Different types and localisations of neurological lesions can produce tinnitus and verbal or musical hallucinations (VMH).
MethodThese symptoms were screened for in 1000 outpatients at a cognitive neurology clinic, and epidemiological and neuroimaging data were recorded.
ResultsTinnitus was present in 6.9% of the total and VMH in 0.9%. The paracusia group was predominantly female but the difference was not statistically significant. Patients with tinnitus were younger and those with VMH were older than the rest of the sample (mean ages). Hearing loss was more prevalent in the paracusia group (difference was significant in VMH subgroup). There were no intergroup differences in the prevalence of psychotic and obsessive-compulsive disorders, or of leukoaraiosis. Treatment with acetylsalicylic acid was more frequent in the VMH group, whereas other non-opioid analgesics and benzodiazepines were more commonly prescribed to patients with tinnitus. The suspected cause of VMH was dementia with Lewy bodies (n=2, one with vascular disease), Alzheimer disease (n=2, one with vascular disease), isolated cerebrovascular disease (n=3), traumatic brain injury (n=1), and surgical brainstem lesion (n=1). All VMH cases displayed an underlying factor that might prompt this symptom, e.g., hearing loss (n=6), a predisposing drug (n=9), and polypharmacy (n=9).
ConclusionsTreatment with benzodiazepines and non-opioid analgesics was more frequent in the tinnitus group, whereas the VMH group showed a higher prevalence of hearing loss and treatment with acetylsalicylic acid. The causes of VMH were dementia with Lewy bodies, Alzheimer disease, and focal lesions in the mesencephalon, pons, left temporal lobe, or left claustrum.
Los tinnitus y las alucinaciones verbales o musicales (AVM) pueden deberse a lesiones neurológicas de naturaleza y topografía diversa.
MétodoSe han rastreado estos síntomas en 1.000 pacientes de una consulta de neurología cognitiva, anotando datos epidemiológicos y de neuroimagen.
ResultadosRefirieron tinnitus el 6,9% y AVM el 0,9%. Hubo predominio femenino no significativo en el grupo con paracusias. La edad media fue menor en los pacientes con tinnitus y mayor en los que tenían AVM. La hipoacusia mostró mayor prevalencia en los enfermos con paracusias (significativo con AVM). No hubo diferencias en la prevalencia de trastorno psicótico u obsesivo-compulsivo, o de leucoaraiosis. El tratamiento con ácido acetilsalicílico mostró mayor frecuencia en el grupo con AVM, y otros analgésicos no opioides y benzodiacepinas en los pacientes con tinnitus. La presunta causa de las AVM fue demencia con cuerpos de Lewy (n=2, uno con enfermedad vascular), enfermedad de Alzheimer (n=2, uno con enfermedad vascular), enfermedad vascular cerebral pura (n=3), lesión cerebral traumática (n=1) y lesión quirúrgica en el tronco encefálico (n=1). En los 9 casos había un elemento facilitador de la aparición de paracusias, como hipoacusia (n=6) o medicación de riesgo (n=9), además de polifarmacia (n=9).
ConclusionesLos pacientes con tinnitus tomaban con frecuencia benzodiacepinas y analgésicos no opioides, y en los que tenían AVM había mayor prevalencia de hipoacusia y de tratamiento con ácido acetilsalicílico. Las causas de AVM fueron demencia con cuerpos de Lewy, enfermedad de Alzheimer y lesiones focales en mesencéfalo, protuberancia, lóbulo temporal izquierdo o claustro izquierdo.
Tinnitus (which comes from the Latin word tinnīre, meaning ‘to ring’) is an auditory perception in the absence of an external stimulus. Tinnitus may manifest as a wide range of sounds (whistling, hissing, shrieking, buzzing, ringing, sizzling, bubbling, hammering, drumming, gasping, thundering, fragments of music, clanging, sounds resembling waves or downpour, a river flowing, a waterfall, the steam valve on a pressure cooker, trees rustling, a grinder or blender, a train, animal noises, an engine, doorbells, wind, etc.) and may be due to multiple causes (Table 1).1
Causes of tinnitus.
| Spontaneous, transient increase in auditory neural activity |
| Pressure on the tympanic membrane (earwax, hair, or liquid) |
| Compression of the eighth cranial nerve |
| Stapedius muscle contractions due to abnormal reinnervation after Bell palsy |
| Semicircular canal dehiscence |
| Otoacoustic emissions |
| Autoimmune inner ear disease |
| Extreme stress or fatigue |
| Perilymph fistula |
| Herpes zoster oticus (Ramsay Hunt syndrome) |
| Hypoacusis (conductive or sensorineural, acute or chronic) |
| Idiopathic |
| Brain lesion affecting circuits involved in auditory information processing |
| Focal brainstem lesions |
| Palatal myoclonus |
| Otosclerosis |
| Postinfectious |
| Medication (including drug withdrawal)a |
| Meniere disease |
| Pulsatile tinnitus secondary to prolonged systolic time due to anaemia, hyperthyroidism, or aortic stenosis; carotid artery dissection, stenosis, or fibromuscular dysplasia; dural arteriovenous fistula; carotid-cavernous sinus fistula; dehiscent jugular bulb; glomus jugulare tumour; benign intracranial hypertension |
| Somatic disorders affecting the head and upper cervical region (Costen syndrome, whiplash injury, etc.) |
| Acute acoustic trauma |
| Head trauma |
| Patulous Eustachian tube |
| Cerebellopontine angle tumour |
| Disabling positional vertigo |
Tinnitus that takes the form of more complex perceptions, such as voices, music, or a combination of both, is referred to as either verbal or musical complex auditory hallucinations (CAH). When the person is aware that no external stimulus exists, the perceptions may also be called hallucinosis (acoustic in the case of tinnitus). Tinnitus is called auditory Charles Bonnet syndrome when hearing loss appears to be the only causative factor2,3 and musical ear syndrome when hallucinations are predominantly musical.4
CAH may be due to a wide range of aetiologies (Table 2), and it may also occur in healthy individuals.5–7 Patients with CAH frequently have both predisposing and trigger factors. For example, hallucinations triggered by medications are more likely in patients with hearing loss. In patients with brain lesions, hallucinations coincide with paroxysmal electrical activity. Furthermore, the literature describes the case of a patient with calcifications in the thalamus and striatum who experienced CAH due to low levels of calcium and phosphorus secondary to hypoparathyroidism. Her hallucinations disappeared once the electrolyte balance had been restored.8 The reduction in cholinergic neurons occurring in old age may also act as a predisposing factor; this process, combined with visual or auditory deficiencies, may cause visual and/or auditory hallucinations (Charles Bonnet syndrome) that may respond to treatment with acetylcholinesterase inhibitors.9
Causes of complex auditory hallucinations according to the literature.
