To update the Spanish Society of Neurology's guidelines for subarachnoid haemorrhage diagnosis and treatment.
Materials and methodsA review and analysis of the existing literature. Recommendations are given based on the level of evidence for each study reviewed.
ResultsThe most common cause of spontaneous subarachnoid haemorrhage (SAH) is cerebral aneurysm rupture. Its estimated incidence in Spain is 9/100,000 inhabitants/year with a relative frequency of approximately 5% of all strokes. Hypertension and smoking are the main risk factors. Stroke patients require treatment in a specialised centre. Admission to a stroke unit should be considered for SAH patients whose initial clinical condition is good (Grades I or II on the Hunt and Hess scale). We recommend early exclusion of aneurysms from the circulation. The diagnostic study of choice for SAH is brain CT (computed tomography) without contrast. If the test is negative and SAH is still suspected, a lumbar puncture should then be performed. The diagnostic tests recommended in order to determine the source of the haemorrhage are MRI (magnetic resonance imaging) and angiography. Doppler ultrasonography studies are very useful for diagnosing and monitoring vasospasm. Nimodipine is recommended for preventing delayed cerebral ischaemia. Blood pressure treatment and neurovascular intervention may be considered in treating refractory vasospasm.
ConclusionsSAH is a severe and complex disease which must be managed in specialised centres by professionals with ample experience in relevant diagnostic and therapeutic processes.
Actualización de la guía para el diagnóstico y tratamiento de la hemorragia subaracnoidea de la Sociedad Española de Neurología.
Material y métodosRevisión y análisis de la bibliografía existente. Se establecen recomendaciones en función del nivel de evidencia que ofrecen los estudios revisados.
ResultadosLa causa más frecuente de hemorragia subaracnoidea espontánea (HSA) es la rotura de un aneurisma cerebral. Su incidencia se sitúa en torno 9 casos por 100.000 habitantes/año y supone un 5% de todos los ictus. La hipertensión arterial y el tabaquismo son sus principales factores de riesgo. Se ha de realizar el tratamiento en centros especializados. Se debe considerar el ingreso en unidades de ictus de aquellos pacientes con HSA y buena situación clínica inicial (grados I y II en la escala de Hunt y Hess). Se recomienda la exclusión precoz de la circulación del aneurisma. El estudio diagnóstico de elección es la tomografía computarizada (TC) craneal sin contraste. Si esta es negativa y persiste la sospecha clínica se aconseja realizar una punción lumbar. Los estudios de elección para identificar la fuente de sangrado son la resonancia magnética (RM) y la angiografía. Los estudios ultrasonográficos son útiles para el diagnóstico y seguimiento del vasoespasmo. Se recomienda el nimodipino para la prevención de la isquemia cerebral diferida. La terapia hipertensiva y el intervencionismo neurovascular pueden plantearse para tratar el vasoespasmo establecido.
ConclusionesLa HSA es una enfermedad grave y compleja que debe ser atendida en centros especializados, con suficiente experiencia para abordar el proceso diagnóstico y terapéutico.
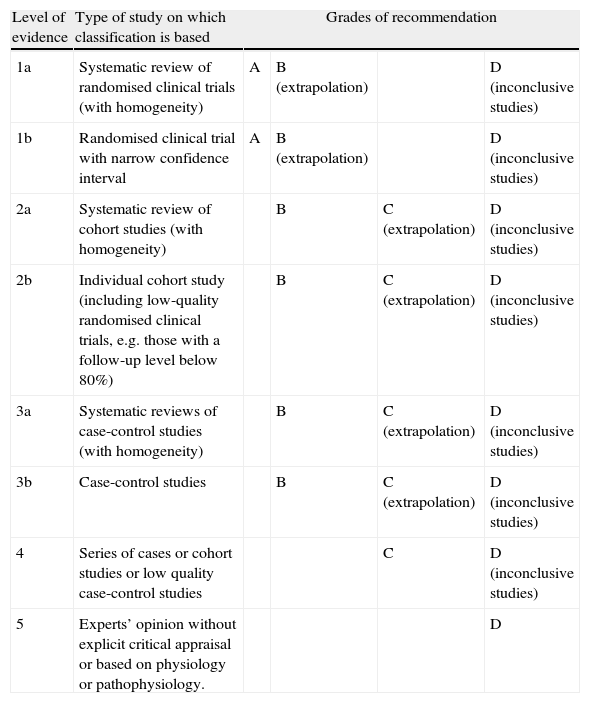
Although subarachnoid haemorrhage (SAH) is the least frequent stroke subtype, its morbidity/mortality rate is the highest. The social and healthcare burden it creates is even heavier when we consider that a sizeable percentage of cases affect younger patients who were previously healthy and completely independent. In this study, we update the recommendations regarding the diagnostic methods and medical treatment for patients with SAH. Grades of recommendation and the scientific evidence supporting them are classified according to Centre for Evidence-Based Medicine (CEBM) criteria (Table 1).
