfarin, acetylsalicylic acid, and the combination of both drugs in 3630 patients with AMI, with a mean follow-up period of 4 years, found that the combined treatment reduced non-fatal AMI recurrence and thromboembolic ischaemic stroke by 29% compared to acetylsalicylic acid only (rate ratio: 0.71; 95% CI, 0.60-0.83; P=.001) and by 13% compared to warfarin only (rate ratio: 0.87; 95% CI, 0.73-1.03; P=.18, not significant). Warfarin was associated with a greater risk of bleeding (particularly gastrointestinal bleeding) than the other treatments.11 The Spanish Society of Cardiology's consensus document on the management of patients with AF and ACS, who have a high risk of haemorrhage (HAS-BLED score≥3) and thromboembolia (CHA2DS2-VASc≥2), recommends administering triple antithrombotic therapy for 4 weeks, followed by dual therapy with oral anticoagulants plus clopidogrel 75mg/day (or acetylsalicylic acid 100mg/day) until month 12, and then oral anticoagulation indefinitely.12,13 A comprehensive literature search on PubMed yielded no reports of patients with AF manifesting as AMI and bilateral ACA infarct. Physicians should consider this possibility in cases initially compatible with this complex and unusual scenario, even when patients have no cerebral vasculature abnormalities or history of thrombophilia, and especially in those with cardiovascular risk factors. This would assist in the early diagnosis and treatment of the condition, reducing the risk of recurrent embolism. There is a need for randomised controlled trials including patients with cardioembolic ischaemic stroke and emboligenic comorbidities associated with AF and AMI, in order to determine the optimal triple antithrombotic therapy in terms of efficacy and safety to prevent recurrent events.
The main limitation for determining the exact aetiology in our patient was the inability to perform a coronarography, which would have provided more reliable information on the sequence of pathophysiological events experienced by our patient. Our patient arrived at the emergency department over 24 hours after symptom onset; the usefulness of this technique for reperfusion was not clear at that point. Furthermore, we opted for multidisciplinary conservative treatment as the patient's relatives refused any type of invasive procedure unable to significantly improve functional independence.
FundingThis study received no public or private funding.
Conflicts of interestAll authors have given their approval for the publication of the manuscript. The authors have no conflicts of interest to declare.
We would like to thank Drs Juan Camilo Rodríguez Carrillo, Clara Aguirre Hernández, Álvaro Ximénez-Carrillo Rico, Francisco Cabrera Valdivia, and José Tejeiro Martínez.
Please cite this article as: León Ruiz M, García Soldevilla MA, Vidal Díaz MB, Izquierdo Esteban L, Benito-León J, García-Albea Ristol E. Infarto agudo de miocardio asociado con ictus subagudo bilateral cardioembólico en el territorio de la arteria cerebral anterior: la cara oculta de una fibrilación auricular de novo. Neurología. 2019;34:127–130.
This study was presented in e-poster format at the 25th European Stroke Conference (Venice, Italy, 14 April 2016).