| Disorders associated with hypoacusis as the main manifestation | Otosclerosis*, cochlear implant*, presbycusis*, lesions to the pontine-mesencephalic portion of the auditory pathway* |
| Primary psychiatric disorders | Schizophrenia*, bipolar disorder*, obsessive-compulsive disorder*, depression*, anorexia nervosa, conversion disorder |
| Disorders linked to detectable focal lesions in the central nervous system | Vascular lesions (stroke*, intraparenchymal haemorrhage*, subarachnoid haemorrhage*, vascular malformations*, cerebral venous sinus thrombosis), neoplasia (meningioma*, glioma*, metastasis*, lipoma), radiotherapy*, epilepsy*a (especially when the epileptogenic area is located in the right temporal lobe), electroconvulsive therapy*, lobectomy for epilepsy*, abscesses*, viral encephalitis*, autoimmune limbic encephalitis, rhombencephalitis due to Listeria*, multiple sclerosis, migraine attacks (with and without aura)*, basal ganglia calcification*, schizencephaly |
| Neurological disorders with no apparent focal signs affecting the auditory pathway | Hallucinosis secondary to head trauma*, Hashimoto encephalopathy*, Lyme disease*, encephalitis due to virus or Taenia solium infection, neurosyphilis; α-synucleinopathies (multiple system atrophy, Lewy body dementia, Parkinson's disease*), Alzheimer disease* |
| Effect of substances | Alcoholic hallucinosis*, other substancesb |
| Idiopathic* |
* Published cases of musical hallucinations.
Amantadine*, tricyclic antidepressants*, antimalarial drugs (quinine, chloroquine, mefloquine), baclofen (sudden withdrawal), benzodiazepines* (and benzodiazepine discontinuation), biphosphonates, cocaine, corticosteroids*, bromocriptine*, digoxin, dipyridamole*, gentamicin*, selective serotonin reuptake inhibitors, ketamine, marijuana, mirtazapine*, opioids*, pentoxifylline*, psychostimulants (cocaine, amphetamines, methylphenidate), pramipexole*, prazosin, propranolol*, ranitidine, salicylates*, topiramate, trimethoprim/sulfamethoxazole, voriconazole*.
We searched for patients with tinnitus or CAH in a sample of neurological patients and analysed predisposing factors, neuroimaging findings, and potential pathophysiological mechanisms for the symptoms.
Patients and methodsWe used a register of 1000 patients seen at a cognitive neurology clinic to search for patients with tinnitus and/or CAH. We performed a descriptive analysis for age and sex and examined any associations with such potential risk factors as hearing loss and leukoaraiosis. Data on pharmacological treatments were also gathered to check for any associations between tinnitus/CAH and medication use. Patients were considered to be positive for white matter alterations in neuroimaging studies when they had scores ≥2 on Blenow's scale (CT) or ≥4 on the Fazekas scale (MRI).10
In patients with CAH, we analysed the potential association between manifestations and the diagnosis (and location) of focal brain lesions, when present.
Results are expressed as either means±SD or absolute frequencies (percentages). We used the chi square, exact Fisher, and t tests to analyse the association between variables. Values of P<0.05 were considered statistically significant.
ResultsOf these 1000 patients, 69 had tinnitus and 9 CAH (including 2 with both entities). Table 3 shows sex, age, and presence of potential risk factors in our sample. We analysed regular pharmacological treatment in the subgroup of patients with symptoms: antiplatelet drugs (acetylsalicylic acid [ASA] was analysed separately), opioid and non-opioid analgesics, nonsteroidal anti-inflammatory drugs, benzodiazepines, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, neuroleptics, beta-blockers (propranolol was analysed separately), angiotensin II type 1 receptor blockers, calcium channel blockers, loop diuretics, statins, and proton pump inhibitors. Table 4 displays demographic and clinical data of the patients with CAH.
Sex, age, and possible risk factors in our sample (n=1000).
| Sex, % womena | Mean age±SD | Hypoacusis, %a | LWM, %a | PSchiz, %a | Treatmentb, %a | |
|---|---|---|---|---|---|---|
| No paracusis (n=924) | 61.1 | 74.0 (13.3) | 16.0 | 31.5 | 1.5 | ASA (26.5) NOA (7.4) BZD (37.7) |
| Paracusis (n=76) | 71.1ns | 68.0 (16.0)** | 26.3* | 29.6ns | 1.3ns | NOA (18.4**) BZD (50.0*) |
| Tinnitus (n=69) | 69.6ns | 66.4 (15.9)** | 23.2ns | 29.8ns | 1.4ns | NOA (18.8**) BZD (52.2*) |
| CAH (n=9) | 80ns | 83.8 (5.7)* | 66.7* | 22.2ns | 0ns | ASA (66.7*) NOA (11.1ns) BZD (44.4ns) |
CAH: complex auditory hallucinations; ASA: acetylsalicylic acid; NOA: non-opioid analgesics; BZD: benzodiazepines; SD: standard deviation; LWM: moderate to severe leukoaraiosis or multiple lacunar lesions in white matter; ns: non-significant differences between patients with the condition analysed in that row and those without (marked in left column); PSchiz: patients with schizophrenia, bipolar disorder, or obsessive-compulsive disorder.