Levels of evidence and grades of recommendation.
| Level of evidence | Type of study on which classification is based | Grades of recommendation | |||
| 1a | Systematic review of randomised clinical trials (with homogeneity) | A | B (extrapolation) | D (inconclusive studies) | |
| 1b | Randomised clinical trial with narrow confidence interval | A | B (extrapolation) | D (inconclusive studies) | |
| 2a | Systematic review of cohort studies (with homogeneity) | B | C (extrapolation) | D (inconclusive studies) | |
| 2b | Individual cohort study (including low-quality randomised clinical trials, e.g. those with a follow-up level below 80%) | B | C (extrapolation) | D (inconclusive studies) | |
| 3a | Systematic reviews of case-control studies (with homogeneity) | B | C (extrapolation) | D (inconclusive studies) | |
| 3b | Case-control studies | B | C (extrapolation) | D (inconclusive studies) | |
| 4 | Series of cases or cohort studies or low quality case-control studies | C | D (inconclusive studies) | ||
| 5 | Experts’ opinion without explicit critical appraisal or based on physiology or pathophysiology. | D | |||
SAH is one of the most feared neurological events due to its associated high mortality and tendency to cause dependence. Its economic impact is more than twice that estimated for ischaemic stroke.1 SAH cases account for 5% of all strokes.2,3 This percentage has risen slightly in the last 30 years due to the decrease in incidence of other stroke subtypes, a tendency associated with improved control over vascular risk factors (VRF). However, this has not affected the incidence of SAH, which remains stable4 at 9 cases/100,000 inhabitants per year according to the European Registers of Stroke study (EROS).2 Similar data are reported by international meta-analyses5 except in Japan and Finland, which report twice this rate. In the case of Spain, the Spanish Society of Neurosurgery reported an increase in incidence after the age of 50, with a slightly higher percentage of women being affected than men. It found no differences related to the day of the week or the month or season of the year, in contrast to results from Rochester,7 where researchers observed increased incidence in the population during winter months. The study by Omama et al.8 found an association between SAH and the hour of onset, with a bimodal incidence curve; this pattern had already been described for haemorrhagic strokes.9 One theory is that this association with circadian rhythm might be explained by the variations in arterial blood pressure throughout the day, and by the increase in platelet aggregation that occurs during the waking process.10 Some 5% of these patients die before reaching the hospital or undergoing imaging studies.3 This percentage is significantly lower than the classic figures for sudden death due to anterior or posterior circulation aneurysms (12% and 44%, respectively).11 Nevertheless, these figures may be biased due to the complexity of defining sudden death and extrapolating data to different countries and cities. In the Spanish study, as many as 68% of patients present in “good clinical condition” at time of arrival, defined as grades I through III on the World Federation of Neurosurgeons Scale (WFNS, Table 2). These grades indicate a score between 13 and 15 on the Glasgow coma scale. This status is a key factor in the decision to hospitalise SAH cases in stroke units.12 After an initial angiography yields negative results, 19% of these events are classified as idiopathic SAH. Of this group, 40% of the cases were perimesencephalic SAH and imaging study results were normal in 10%. As many as 90% of all patients with perimesencephalic SAH and a normal neuroimaging study achieve complete recovery by the 6-month mark.13
In cases of aneurysmal SAH, the most common aneurysm localisation is the anterior communicating artery (36%), followed by the middle cerebral (26%), posterior communicating (18%), and internal carotid arteries (10%). Posterior circulation aneurysms constitute 9% of all cases, while multiple aneurysms are present in 20%. The incidence of associated arteriovenous malformation (AVM) is less than 2%. In Spain, aneurysm repair is performed in 85% of aneurysmal SAH cases; this figure is significantly higher than that reported in series from the United States.14,15 These procedures were performed within 72hours of onset in 64% of the patients, and endovascular treatment was chosen in 56% of the cases.
