The Addenbrooke's Cognitive Examination III (ACE-III), an adaptation of the ACE cognitive screening test, has been demonstrated to have high sensitivity and specificity in detecting cognitive impairment in patients with dementia and other neurological and psychiatric disorders. Although the Spanish-language version of the ACE-III has already been validated in Spain, it is yet to be validated in Latin America. The aim of this study was to validate the ACE-III test in an Argentinean and Chilean population.
MethodsACE-III was administered to 70 patients with Alzheimer disease, 31 patients with behavioural variant frontotemporal dementia, and a control group of 139 healthy volunteers. Participants were recruited at centres in both countries.
ResultsThe Spanish-language version of ACE-III was found to have good internal consistency (Cronbach's alpha=0.87). We found significant differences in total ACE-III scores between patients with Alzheimer disease and controls (P<.05) and between patients with Alzheimer disease and bvFTD (P<.05). With a cut-off point of 86, 98.6% of AD patients, 83.9% of behavioural variant frontotemporal dementia patients, and 84.2% of controls were correctly classified.
ConclusionsThis study shows that the Spanish-language version of ACE-III continues to be an effective tool for detecting cognitive dysfunction in patients with dementia.
El Addenbrooke's Cognitive Examination III (ACE-III) es una adaptación del test de cribado ACE, la cual ha demostrado tener una alta sensibilidad y especificidad para detectar disfunción cognitiva en pacientes con demencia y otras patologías neurológicas y psiquiátricas. Si bien el ACE-III ya ha sido validado en castellano (España), este no ha sido validado en Latinoamérica. El objetivo del presente estudio fue validar el ACE-III en una población argentina y chilena.
MétodosSe evaluó un grupo de pacientes con enfermedad de Alzheimer (n=70), un grupo de pacientes con la variante conductual de la demencia frontotemporal (n=31) y un grupo control (n=139) con la versión en español del ACE-III; reclutados en centros médicos de ambos países.
ResultadosLa versión argentina-chilena del ACE-III presentó una buena consistencia interna (alfa de Cronbach=0,87). Se hallaron diferencias significativas en los valores totales del ACE-III entre el grupo control y el grupo de demencias (p<0,05) y entre ambos grupos de demencia (p<0,05). Con un punto de corte de 86, el 98,6% de los pacientes con DTA, el 83,9% de pacientes con variante conductual de la demencia frontotemporal y el 84,2% de los controles fue correctamente clasificado.
ConclusionesEl presente estudio ha demostrado que el ACE-III continúa siendo una herramienta útil para la detección de la disfunción cognitiva en la demencia.
The number of consultations due to memory complaints has significantly increased in recent years; this trend is probably associated with the increased prevalence and greater knowledge of dementia. The implication of this is a need for diagnostic tests that improve the early detection of dementia.1–3 It is imperative that these tests should be brief, easy to administer, and accessible in daily clinical practice. The availability of valid screening tests for detecting cognitive deficits is therefore also of great importance.
Over the years, several cognitive screening instruments have been developed to enable easy, objective estimation of an individual's risk of developing dementia-like symptoms. One of the earliest and most widely used is the Mini-Mental State Examination (MMSE).4 However, the MMSE has shown several limitations, the most important being its poor sensitivity for detecting early-stage dementia and non-Alzheimer dementia.5 These limitations led to the development of the Addenbroke's Cognitive Examination (ACE).6 This instrument included the MMSE tests and added other more sensitive and specific tests, enabling a more complete description of the patient's overall cognitive performance. The ACE described cognitive performance in 6 domains: orientation, attention, memory, verbal fluency, language, and visuospatial abilities; the maximum possible score was 100, including the 30 points from the MMSE. It also included a coefficient called the VLOM ratio that enabled differentiation between Alzheimer-type dementia (ATD) and behavioural variant frontotemporal dementia (bv-FTD). Thus, this new instrument more accurately determined the presence or absence of cognitive impairment and facilitated differential diagnosis between different types of dementia.