Patients with complex auditory hallucinations.
| Patient | Sex-age | Symptoms | Diagnosis | Focal lesion | Other data |
|---|---|---|---|---|---|
| BLM | W-83 | Sees people and hears voices | Lewy body dementia | No | 5 AI. L-dopa |
| CMC | W-81 | Always hears the same 2 songs | Cerebrovascular disease | Infarct in the pontine-mesencephalic region bilaterally | Hypoacusis. 8 AI. ASA and BZD |
| CAM | W-85 | Unspecified visual and auditory hallucinations | Cerebrovascular disease | Lacunar lesions in the centrum ovale and pons | Leukoaraiosis. 11 AI. ASA |
| CRM | W-84 | Hears television | Alzheimer disease | No | Hypoacusis. 10 AI. ASA, BZD, NOA, and OA |
| FMS | M-78 | Hears people talking | Encephalopathy secondary to head trauma associated with complex partial seizures | Cortico-subcortical encephalomalacia in the left temporal lobe | Hypoacusis. Leukoaraiosis. 12 AI. ASA |
| GPM | W-83 | Sees people and hears voices | Lewy body dementia, cerebrovascular disease | No | Leukoaraiosis. 5 AI. ASA |
| RGD | W-91 | Sees people and hears voices | Alzheimer disease, cerebrovascular disease | No | Hypoacusis. 7 AI. ASA |
| SMA | W-76 | Hears songs | Systemic lupus erythematosus, surgical lesion to the brainstem | Surgical malacic lesion in the left side of the pons | Hypoacusis. 6 AI. BZD |
| VRC | W-94 | Hears voices and a gramophone playing music | Cerebrovascular lesion | Lacunar infarction in left claustrum | Hypoacusis. 5 AI. BZD |
ASA: acetylsalicylic acid; NOA: non-opioid analgesics; OA: opioid analgesics; BZD: benzodiazepines; W: woman; M: man; AI: active ingredients.
Auditory hallucinations were found in 7.6% of the patients included in our register (6.9% had tinnitus and 0.9% had CAH). Over 80% of the adult population has experienced tinnitus at some point in their lives; however, prevalence of persistent and obtrusive tinnitus ranges from 4.4% to 27.9%.11–13 In neurology consultations, patients with tinnitus describe their acoustic perceptions as disturbing and believe them to be linked to the reason for consultation. Two series of patients with no psychotic disorders reported CAH prevalences of 0.7% and 0.8%, respectively.14,15 Therefore, frequencies of tinnitus and CAH in our sample are in line with those described in other studies.
The literature reports no sex-related differences in the presence of tinnitus11–13; in contrast, CAH, both verbal16 and musical,3,17 are more frequent in women. In our sample, most of the patients with paracusis were women, especially in the group with CAH (Table 3); however, sex-related differences between the groups with and without paracusis were not significant (there were also more women in the group without paracusis).
Prevalence of tinnitus increases until the age of 70, after which it either stabilises or decreases.12,13 Verbal auditory hallucinations are more frequent in middle-aged patients, whereas7,14 a study of 132 patients with musical hallucinations reported a mean age of 61.5 years.3 In our sample, patients with tinnitus were younger than those with no auditory phenomena and patients with CAH were older than the other patients (Table 3). Prevalence of cognitive disorders increases with age, which explains the advanced mean age of our sample (73.6±13.6, median 77). This is consistent with the finding of tinnitus in the youngest patients in the sample (66.4±15.9): incidence of tinnitus does not increase in elderly patients, in contrast with the incidence of cognitive impairment. However, patients with CAH were older than the other patient group, which seems to contradict published evidence. A potential explanation is that most epidemiological studies include samples from the normal population; in these individuals, CAH may be caused by schizophrenia and other disorders that are more prevalent among young or middle-aged patients, such as epilepsy, multiple sclerosis, and alcoholic hallucinosis (Table 2). In our subset of 9 patients with CAH, the concomitant disorders that are more frequent in older individuals (cerebrovascular disease, dementing degenerative disorder [Table 4]), may be responsible for both auditory hallucinations and cognitive impairment.
Hearing loss is a risk factor for paracusis2,3,11,18,19: in our sample, this symptom was more frequently observed in patients with auditory hallucinations (differences were significant for the subgroup of patients with CAH) (Table 3). In most published cases of CAH, patients have a trigger factor plus one or more predisposing factors; hypoacusis is a frequent predisposing factor, especially in the case of musical CAH.1–3,18,19 In our sample, 66.6% of the patients with CAH presented hypoacusis (Table 4).
Studies mainly including patients with schizophrenia report white matter changes affecting connectivity among certain areas of the brain (especially in the left frontotemporal area); these changes cause auditory hallucinations.20,21 We may therefore hypothesise that multiple extensive or lacunar leukoaraiosis lesions may predispose to CAH. We observed leukoaraiosis in 31.3% of the sample; there were no significant differences between patients with and without paracusis (Table 3). The potential association between leukoaraiosis and paracusis may be attenuated in our sample given that leukoaraiosis is a frequent cause of cognitive and behavioural symptoms.22,23
CAH are frequent in patients with schizophrenia (≅70%),5 bipolar disorder (11%-67%),5,24 or obsessive-compulsive disorder.25 They may be verbal or musical.26,27 While our sample contained 15 patients with any of the above diagnoses, none of them reported CAH. Auditory hallucinations in these patients are usually assessed by psychiatrists rather than neurologists, unless hallucinations have changed alongside neurological symptoms.