The overall mortality rate, measured at hospital discharge, is 22%, or as high as 26% if we consider only aneurysmal SAH. These results are comparable to those found in published meta-analyses, which show mortality rates of 25% to 30% calculated at 30 days from onset in countries with a high per capita income.4,16 The last 30 years have shown a clear tendency towards a decrease in mortality, and this is not associated with a higher rate of dependence.3 Patients experience complete recovery (defined as a score of 5 on the Glasgow Outcome Scale [GOS]) in 49% of these cases. Up to 64% of these patients will achieve independence (defined as a GOS score of 4 or 5, comparable to a modified Rankin scale score of 0 to 2). This percentage is only 58%, however, for aneurysmal SAH, a figure which coincides with international results.3,16
Prevention and risk factorsThe main modifiable VRFs are tobacco use, alcohol use, and arterial hypertension (AHT)17; the risk is twice as high if systolic blood pressure (SBP) exceeds 130mm Hg and three times as high if SBP exceeds 170mmHg.18 Other risk factors, such as intense physical exercise, have been described in series that include ACROSS, an Australian study.19 On the other hand, diabetes mellitus is only related to the perimesencephalic SAH subtype.13 The main non-modifiable VRFs are family history in a first-degree relative (which increases incidence by a factor of up to 4)20 and the following connective tissue disorders: polycystic kidney disease, Ehlers–Danlos syndrome type IV, hereditary haemorrhagic telangiectasia, pseudoxanthoma elasticum, multiple endocrine neoplasia type 1, and neurofibromatosis type 1.21 Modifiable VRFs must be corrected in the entire population, especially in patients with SAH.22 This is not only due to the causal relationship described previously, but also to the higher incidence of vascular diseases that are present after a diagnosis of SAH; such cases have nearly twice the level of risk found in the healthy population.16 While use of oral anticoagulants is linked to an increased risk of haemorrhage,23 use of antiplatelet drugs was an unknown factor that required the doctor to weigh the risk of recurrence against the demonstrated increase in incidence of vascular diseases. A sub-analysis of the ISAT study,24 and recent European articles on the same topic,23 provide evidence that antiplatelet drugs can be used safely in these patients. Regarding use of acetylsalicylic acid (ASA), a recent study seems to show that patients with a brain aneurysm who take ASA regularly have a lower risk of rupture than patients who do not.25
Although the prognosis of this disease has shown a tendency towards improvement in the past 30 years, thanks to advances in treatment, the morbidity and mortality rates remain at nearly 60% in the case of aneurysmal SAH. Added to the data for chronic cognitive impairment,16 this situation should urge us to complete additional studies and improve our management of the disease.
Clinical manifestations and systematic diagnostic proceduresRecommendations for diagnosing aneurysmal subarachnoid haemorrhage are summarised in Table 3.