The original version of the ACE was revised and updated on several occasions, overcoming the limitations observed in its daily use. In this context, the Addenbrooke's Cognitive Examination-Revised (ACE-R)7 was developed, followed several years later by the Addenbrooke's Cognitive Examination III (ACE-III).8 The ACE-R was validated for the Argentinian and Chilean populations and was shown to be very useful in detecting and differentiating between the cognitive deficits characteristic of dementias including ATD and bv-FTD.9,10 It has been used not exclusively in the field of dementias, but also for such other disorders as Parkinson's disease,11 progressive supranuclear palsy,12 corticobasal degeneration,13 multiple system atrophy,14 and psychiatric disorders, such as depression.15
Adaptations of Addenbrooke's Cognitive Examination IIIThe ACE has become highly popular in clinical practice, as it is brief, easy to administer, and sensitive for detecting cognitive impairment; as a result, it has been used in several countries and translated into various languages. Several alternative forms (versions A, B, and C) have been created with different stimuli for recalling the name and address to avoid the learning effect in repeated evaluations. It includes 5 subscales representing specific cognitive domains (orientation and attention, memory, verbal fluency, language, and visuospatial abilities). The total maximum score is 100, with similar scores for all subscales, including the 30 points from the MMSE. This version was revised on 2 occasions, giving rise to the ACE-R and the recently published ACE-III, which was the result of work by the Australian researchers Hsieh et al.8
The previous versions of the ACE-III (ACE and ACE-R) included all items from the MMSE, thereby providing the total scores for both tests. Due to conflict regarding the MMSE copyright, the ACE-III replaces all items from the MMSE with analogous items, keeping a maximum score of 100.8
The following changes were made to each subscale: the orientation and attention subscales were unified, and the task of backward spelling of the word “mundo” (world) was removed, keeping serial subtraction only. For the language subscale, the sentence “close your eyes” was removed and the task of writing a sentence was changed to writing 2 sentences on a common subject. Complex command tasks were replaced by tasks with 3 simple commands of increasing syntactic complexity, the 2 sentences previously used in the repetition task were modified, and in the naming test, the 2 first objects (“clock” and “pencil”) were replaced by 2 very common objects (“spoon” and “book”). In the visuospatial abilities domain, pentagons were replaced with the infinity symbol. The memory and verbal fluency domains of the ACE-R were not modified.8
Published data suggest that the ACE-III overcomes many limitations of the original instrument. Given the absence of a Spanish-language version of the ACE-III, Matías-Guiu et al.16 produced a Spanish-language adaptation, provided normative data,17 and developed a new abbreviated version (the Mini-ACE).18 The group demonstrated the validity of both instruments in detecting cognitive impairment. Furthermore, the Spanish-language version of the ACE-III has been shown to be superior to other screening tests in terms of its diagnostic accuracy for screening for dementia,19 as well as its validity in detecting amnestic mild cognitive impairment and Alzheimer disease in early stages.20
Although the validity of the Spanish-language version of the ACE-III has been demonstrated,16 we suspect that the cultural, social, and socioeconomic differences between Spanish and Argentinian/Chilean populations may affect the interpretation and conclusions drawn from the test results.21,22 For this reason, the aim of the present study was to validate the ACE-III for the Argentinian/Chilean population.
Patients and methodsWe assessed 240 individuals recruited from the Cognitive Neurology Institute in Argentina and the Cognitive Neurology and Dementia Unit at the Neurology Department at Hospital del Salvador in Chile. The study period was 2 years. We divided participants into 3 groups: patients diagnosed with bv-FTD (n=31), patients diagnosed with ATD (n=70), and healthy controls matched for age, sex, and level of education (n=139). ATD was diagnosed according to the NINCDS-ADRDA criteria,23 and bv-FTD according to the consensus criteria established by Neary et al.24 The healthy control group did not present history of neurological or psychiatric diseases.
InstrumentWe used the Spanish-language version of the ACE-III, adapted to the Argentinian and Chilean populations by an expert committee. By consensus, we created a version for both countries including 5 subscales (attention, memory, language, verbal fluency, and visuospatial ability), with a maximum score of 100 points.
Procedure and statistical analysisThe study was previously approved by the ethics committees of the institutions involved and complies with the international regulations for human research. All participants signed an informed consent form for inclusion in the study. Specialist neurologists conducted interviews to confirm that patients met the inclusion criteria, and patients were then assessed by a specialist neuropsychologist using the Spanish-language version of the ACE-III. Statistical analysis was performed using SPSS computer software version 22. We used a one-way analysis of variance (ANOVA) and the Tukey test to compare mean values for demographic variables in the different groups. Internal validity was determined using the Cronbach alpha coefficient. Sensitivity and specificity values were determined using the receiver operating characteristic (ROC) curve.