Some drugs have been associated with tinnitus or CAH (Tables 1 and 2). This study found a higher rate of use of ASA in patients with CAH (66.7% vs 26.5% in those without), non-opioid analgesics in patients with tinnitus (18.8% vs 7.4% in those without), and benzodiazepines in both paracusis groups (52.2% in patients with tinnitus vs 37.6% in those without; 44.4% in patients with CAH vs 38.5% in those without) (Table 3). ASA in high doses or as long-term treatment leads to spiral ganglion neuron apoptosis, resulting in turn in hypoacusis and tinnitus, which are reversible in many cases.28 In our sample, ASA was more frequently used by patients without tinnitus. The use of low doses (100mg/day) administered shortly before symptom onset in many cases, and the fact that these symptoms may be transient may explain why the frequency of ASA use is not higher in patients with tinnitus. However, ASA is more frequently given to patients with CAH than to those without. Six of the 9 patients with CAH were receiving ASA as antiplatelet therapy. Differences in rates of antiplatelet treatment between patients with and without CAH were not significant (66.7% vs 40.1%). We hypothesise that the effects of vascular disease on the brain, rather than treatment with ASA, may be involved in the pathogenesis of CAH. However, Allen29 reported 18 cases of musical CAH or tinnitus attributable to ASA use. In any case, the size of our sample is insufficient to draw robust conclusions on the association between ASA and paracusis.
Of the 69 patients with tinnitus, 13 (18.8%) were taking non-opioid analgesics regularly (ASA at antiplatelet doses in all cases; nonsteroidal anti-inflammatory drugs were analysed separately). Ten of the 69 were taking paracetamol. Of the patients without tinnitus, 7.4% were taking analgesics (statistically significant difference, P=.001). This finding may be explained by the little known fact that paracetamol has ototoxic effects and may cause both tinnitus and hypoacusis.30
Tinnitus is also among the symptoms associated with benzodiazepine withdrawal.31 While these drugs constitute a treatment option for tinnitus, their effectiveness is unknown.32 However, the literature describes some cases of CAH triggered by benzodiazepines, even though they are cited as a possible treatment option for this condition.33,34 In some of our patients, benzodiazepines were prescribed before onset of paracusis. In others they were administered to treat anxiety or dysphoria, symptoms sometimes triggered or aggravated by paracusis. Although we do not know whether benzodiazepines triggered paracusis in some of the cases, no patients developed symptoms soon after treatment onset.
Four of the 9 patients with CAH had a degenerative disease (2 with Lewy body dementia and 2 with Alzheimer disease [AD]); 5 had a cerebrovascular disease (associated with degenerative disease in 2 cases), and the remaining 2 had focal brain lesions secondary to either trauma or surgery (Table 4). Patients with Lewy body dementia frequently develop visual hallucinations and may also present other psychotic symptoms, including auditory hallucinations; these symptoms support diagnosis of the condition.35 Psychotic symptoms are less frequent in AD, occurring in 12% of the cases (median),36 and probably in one specific phenotype37,38 in which an association with the C102 allele of the gene coding for the 5-HT2A receptor has been observed.39 In any case, CAH are more frequent in patients with α-synucleinopathies or AD than in those with other types of degenerative dementia; this may explain why none of the 9 patients with CAH displayed other degenerative diseases.
Non-degenerative focal lesions affecting the circuits involved in auditory information management may cause auditory hallucinations. These lesions may result from tumours, infections, trauma, or surgery, among other causes, although they are usually vascular in origin.40 It was therefore not surprising to find cerebrovascular disease in 5 of the 9 patients with CAH. Three of them had focal lesions potentially responsible for these symptoms whereas the remaining 2 also presented degenerative disease (Table 4).
Lesions associated with paracusis may appear in a wide range of locations since the auditory pathway is long and includes multiple modulatory circuits. First, the information gathered by the organ of Corti travels to the cochlear nucleus. Some fibres of this pathway then pass through the nuclei of the superior olivary complex, after which the pathway ascends through the lateral lemniscus (mostly along the contralateral structure). Some axons terminate at the nucleus of the lateral lemniscus and the inferior colliculus. The pathway continues towards the medial geniculate nucleus, where it sends projections to the first temporal gyrus and subsequently to the associative areas. Regarding the modulatory aspect of this pathway, its peripheral fragment contains an olivocochlear bundle that protects against damage caused by excessive noise and mediates selective attention. Alterations in the peripheral afferent section cause ipsilateral hearing loss for certain frequencies; by a phenomenon of homeostatic plasticity, they also induce reactive hyperactivity in intact neurons of the cochlear nucleus which is transmitted to the brain and may cause unilateral tinnitus.41 Furthermore, somatosensory afferences from the head and upper cervical region converge in the dorsal cochlear nucleus; the inputs are necessary to locate sound with respect to the position of the head. Both partial deafferentation of the auditory nerve fibres and excessive somatosensory stimulation of the head or neck may cause tinnitus (even in cases of normal auditory function).1,41 If alterations are mild, tinnitus appears when both factors co-occur. Tinnitus may also appear in patients with normal hearing due to dysfunctions of the outer hair cells as a result of excessive ‘otoacoustic emissions’ that surpass the compensatory capacity of the olivocochlear system.42 This cause is responsible for less than 10% of all cases of tinnitus and generally appears in association with other causes. In summary, hypoacusis promotes tinnitus, but it is not a necessary or sufficient condition for the development of this disorder. In our sample, nearly one fourth of all patients with tinnitus also had hypoacusis (Table 3).
The section of the auditory pathway running through the brain contains several modulatory tracts. Multiple fibres run from the auditory cortex to the medial geniculate nucleus, the inferior colliculus, and other nuclei in the thalamus. Other neurons reach the cochlear nucleus from the inferior colliculus and the lateral lemniscus. The reticular formation manages bidirectional communication between the cochlear nucleus and the thalamus. These circuits are in turn influenced by other regions (prefrontal and parietal cortex, cingulate, insula, parahippocampus, amygdala, basal nuclei, and cerebellum).2,43,44 Hypofunction of the auditory pathway or the ascending reticular formation leads to thalamocortical dysrhythmia45,46; deafferentation promotes hyperactivity in some cortical areas as a compensatory mechanism. Marked deafferentation may result in release of auditory information stored in mnestic circuits. When no deafferentation occurs, paracusis may be mediated not by thalamocortical dysrhythmia, but rather by deficiencies in descending modulatory circuits. In many cases, both mechanisms are involved. Paracusis secondary to alterations rostral to the cochlear nucleus may be simple or complex, and bilateral (most frequently) or unilateral.