Recommendations for diagnosing aneurysmal subarachnoid haemorrhage: levels of evidence and recommendations.
| Recommendations | Level of evidence and recommendation grade |
| Diagnosis of SAH | |
| If there is a clinical suspicion of SAH, immediately refer the patient to an adequately equipped specialist centre | Level of evidence 3–5. Grade of recommendation C |
| SAH is a serious event with a wide range of clinical presentations. As a result, some 25% to 30% of all cases are diagnosed incorrectly, and repercussions are severe. Doctors must have a good knowledge of its forms of presentation, and all sudden-onset headaches with or without other symptoms should be regarded with a high level of clinical suspicion | Level of evidence 2. Grade of recommendation B |
| The diagnostic study of choice is CT without contrast. The procedure is more accessible than MRI and certain sequences are equally sensitive. The procedure's value decreases over time; CT may be highly sensitive in the first 5 days, but gradually lose sensitivity subsequently | Level of evidence 2. Grade of recommendation B |
| If CT results are negative and there is still a clinical suspicion of SAH, performing a lumbar puncture a few hours after onset is recommended. Presence of red blood cells and/or xanthochromia confirms the diagnosis. After 3 weeks, results from both neuroimaging scans and CSF analysis return to normal | Level of evidence 2. Grade of recommendation B |
| MRI is the study of choice for identifying the source of the bleed; angiography is used if an aneurysm is suspected. MR and CT angiography are very sensitive for detecting aneurysms in the circle of Willis larger than 5mm in diameter. If results from the first angiography are negative, experts recommend repeating it in 2 weeks except in perimesencephalic SAH | Level of evidence 2. Grade of recommendation B |
| Ultrasonography studies are useful for vasospasm diagnosis and monitoring | Level of evidence 2. Grade of recommendation B |
| Screenings are recommended for patients with at least 2 family members with aneurysms. These patients should have an MR angiography study every 7 years between the ages of 18 and 80 | Level of evidence 4. Grade of recommendation C |
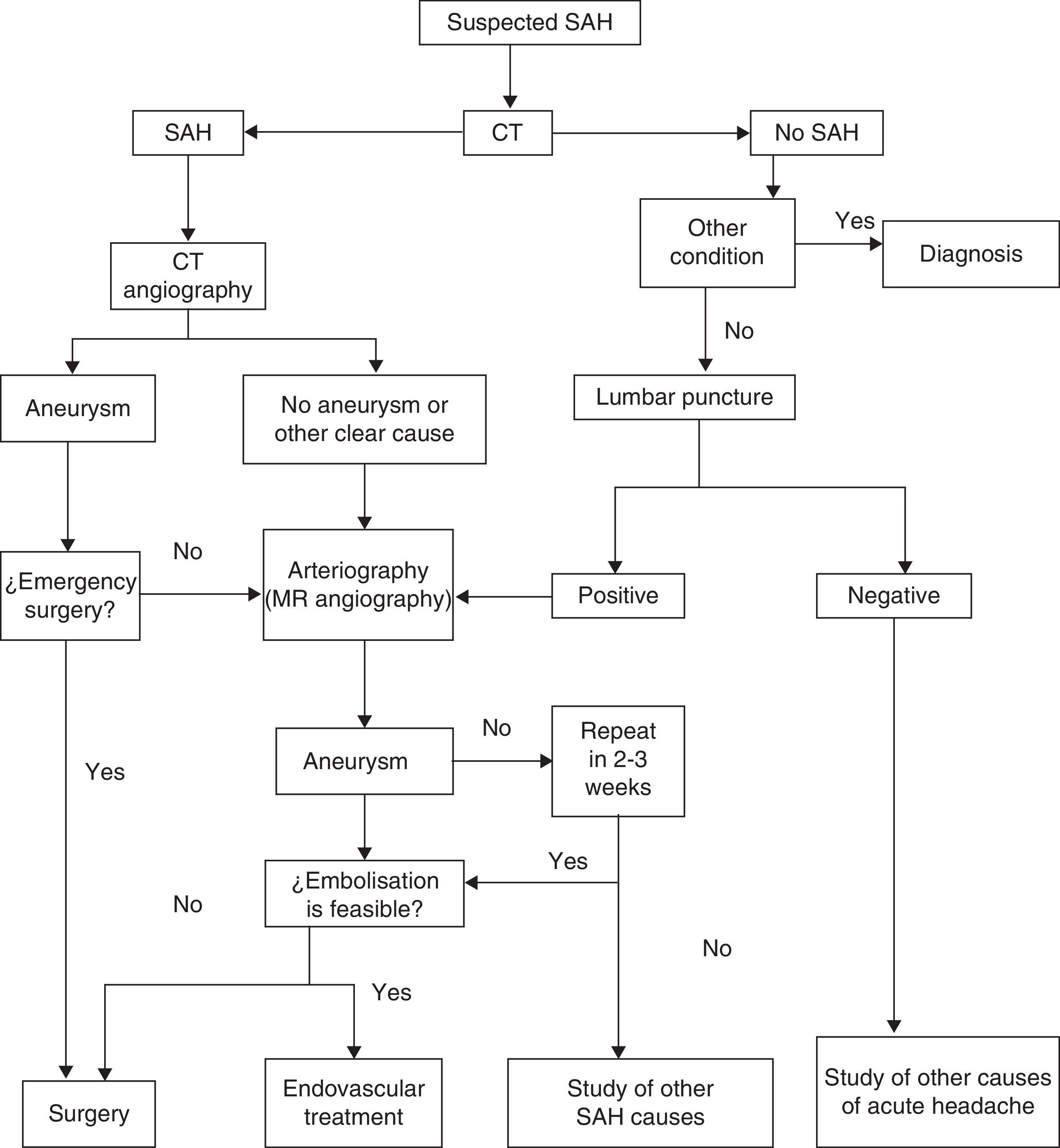
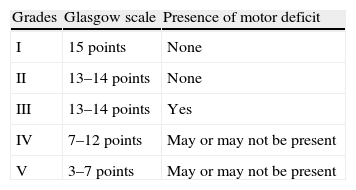
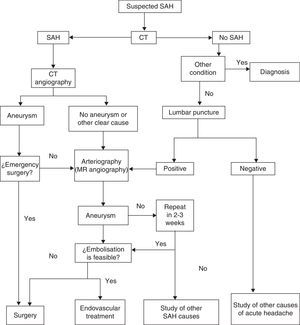
The most common initial sign of SAH is headache, usually presenting as an intense, sudden-onset headache that reaches maximum intensity in seconds or minutes. This is the only manifestation in a third of all cases, and these are the cases most likely to be misdiagnosed. Other symptoms may appear, including loss of conscience (which predicts aneurysmal haemorrhage),26 nausea or vomiting, focal neurological signs, or seizures. Not all sudden-onset or thunderclap headaches indicate SAH,27 and not all cases of SAH present