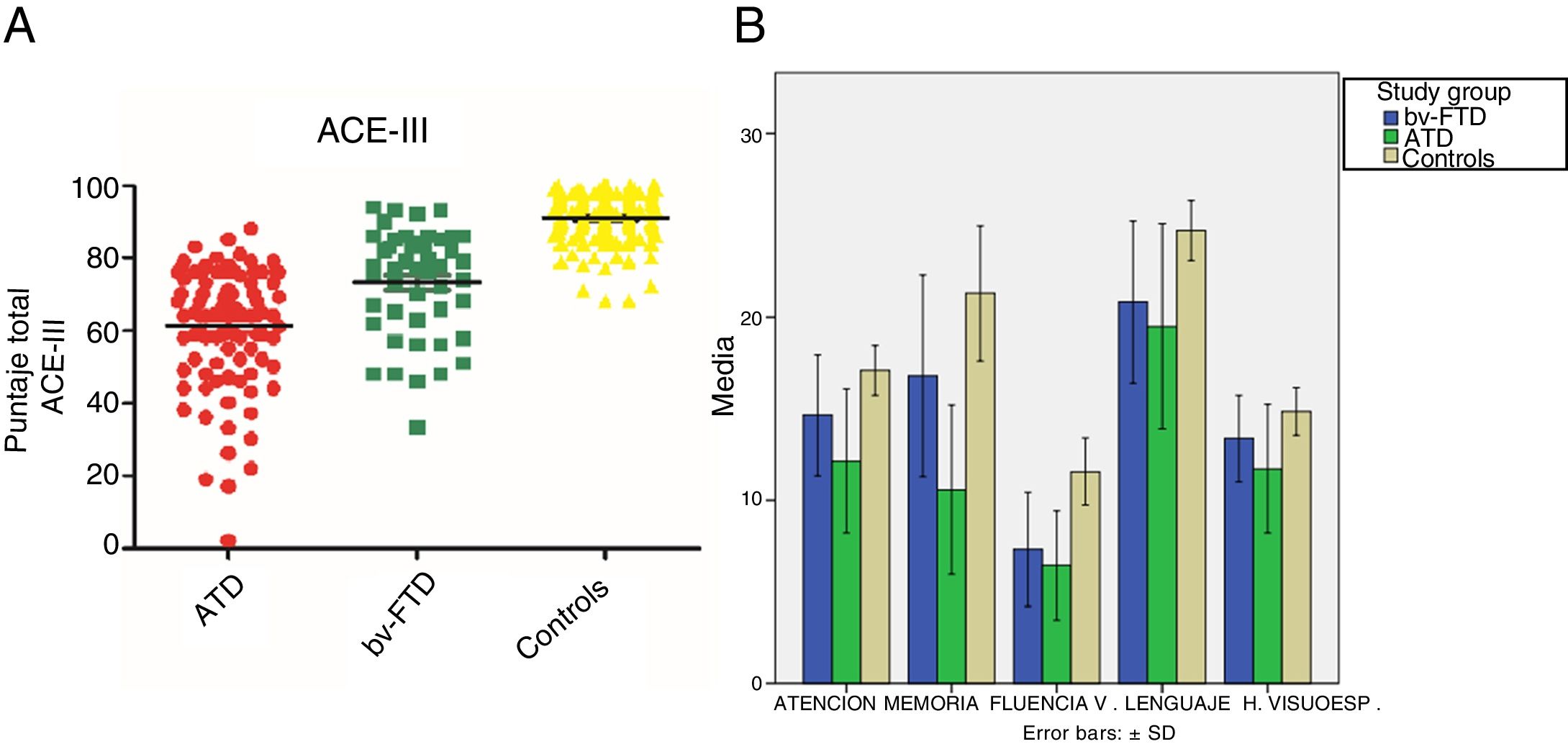
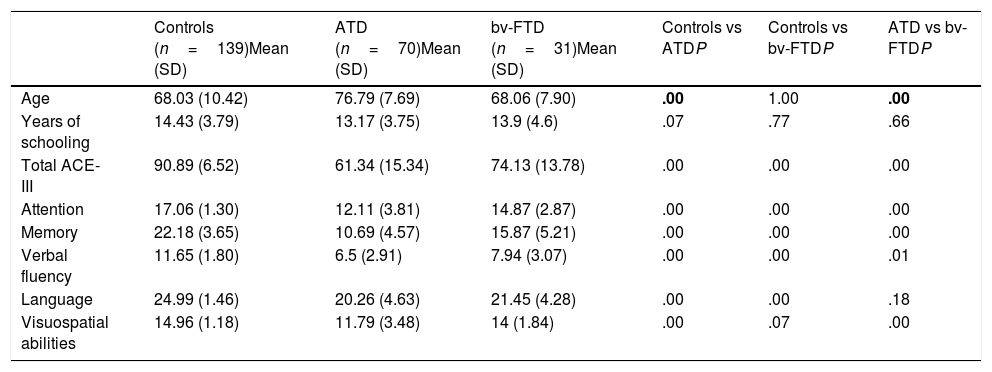
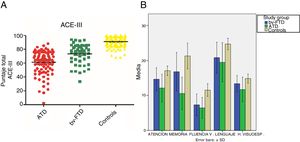
ResultsDemographic variablesWe did not find significant differences in age between the control group and the bv-FTD group, although significant differences were observed when comparing the ATD group with the control and bv-FTD groups. This is due to clinical variables associated with the age of onset of both conditions, which was later for ATD than for bv-FTD. Regarding years of schooling, no significant differences were found between the study groups (Table 1).
Age, years of schooling, and ACE-III total and subscale scores for the control, ATD, and bv-FTD groups.
| Controls (n=139)Mean (SD) | ATD (n=70)Mean (SD) | bv-FTD (n=31)Mean (SD) | Controls vs ATDP | Controls vs bv-FTDP | ATD vs bv-FTDP | |
|---|---|---|---|---|---|---|
| Age | 68.03 (10.42) | 76.79 (7.69) | 68.06 (7.90) | .00 | 1.00 | .00 |
| Years of schooling | 14.43 (3.79) | 13.17 (3.75) | 13.9 (4.6) | .07 | .77 | .66 |
| Total ACE-III | 90.89 (6.52) | 61.34 (15.34) | 74.13 (13.78) | .00 | .00 | .00 |
| Attention | 17.06 (1.30) | 12.11 (3.81) | 14.87 (2.87) | .00 | .00 | .00 |
| Memory | 22.18 (3.65) | 10.69 (4.57) | 15.87 (5.21) | .00 | .00 | .00 |
| Verbal fluency | 11.65 (1.80) | 6.5 (2.91) | 7.94 (3.07) | .00 | .00 | .01 |
| Language | 24.99 (1.46) | 20.26 (4.63) | 21.45 (4.28) | .00 | .00 | .18 |
| Visuospatial abilities | 14.96 (1.18) | 11.79 (3.48) | 14 (1.84) | .00 | .07 | .00 |
P values < .01 are shown in bold.
ATD: Alzheimer-type dementia; bv-FTD: behavioural variant frontotemporal dementia; SD: standard deviation.
Sex distribution was equal in the study groups, with the exception of the bv-FTD group, where sex was associated with epidemiological and clinical variables (Table 2).
Performance on the ACE-III: total score and subtestsWe found significant differences in total ACE-III score between the control group and the dementia groups (P<.05) and between both dementia groups (P<.05). Furthermore, the ATD group scored significantly lower than the other study groups. Regarding the scores obtained in the different ACE-III subtests, we did find significant differences between the 3 study groups in the domains attention, memory, and verbal fluency (P<.05). However, in the language subtest, while we did observe significant differences between the control group and the ATD group (P<.05) and the bv-FTD group (P<.05), none were observed between the 2 dementia groups (P=.18). In the visuospatial abilities subtest, the bv-FTD group obtained similar values to those of the control group, with no significant differences (P=.07). However, the ATD group scored significantly lower than patients with bv-FTD and controls (P<.05) (Table 1 and Fig. 1).