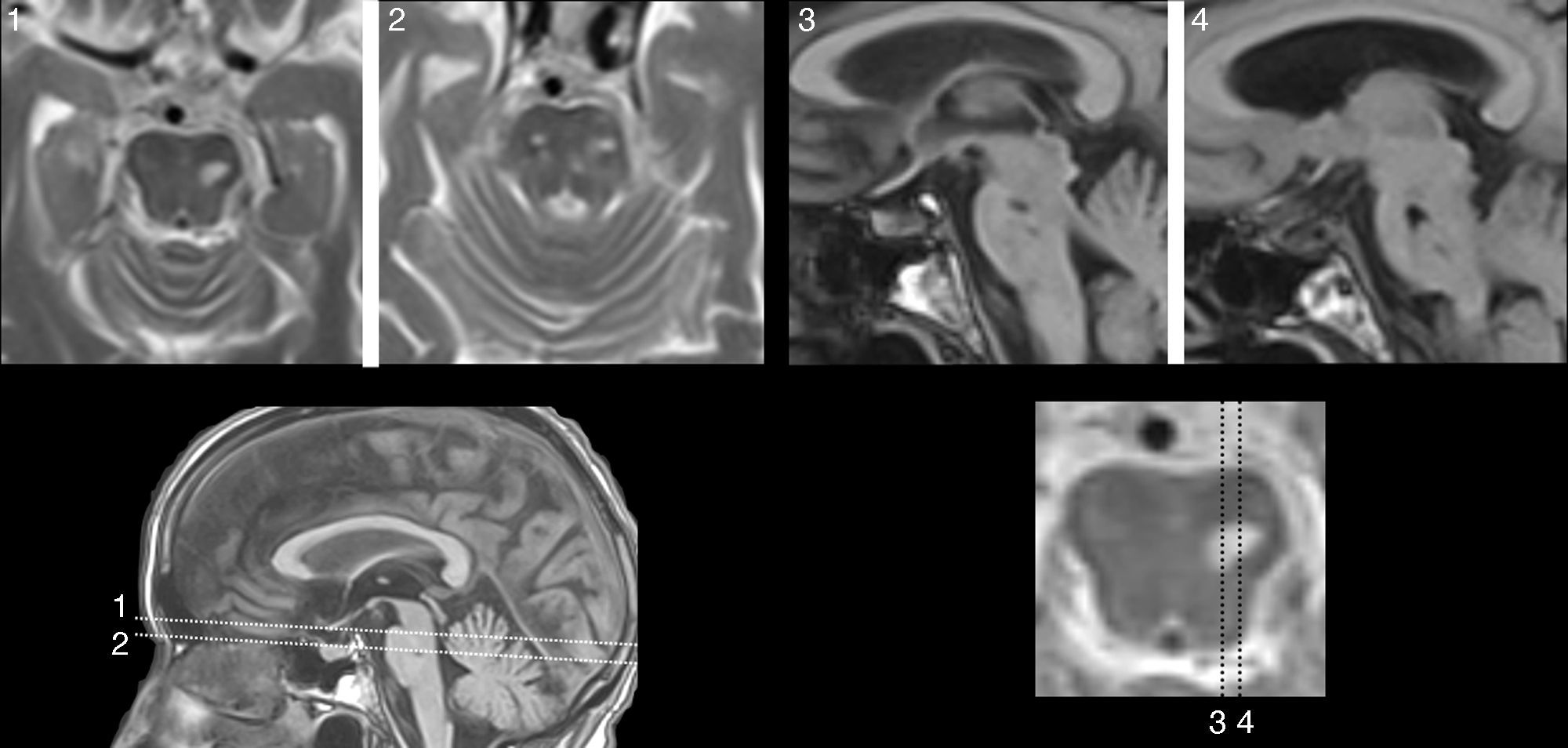
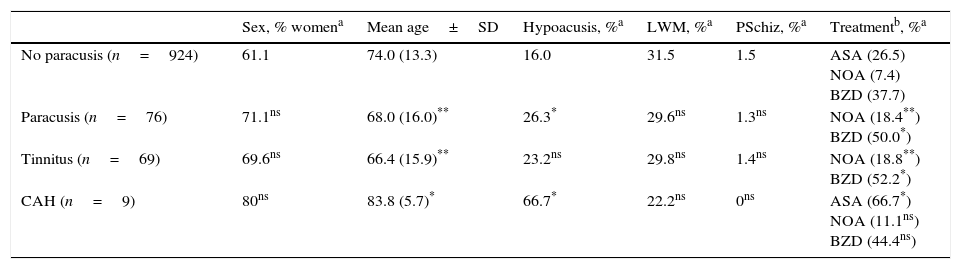
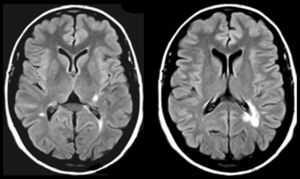
Lesions causing CAH may be located at any part of the auditory pathway (generally rostral to the cochlear nucleus), in adjacent areas (affecting modulatory fibres), and even in other regions linked to the ones mentioned previously due to a mechanism of diaschisis.47–50 The literature includes multiple cases of CAH secondary to lesions to the portion running from the pontine-mesencephalic tegmentum to the thalamus, the thalamus itself, and the area containing fibres which connect the thalamus to the temporal cortex. In other cases, lesions are located in the superior temporal region or adjacent areas (left or right) and have a stimulating effect. On rare occasions, lesions are located in other areas of the connectome that are functionally related to the regions mentioned previously (frontal lobe,51,52 parietal lobe,53 hippocampus,54 cerebellum55). Five of the 9 patients with CAH displayed focal lesions potentially responsible for the condition (Table 4). In 3 of these cases, lesions were located in the pontine or mesencephalic tegmentum. The literature reports many cases of verbal or musical CAH secondary to lesions to these regions. Symptoms are similar to those associated with peduncular hallucinosis, which is characterised by visual alterations and may also present with tactile or auditory dysfunction.46Fig. 1 shows neuroimages taken of a lesion at this location in a patient who had reported hearing fragments of the same songs for several years.