with this type of headache, which would correspond more specifically to SAH due to aneurysm rupture; this type accounts for about 85% of these cases. Types of SAH such as the purely cortical subtype (non-traumatic convexity SAH)28 may have a more subtle presentation with a dull headache, a seizure, or neurological focal sign related to the localisation.29–32Table 4 lists the different causes of SAH. Examination may yield completely normal results, or else the patient may present neck rigidity. This may be absent at first, or in mild cases, or in patients in coma. Another sign to present eventually, in addition to focal signs indicating cranial nerve, cortical, or pathway injury, is subhyaloid haemorrhage in the fundus. SAH is associated with a high rate of diagnostic errors which exceeds 30% in some studies. Errors essentially arise from failure to order a CT because the headache was interpreted as ‘benign’, or else from the headache being masked by an initial disorder of consciousness or by the confusion experienced by the patient.33–36 It should be stressed that SAH events preceded by a ‘warning’ (sentinel haemorrhage) are more serious; in fact, such events actually constitute rebleeding.6,37–44 Complementary studies are run to confirm the diagnosis on the one hand and to identify the source of the bleed on the other. Suspected SAH calls for performing CT without contrast. Some MRI sequences (especially T2-weighted gradient echo and FLAIR) may be as sensitive or even more sensitive than CT for detecting blood in the subarachnoid space,45 but access is poorer and the scan may be compromised if the patient does not cooperate. The diagnostic yield of imaging techniques decreases as time from onset increases. If results are negative but the suspicion of SAH remains, the next step is performing a lumbar puncture.39,46,47 This procedure should be carried out between 6 and 12hours after symptom onset so that blood will diffuse throughout the subarachnoid space; red blood cell lysis will then reveal xanthochromia if it is unclear whether the blood residue is due to SAH or to a traumatic puncture. About 3 weeks after the event, cerebrospinal fluid (CSF) will return to its normal state.48 The Hunt and Hess scale (Table 5)49 and the WFNS scale (Table 2)50 let us quantify the severity of the patient's clinical state; the Fisher scale, based on the quantity and distribution of the haemorrhage, is helpful for predicting the risk of vasospasm (Table 6).51 A more recent version of this scale, the modified Fisher scale, assigns a qualitative value to the presence of bilateral intraventricular haemorrhage and cisternal clots, which indicate a higher risk of vasospasm.52 The source of the SAH is identified with CT, MRI, or conventional 4-vessel digital subtraction angiography. In cases of suspected fistula or AVM, studying both external carotid