Atrial fibrillation (AF), the most common sustained arrhythmia, is estimated to affect 5% of individuals older than 65 years of age.1 AF is frequently asymptomatic, and 15% of strokes are associated with untreated AF.2 This has led researchers to search for potential predictors of AF and of the risk of embolism. We present a case of ischaemic stroke in the context of paroxysmal AF.
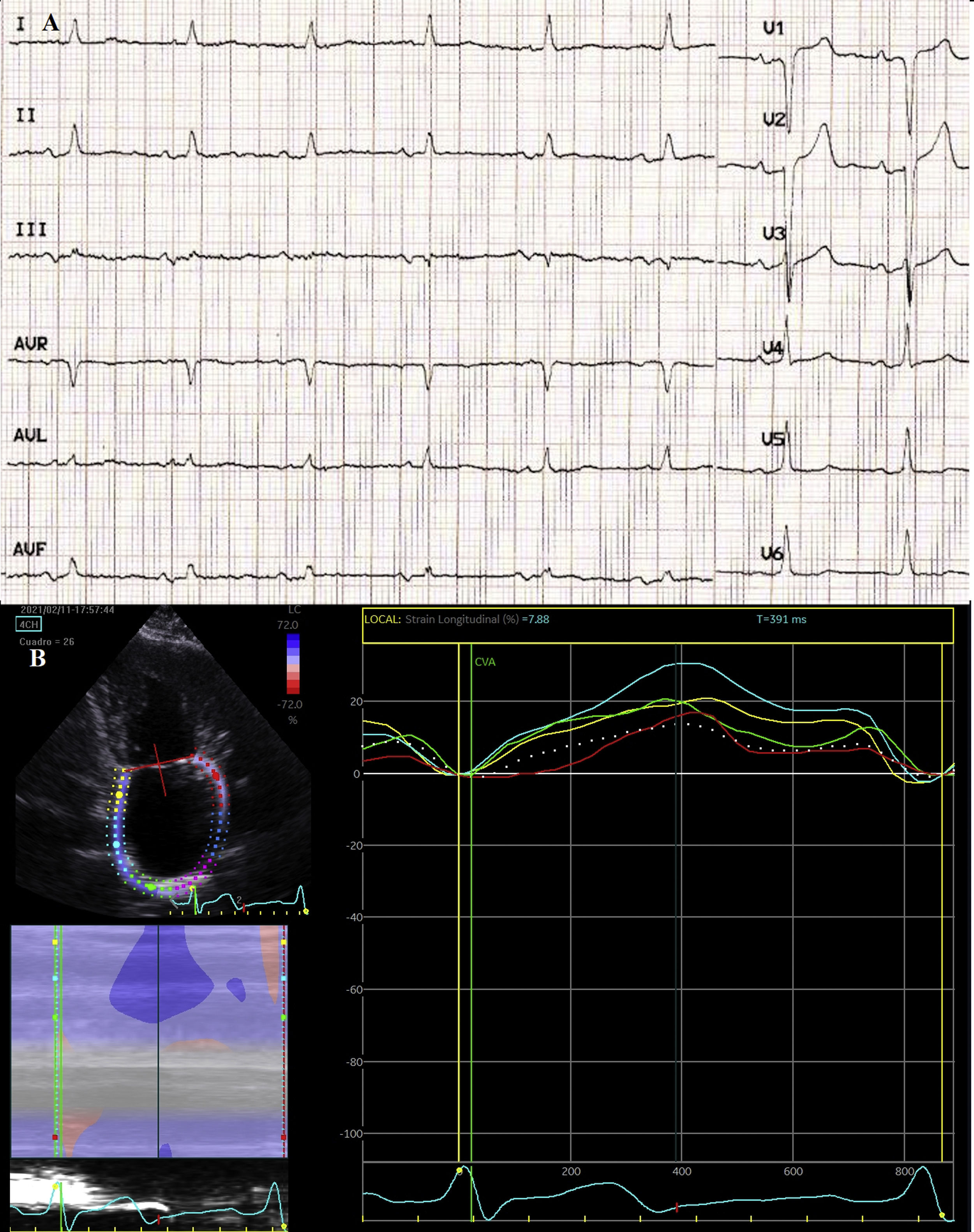
The patient was a 73-year-old woman with arterial hypertension and type 2 diabetes mellitus, who was independent for daily living activities and was under follow-up for dyspnoea. Electrocardiography (ECG) revealed sinus rhythm and P-wave duration > 120 ms with biphasic morphology (+/–) in the inferior leads. Echocardiography revealed normal left ventricular ejection fraction, moderate left atrial enlargement (43 mL/m2), and a significantly decreased peak atrial longitudinal strain (13.5%) (Fig. 1). The patient was diagnosed with heart failure with preserved ejection fraction, and her symptoms improved with diuretic treatment. Several months later, she visited the emergency department due to predominantly sensory mixed aphasia and right central facial paresis of 6 hours’ duration. She scored 6 on the NIHSS at arrival. A CT scan revealed established ischaemic stroke in the territory of the middle cerebral artery, and ECG detected AF. A Doppler ultrasound of the supra-aortic trunks revealed atheromatous plaque, with no haemodynamic involvement. The patient was admitted to our department, where we started heart rate monitoring and administered anticoagulation treatment, after ruling out haemorrhagic transformation in a follow-up CT scan. She was discharged with a diagnosis of cardioembolic stroke.