81-year-old woman with hypoacusis, diabetes, and dyslipidaemia who began to experience verbal and musical complex auditory hallucinations at the age of 78. The examination revealed upward gaze palsy. An MRI scan showed a lesion in the anterior part of the lateral lemniscus, at the level of the left pontomesencephalic junction (1 and 2: T2-weighted TSE sequences; 3 and 4: T1-weighted SE sequences).
Another 2 cases of paracusis (known as VAA and SMA) display pathophysiological differences. In the case of VAA, hypoacusis and unilateral tinnitus are secondary to vestibular schwannoma. SMA began to experience CAH after surgical resection of a vestibular schwannoma: postsurgical MR images reveal signal alterations in the adjacent pontine region. In VAA, in addition to hyperactivity of preserved fibres as a reaction to hypoacusis, paracusis may also be due to a transmission phenomenon caused by contact between adjacent compressed fibres.1 In SMA, paracusis had to do with modulatory tracts of the central nervous system stemming from cortical association areas.
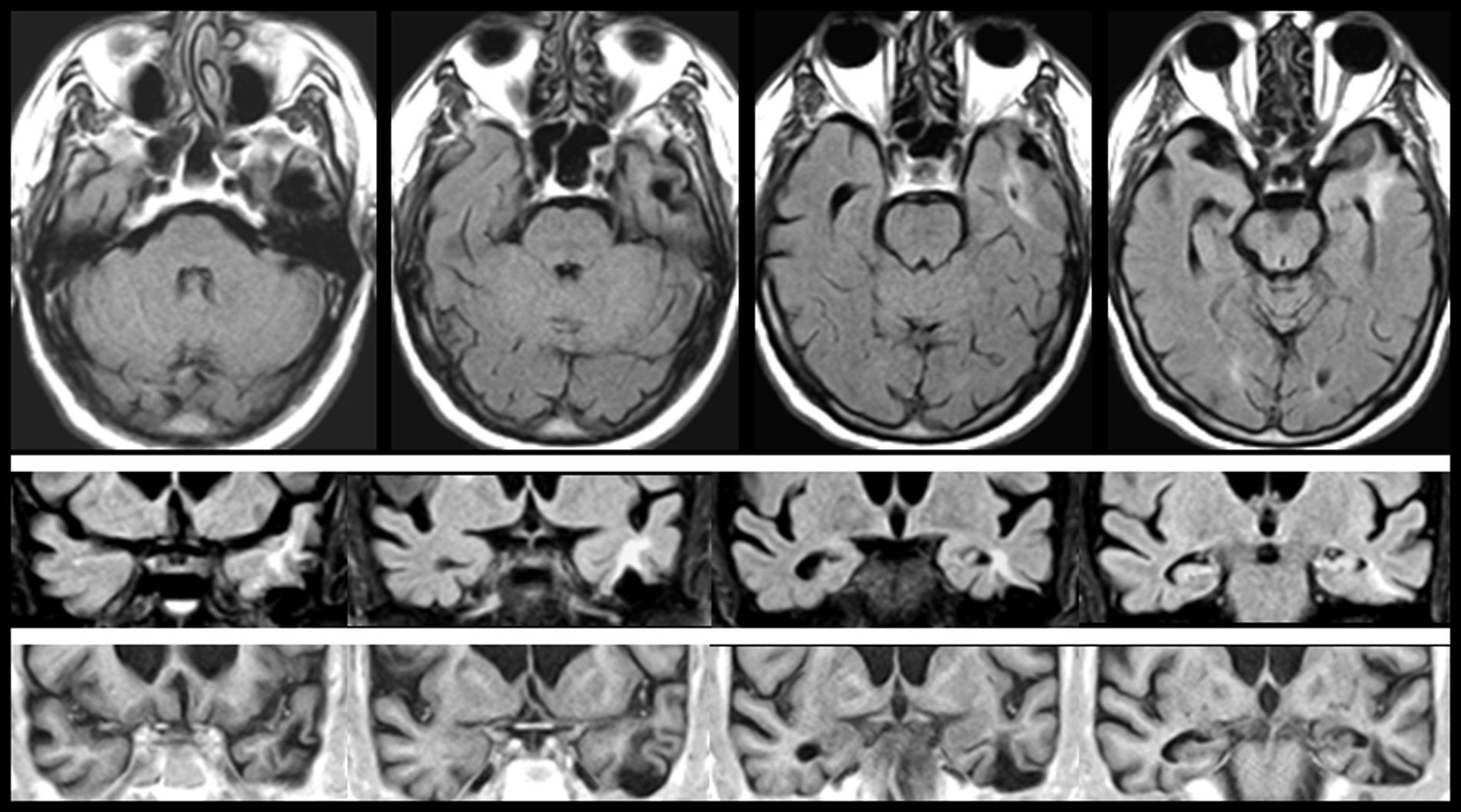
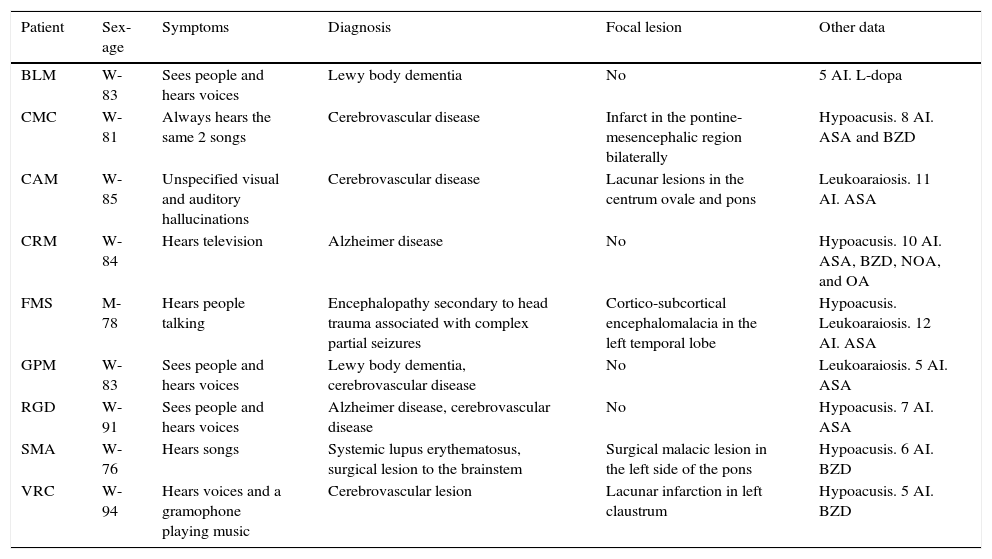
In FMS (Table 4), partial seizures and CAH were caused by paroxysmal bioelectrical activity in a temporal cortical area with encephalomalacia surrounded by gliosis (Fig. 2). Epileptic activity in the superior temporal gyrus is a well-known cause of CAH.56