arteries is recommended. The bleed pattern may lead us to suspect an aneurysmal component (the pure cortical pattern and especially the perimesencephalic pattern are less indicative of aneurysm).53,54 It may also call for an evaluation of the bleed site in cases with multiple aneurysms. With current equipment, CT or MRI angiography is becoming nearly as sensitive as conventional angiography for detecting aneurysms, especially those larger than 5mm.55,56 Imaging studies also let us identify non-aneurysmal causes of bleeding (AVM, tumours, venous thrombosis, angiitis, amyloid angiopathy, or arterial dissection).57,58 They are also helpful in screening for associated entities (intraparenchymal, epidural, or subdural haemorrhage; hydrocephalus; or early-onset vasospasm). In the case of aneurysms, both CT angiography and MRI angiography allow doctors to identify the structure and study its morphology. This being the case, conventional angiography, which is not without its risks, is increasingly relegated to use in later endovascular treatment of the causal aneurysm or AFV. If an aneurysm is suspected but not identified during the initial study, we recommend repeating the study in a period of no less than 2 weeks59 to avoid the problem of aneurysms being masked by an early vasospasm. Perimesencephalic SAH would constitute the exception excluded from this procedure.53,54 It has been calculated that a second angiography study will detect about 5% of all new aneurysms.60 Another useful technique for diagnosing and managing SAH is Doppler ultrasonography; ultrasound techniques are the most useful for diagnosing and monitoring secondary vasospasm.61,62 Transcranial Doppler ultrasound (TCD) is a non-invasive method that is quite useful for diagnosing and following up on vasospasm. This technique is limited by the difficulty of detecting vasospasm in the most distal branches and lack of a good ultrasound window in up to 10% of patients, but it does show good angiographic correlation. We must keep in mind that this technique must be performed by an experienced operator, and that several sequential studies are necessary to deliver the right diagnosis. MRI angiography is another technique that may be useful in detecting, locating, measuring, monitoring, and diagnosing the repercussions of vasospasm (ischaemia). Its specificity, sensitivity, positive predictive value, and negative predictive value range between 92% and 98% of those delivered by conventional angiography. Screening individuals with a family history of aneurysms has been found cost-efficient for those with at least 2 first-degree relatives with aneurysms. The best strategy is performing MRI angiography every 7 years between the ages of 20 and 80, followed by angiography where indicated.63,64Fig. 1 shows an algorithm for diagnostic management in cases of suspected aneurysmal SAH.
