We describe a case of isolated idiopathic hypoglossal nerve palsy in a young male patient and discuss several of the aetiopathogenic mechanisms which may cause this entity.
Our patient was a 36-year-old man with no relevant medical history who attended the emergency department with a tongue motility disorder, which caused difficulty chewing food. A week before, he developed a right-sided hemicranial headache, reported to be of moderate-high intensity, starting in the cervical region and radiating to the occipital region; he did not present nausea, vomiting, photophobia, or phonophobia. The previous day, he had experienced discomfort in the right pharyngeal region, which he described as an oppressive and inflammatory sensation.
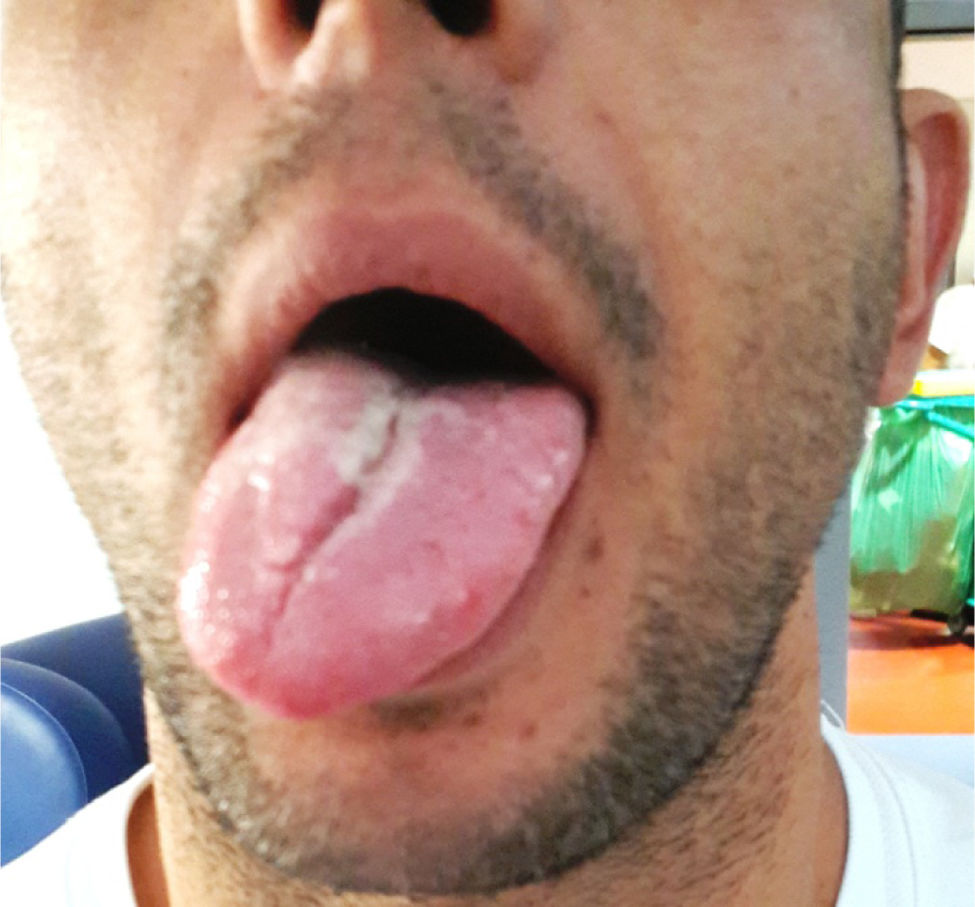
The neurological examination revealed right-sided deviation of the tongue; the patient had difficulty moving it further to the right (Fig. 1). An examination of the remaining cranial nerves, motor function, sensitivity, static coordination, and gait revealed no abnormal findings. The general physical examination obtained normal results, with no palpable pathological peripheral adenopathies.
We performed an analytical study including a complete blood count, urea, creatinine, uric acid, electrolytes, transaminases, alkaline phosphatase, LDH, total and direct bilirubin, creatin kinase, C reactive protein (CRP), rheumatoid factor, and angiotensin-converting enzyme (ACE). As part of the autoimmunity study, we requested anti-nuclear antibodies (ANA) and anti-neutrophil cytoplasmic antibodies (ANCA) tests, as well as serology tests for HIV, EBV, HSV, and VZV. A lumbar puncture was performed; CSF samples were sent for cytobiochemical study, Tibbling index, oligoclonal band testing, microbiological studies, and serology tests. All these parameters were normal or negative.
A chest radiography showed no pathological findings. A head magnetic resonance imaging (MRI) scan only revealed a cyst of the septum pellucidum. MRI angiography of the supra-aortic trunks and brain revealed no stenotic lesions or other findings of pathological significance in the carotid and vertebrobasilar systems; the brain vascular tree presented normal morphology and distribution. A neck CT scan was also performed, identifying no significant locoregional ganglion growth or other relevant findings. We requested an examination by the otorhinolaryngology department, which did not identify any locoregional cause that may explain the patient's symptoms.