MRI scan of a 78-year-old patient with arterial hypertension and presbycusis who at the age of 53 experienced head trauma resulting in coma. When he was 76, he began to experience recurrent paroxysmal episodes of delusions with visual and auditory hallucinations. Neuroimages revealed encephalomalacia surrounded by gliosis in the left temporal area (upper and middle rows: FLAIR sequences; lower row: T1-weighted IR sequences).
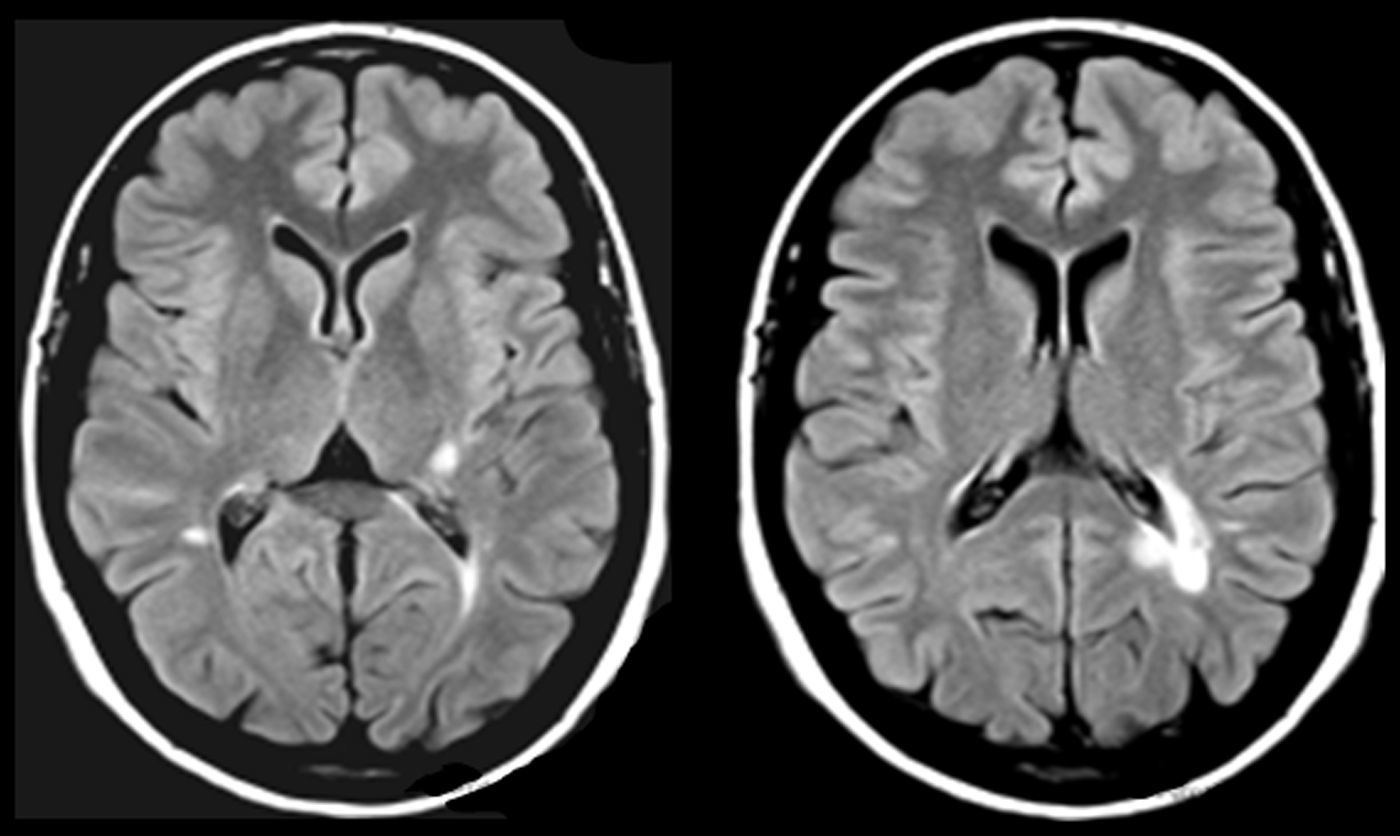
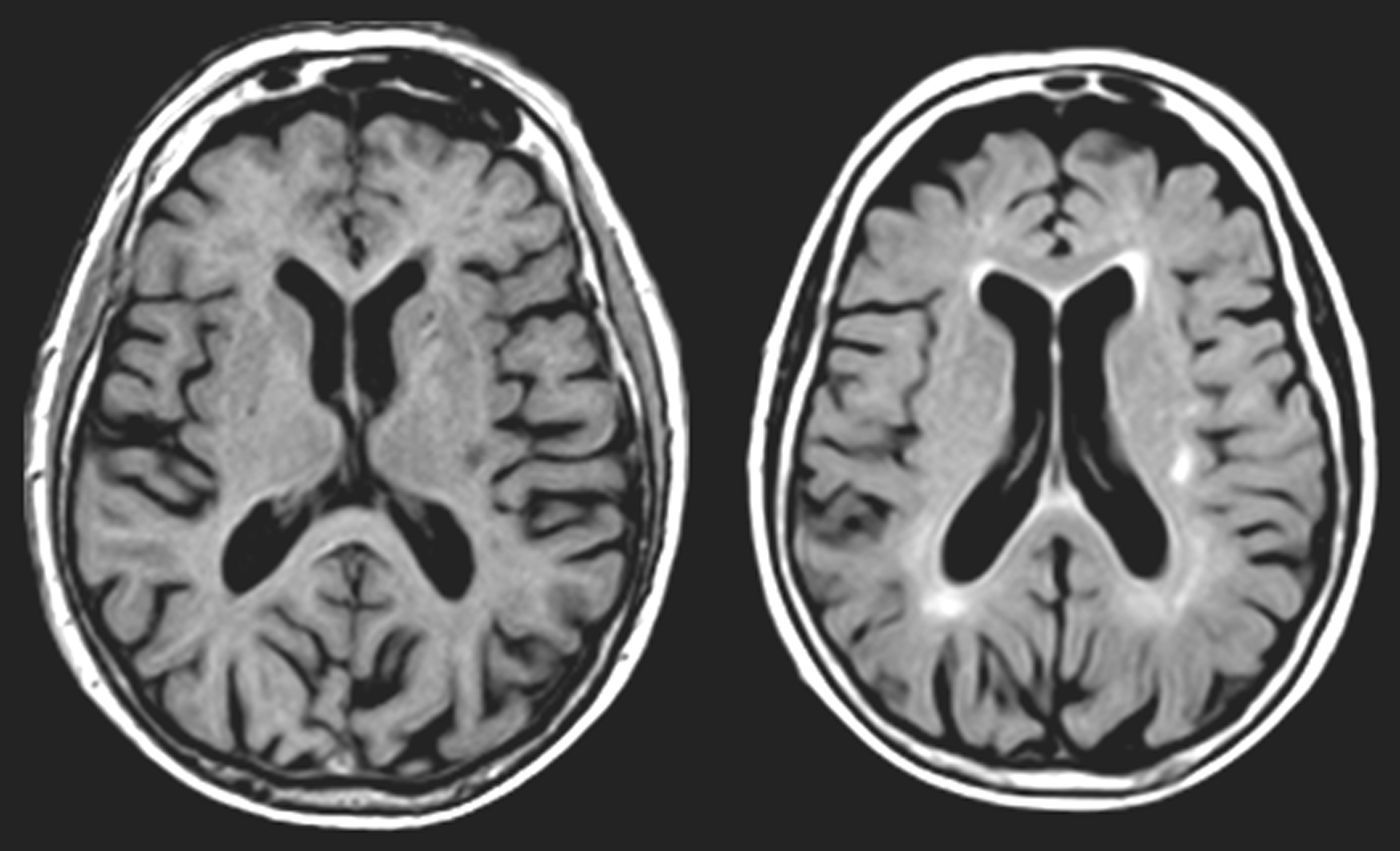
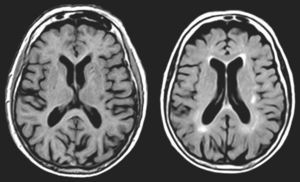
One of our patients (AVM) displayed a lacunar lesion along the path of the projections running from the pulvinar nuclei and medial geniculate body to the temporal cortex (Fig. 3). VRC showed a lacunar lesion in the posterior area of the left claustrum, adjacent to the insula, which caused musical CAH (Table 4, Fig. 4). According to a meta-analysis, some of the brain areas that activate during CAH include the insulae and the left claustrum,44 which are connected to auditory cortical areas57 and involved in processing auditory information.57,58
MR images (left: T1-weighted sequence; right: FLAIR sequence) from a 94-year-old woman with arterial hypertension, a 2-year history of bilateral hypoacusis and tinnitus, and a 9-month history of bilateral musical and verbal hallucinations that were repetitive but did not alter the patient's behaviour or mood.