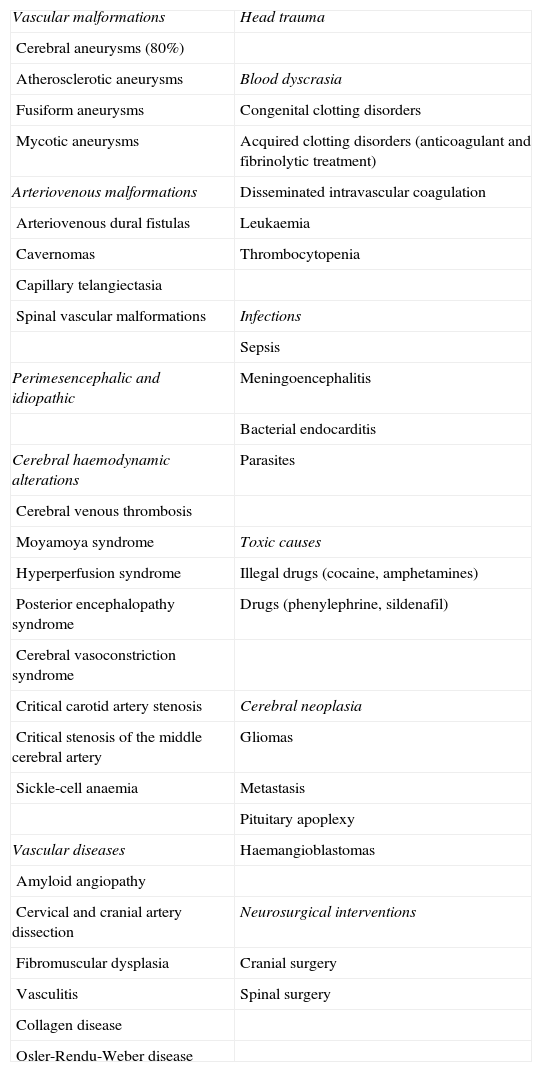
Aetiology of subarachnoid haemorrhages.
| Vascular malformations | Head trauma |
| Cerebral aneurysms (80%) | |
| Atherosclerotic aneurysms | Blood dyscrasia |
| Fusiform aneurysms | Congenital clotting disorders |
| Mycotic aneurysms | Acquired clotting disorders (anticoagulant and fibrinolytic treatment) |
| Arteriovenous malformations | Disseminated intravascular coagulation |
| Arteriovenous dural fistulas | Leukaemia |
| Cavernomas | Thrombocytopenia |
| Capillary telangiectasia | |
| Spinal vascular malformations | Infections |
| Sepsis | |
| Perimesencephalic and idiopathic | Meningoencephalitis |
| Bacterial endocarditis | |
| Cerebral haemodynamic alterations | Parasites |
| Cerebral venous thrombosis | |
| Moyamoya syndrome | Toxic causes |
| Hyperperfusion syndrome | Illegal drugs (cocaine, amphetamines) |
| Posterior encephalopathy syndrome | Drugs (phenylephrine, sildenafil) |
| Cerebral vasoconstriction syndrome | |
| Critical carotid artery stenosis | Cerebral neoplasia |
| Critical stenosis of the middle cerebral artery | Gliomas |
| Sickle-cell anaemia | Metastasis |
| Pituitary apoplexy | |
| Vascular diseases | Haemangioblastomas |
| Amyloid angiopathy | |
| Cervical and cranial artery dissection | Neurosurgical interventions |
| Fibromuscular dysplasia | Cranial surgery |
| Vasculitis | Spinal surgery |
| Collagen disease | |
| Osler-Rendu-Weber disease |
Hunt and Hess scale.
| Grade I | Asymptomatic, mild headache, slight nuchal rigidity |
| Grade II | Moderate to severe headache, nuchal rigidity, cranial nerve palsy |
| Grade III | Drowsiness/confusion, mild motor deficit |
| Grade IV | Stupor, moderate-severe hemiparesis, early decerebrate rigidity, or neurovegetative disorders |
| Grade V | Coma, decerebrate rigidity |
Diagnostic algorithm for suspected aneurysmal SAH. (Modified from Guerrero et al.65).