Psychometric propertiesThe internal validity of the Spanish-language version of the ACE-III was very good (Cronbach alpha value=0.87).
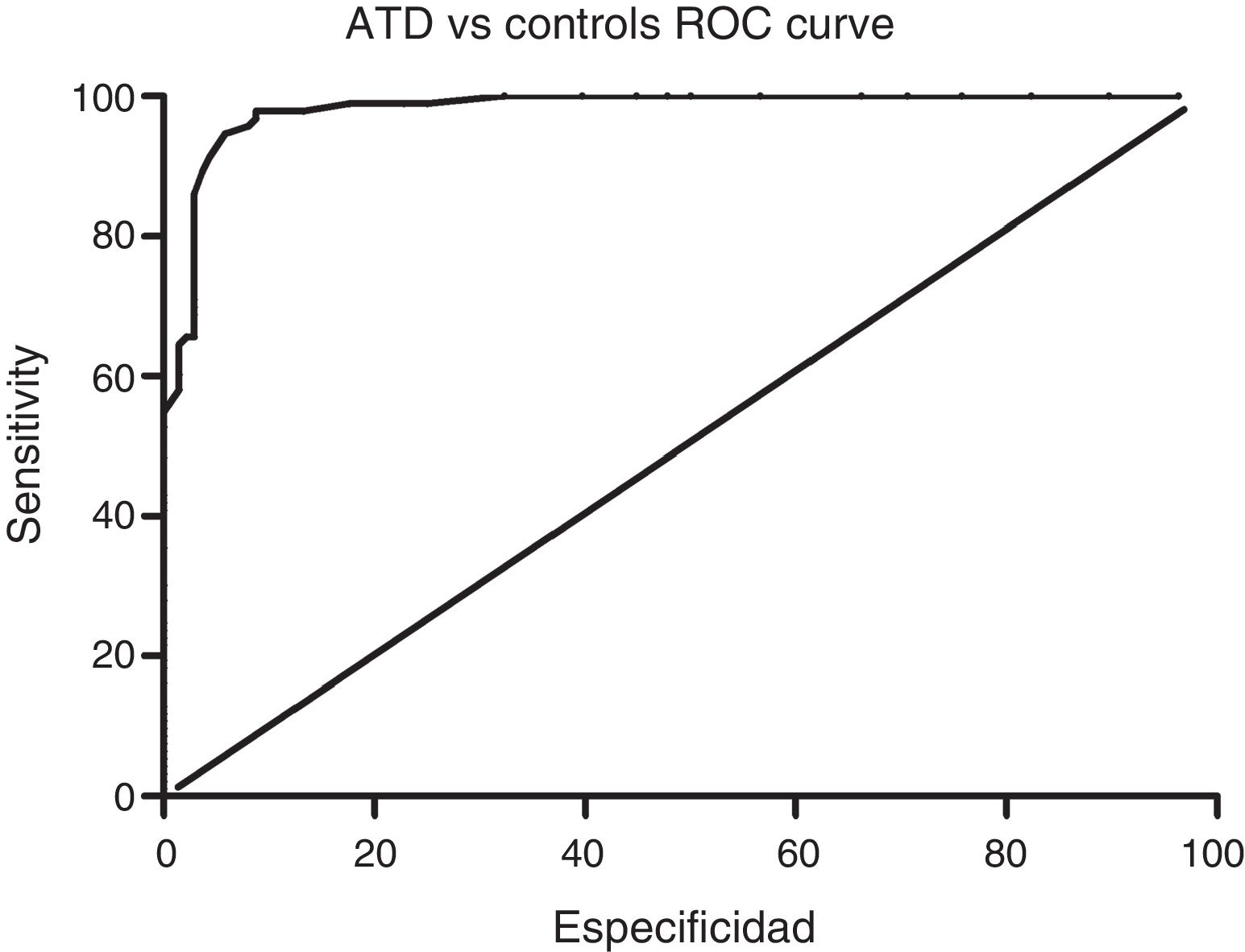
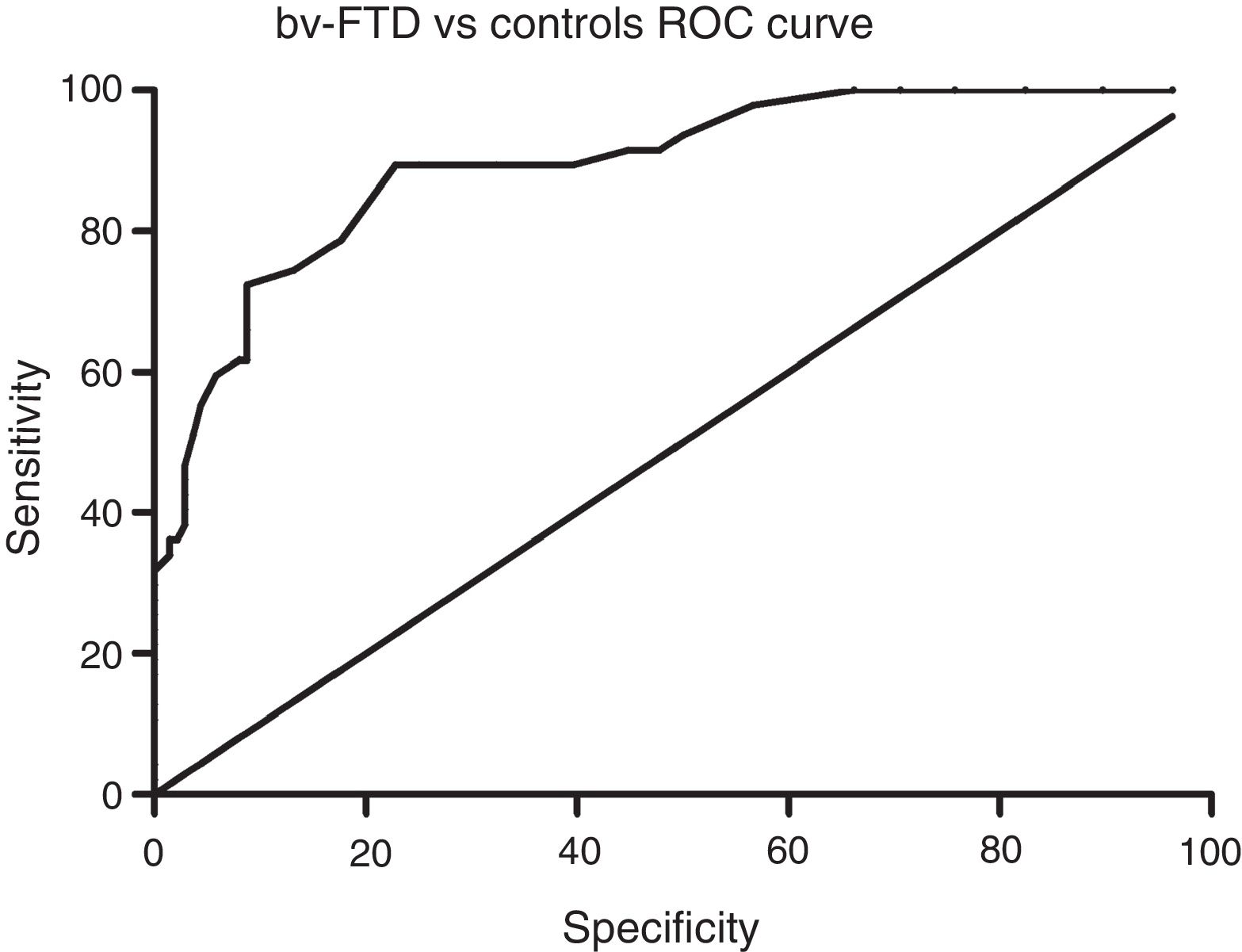
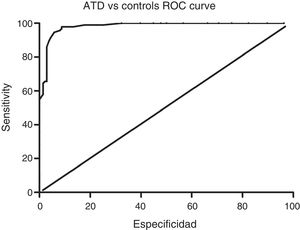
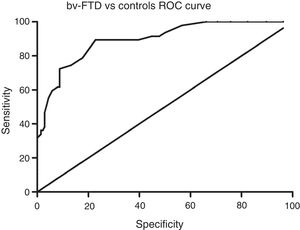
Sensitivity and specificity of the ACE-III for screening for cognitive impairment in ATD and bv-FTDAnalysis of the ROC curve for total ACE-III scores in the control group and in the ATD group suggests that, for a cut-off point of 86, the instrument shows a sensitivity of 98.5% and a specificity of 82.01%. The area under the curve was 0.98 (CI: 0.96-0.99; P<.01) (Fig. 2). Analysis of the ROC curve for total ACE-III score in the control group and in the bv-FTD group suggests that, for a cut-off point of 87, the instrument shows a sensitivity of 93.55% and a specificity of 77.7%. The area under the curve was 0.90 (CI: 0.85-0.96; P<.01) (Fig. 3).