All 9 cases of CAH displayed more than one known cause of CAH (Table 2). On many occasions, co-presence of 2 or 3 causative factors is necessary to exceed the brain's ability to adapt. In addition to the condition causing CAH (α-synucleinopathy, AD, or a focal lesion in a risk area for paracusis), the 9 patients with CAH displayed hypoacusis or were taking drugs potentially predisposing them to paracusis (Table 4). All patients were taking multiple drugs, which suggests that polymedication may be another risk factor for CAH; further studies with greater sample sizes are necessary to confirm this association.
Paracusis, which is often not reported if doctors do not ask about it specifically, is one of a host of symptoms arising from a number of very different neurological disorders. Many tests and procedures are available for determining the multiple causes of paracusis (audiometry, auditory reflexes, dichotic listening test, auditory brainstem evoked potentials).17,59,60 However, working conditions in clinical practice force clinicians to use only such tests as are indispensable to determine the aetiology and choose the most appropriate treatment. In any case, brain MRI scans should be performed when patients report CAH or tinnitus without a well-defined peripheral cause, especially in cases of pulsatile tinnitus or paracusis accompanied by focal neurological signs.
FundingThis study has not been presented at any scientific conferences nor has it received funding from any institutions.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Robles Bayón A, Tirapu de Sagrario MG, Gude Sampedro F. Alucinaciones auditivas en neurología cognitiva. Neurología. 2017;32:345–354.