The patient progressed favourably and recovered tongue motor function in 10 days. After one year of follow-up, he has experienced no further focal neurological signs.
The hypoglossal nerve may be affected at any point of its length. Due to the close anatomical relationship with other medullary pathways and nuclei and with the other lower cranial nerves in their path outside the medulla oblongata, hypoglossal nerve palsy usually manifests with simultaneous involvement of other cranial nerves as the glossopharyngeal, vagus, or spinal nerves. Diagnosis requires precise knowledge of the path of the hypoglossal nerve and its relationships with other structures along that path.1 Numerous causes of isolated hypoglossal nerve palsy have been described. Intracranial neoplasms, skull base neoplasms, and vertebral trauma have classically been reported as the most frequent causes, accounting for up to 50% of cases.1 Furthermore, isolated hypoglossal nerve palsy has recently been reported as the sole manifestation of cervical artery dissection.2,3 On some occasions, however, isolated hypoglossal nerve palsy resolves spontaneously and no underlying cause is determined, despite an exhaustive study. These cases of isolated hypoglossal nerve palsy are infrequent; very few case series have been published in the literature.
Keane et al.1 analysed 100 cases of hypoglossal nerve palsy and determined that in most cases, other cranial nerves were simultaneously affected; neoproliferative processes are the main cause in this series (49%), followed by trauma-related (12%), vascular (6%), psychiatric (6%), post-surgical iatrogenic (5%), and infectious aetiologies (4%). Multiple sclerosis (6%) and Guillain-Barré syndrome (4%) were also listed among the identified causes; 3% of cases were cryptogenic.
However, few series of isolated hypoglossal nerve palsy have been published.4–6 Combarros et al.4 published 9 cases with similar presentation to that of our patient. In that study, researchers identified the following causes: a primary or metastatic proliferative process in 3 patients, an Arnold–Chiari malformation, and dural arteriovenous fistula of the transverse sinus.4 Giuffrida et al.5 described 3 cases of isolated hypoglossal palsy, of which one was due to a head trauma. Boban et al.6 reported 4 patients with isolated hypoglossal palsy caused by carotid pseudoaneurysm, metastatic cancer, and acute disseminated encephalomyelitis. The list of causes of isolated hypoglossal nerve palsy is very extensive (Table 1). However, cases with no identified underlying cause have also been published. In the series published by Keane et al.1 only 3% of cases were idiopathic; however, the series also included cases that were not isolated. If we consider series limited to isolated hypoglossal nerve palsy, the frequency of idiopathic cases significantly increases. In the article published by Combarros et al.,4 no aetiological diagnosis was obtained for 4 of the 9 patients with isolated hypoglossal nerve palsy. Similarly, the series of 3 cases published by Giuffrida et al.5 included 2 idiopathic cases. Combining data from several published series, some authors suggest that approximately half of cases of isolated hypoglossal nerve palsy are idiopathic.7
Reported causes of isolated hypoglossal nerve palsy.
| Aetiology |
|---|
| Head and neck trauma |
| Neoplasms |
| Epstein–Barr virus infection |
| Herpes simplex virus infection |
| Pyogenic meningitis |
| Tuberculosis |
| Cervical artery dissection |
| Internal carotid artery pseudoaneurysm |
| Dural arteriovenous fistula of the transverse sinus |
| Iatrogenesis |
| Wegener granulomatosis |
| Rheumatoid arthritis |
| Behçet disease |
| Arnold–Chiari malformation |
| Demyelinating diseases |
| Multiple myeloma |
| Parainfectious |
Isolated hypoglossal nerve palsy may manifest at any time, from childhood to old age,4,8,9 although the great majority of reported cases manifest at 20-45 years. Some authors compare isolated and idiopathic hypoglossal nerve palsy with Bell palsy. Prognosis of cryptogenic cases is excellent, with tongue mobility recovering spontaneously within a few months.4,8,9 Persistence of palsy is very rare; very few articles in the literature describe this situation.4,8,10
Isolated idiopathic hypoglossal nerve palsy is an infrequent condition representing a challenge in everyday clinical practice, and should be considered a diagnosis of exclusion. Diagnosis requires a systematic search for skull base lesions, especially neoproliferative processes, vascular diseases, and traumatic disease. It usually presents partial or total spontaneous remission. In our case, we observed unilateral, idiopathic, isolated hypoglossal nerve palsy. The study to rule out other processes obtained negative results, and progression was excellent, with the patient recovering in 10 days; furthermore, after one year of follow-up, no neurological events have recurred; we therefore consider that idiopathic isolated hypoglossal nerve palsy is a self-limited disorder, with a favourable clinical prognosis.
Please cite this article as: Robaina Bordón JM, González Hernández A, Curutchet Mesner L, Gil Díaz A. Paresia idiopática del nervio hipogloso. Neurología. 2019;34:125–127.