With a cut-off point of 86, 98.6% of patients with ATD, 83.9% of patients with bv-FTD, and 84.2% of controls were correctly classified.
DiscussionOur findings show that the recently published Spanish-language version of the ACE-III is a useful tool for screening for cognitive impairment in 2 of the most prevalent dementia disorders, AD and bv-FTD. With a cut-off point of 86, the ACE-III shows a sensitivity of 98% and a specificity of 82%.
When comparing each study group's results in the different subtests, the ATD group scored significantly lower in the attention, memory, verbal fluency, and visuospatial abilities subscales. Language was the only domain where no significant differences were observed between the ATD group and the bv-FTD group.
On most of the scales (attention, memory, verbal fluency, and visuospatial abilities), patients with ATD scored significantly lower than patients with bv-FTD. This discrepancy is most likely due to the fact that bv-FTD initially manifests with behavioural changes, and cognitive impairment would predominantly consist of an alteration of executive function, a domain which is not deeply assessed by the ACE-III.25
When comparing the results for the different subtests of the ACE-III with those of the previous version, we found 2 main differences. Previous studies described a significant difference between patients with ATD and patients with bv-FTD on the language subscale.9,26 However, this difference was not observed in the present study. Although both dementia groups scored significantly lower than controls, the results obtained by both groups are not significantly different. This lack of difference might be explained by the fact that both semantic and phonological verbal fluency tasks are scored on a single scale. In this line of research, it has been reported that patients with ATD present greater semantic memory involvement, showing a deficit in semantic verbal fluency, whereas patients with bv-FTD present errors in the search for efficient strategies for the production of words corresponding to a certain condition, which is reflected in the phonological verbal fluency task.27,28
We also observed differences with previous studies in the visuospatial abilities subscale: while no significant differences are reported between dementia groups on the ACE-R,9 our study described significant differences between patients with ATD and patients with bv-FTD and controls. The bv-FTD group showed no differences with the control group in the visuospatial abilities subscale, unlike the ATD group, which did show differences when compared with the control group and the bv-FTD group for the same subscale. These results support the capacity of this instrument to differentiate between both groups of patients, considering that in addition to the classic alteration in anterograde memory, patients with ATD generally also present impaired visuospatial abilities,29 which is not frequent in patients with bv-FTD.
The main limitation of this study is the heterogeneous distribution of individuals from both countries in each diagnostic group; therefore a comparison between participants from Chile and Argentina was not possible. Future studies should test for the presence of an inter-country factor. However, recent normative data for 10 Spanish-language neuropsychological tests in 11 Latin American countries suggest that most differences in test performance are explained by age and educational factors. Intercountry factors only account for a small proportion of the variance.30 Furthermore, study participants were recruited from centres specialising in dementia care; future studies should assess the usefulness of the ACE-III in other clinical contexts, such as primary care services.
In conclusion, our results show that the ACE-III may be considered a useful tool for detecting cognitive impairment in patients with ATD and bv-FTD. Thus, this brief, sensitive test may be of great value in clinical practice for the initial assessment of patients with suspected dementia and for determining the need for complementary examinations.
FundingCONICYT/FONDECYT Regular/1140423 (AS); CONICYT/FONDAP/15150012 (AS and PL) CONICYT/Associative Research Program/Grant Basal Funds for Centers of Excellence FB 0003 (AS).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Bruno D, Slachevsky A, Fiorentino N, Rueda DS, Bruno G, Tagle AR, et al. Validación argentino-chilena de la versión en español del test Addenbrooke's Cognitive Examination III para el diagnóstico de demencia. Neurología. 2020;35:82–88.