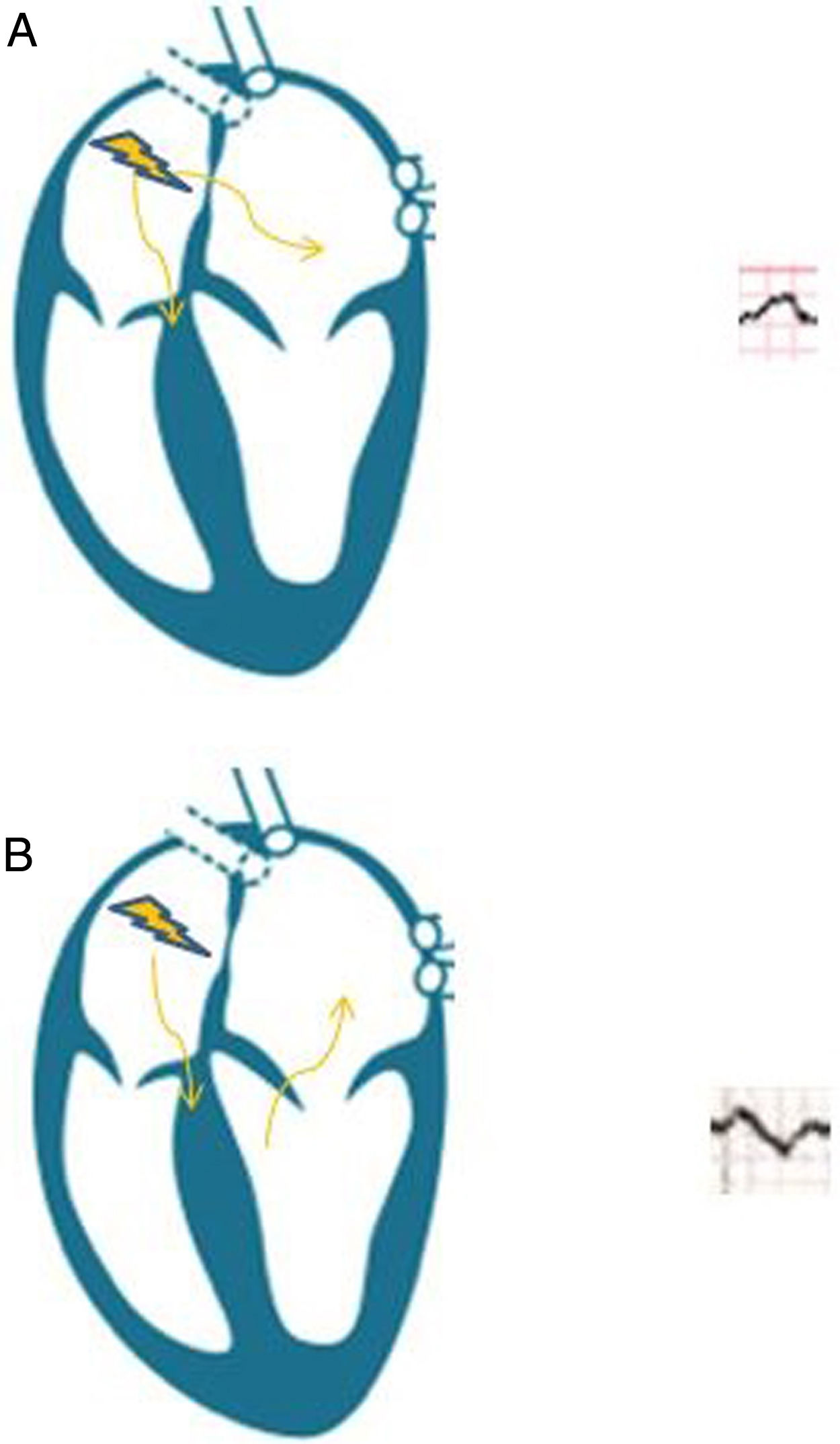
Our patient presented advanced interatrial block (AIB), and subsequently developed AF and stroke. AIB is an ECG pattern first described by Bayés de Luna3 in the late 1970s, and is characterised by conduction delay through the Bachmann bundle, the main conduction pathway between the 2 atria (Fig. 2). Partial interatrial block is more common, and is characterised exclusively by prolonged P-wave duration (> 120 ms) in the inferior leads. AIB involves retrograde activation of the left atrium from the coronary sinus, which results in abnormal electrical activity (P-wave duration > 120 ms, associated with a negative terminal component of the P-wave in inferior leads).4 The association between AIB and paroxysmal AF is known as Bayés syndrome.5 Atrial fibrosis and atrial dyssynchrony constitute the anatomical substrate for AIB and AF, promoting systemic embolism, although atypical forms have also been described in patients with diabetes or chronic kidney disease, with no impact on the risk of AF or stroke.6 The concept of atrial failure has been proposed to underscore the association between this entity and thromboembolic events, with AF being considered a marker of atrial dysfunction, rather than a direct emboligenic mechanism.7 Speckle-tracking echocardiography is a novel technique enabling the assessment of atrial fibrosis in order to predict emboligenic events.8 Although no clinical trial performed to date supports the use of anticoagulants in patients with AIB but not AF, a thorough ECG study is essential. Detecting AIB allows us to identify patients at high risk of embolism who may benefit from prolonged monitoring (external loop recorder, implantable Holter monitor, portable mHealth system); this strategy may in future contribute to deciding whether anticoagulant or antiarrhythmic treatment is needed.4
Please cite this article as: Lopez Perales CR, Perez Guerrero A, Grados Saso D, Salvador Casabona JM. Bloqueo interauricular avanzado como predictor de ictus cardioembólico: ¿es hora de cambiar nuestra práctica clínica? Neurología. 2022;37:413–415.