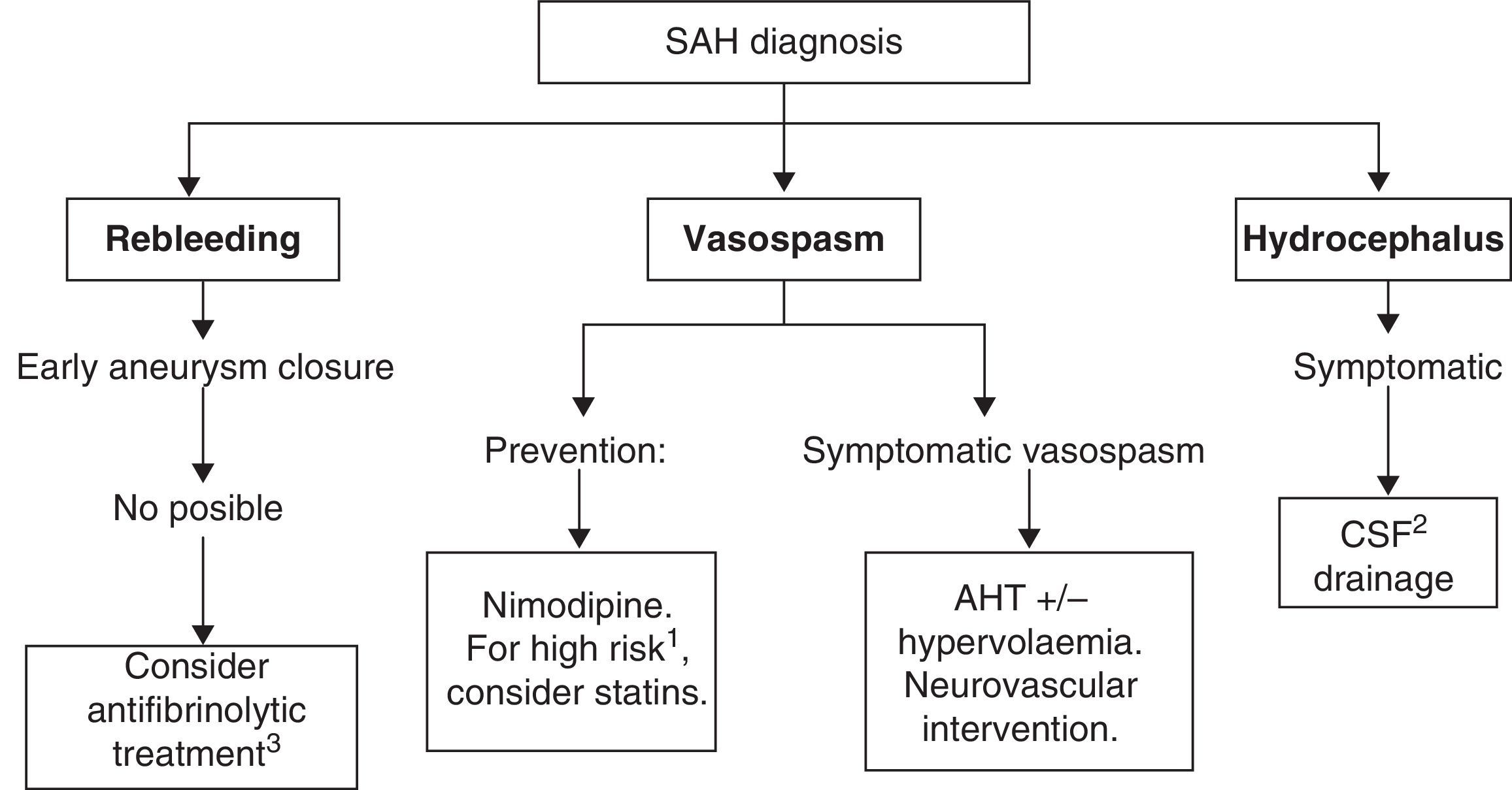
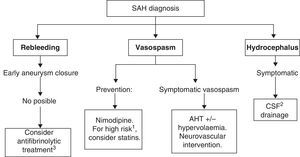
Recommended medical treatments for aneurysmal subarachnoid haemorrhage and its complications are summarised in Table 7. See Fig. 2 for a treatment algorithm for aneurysmal SAH.
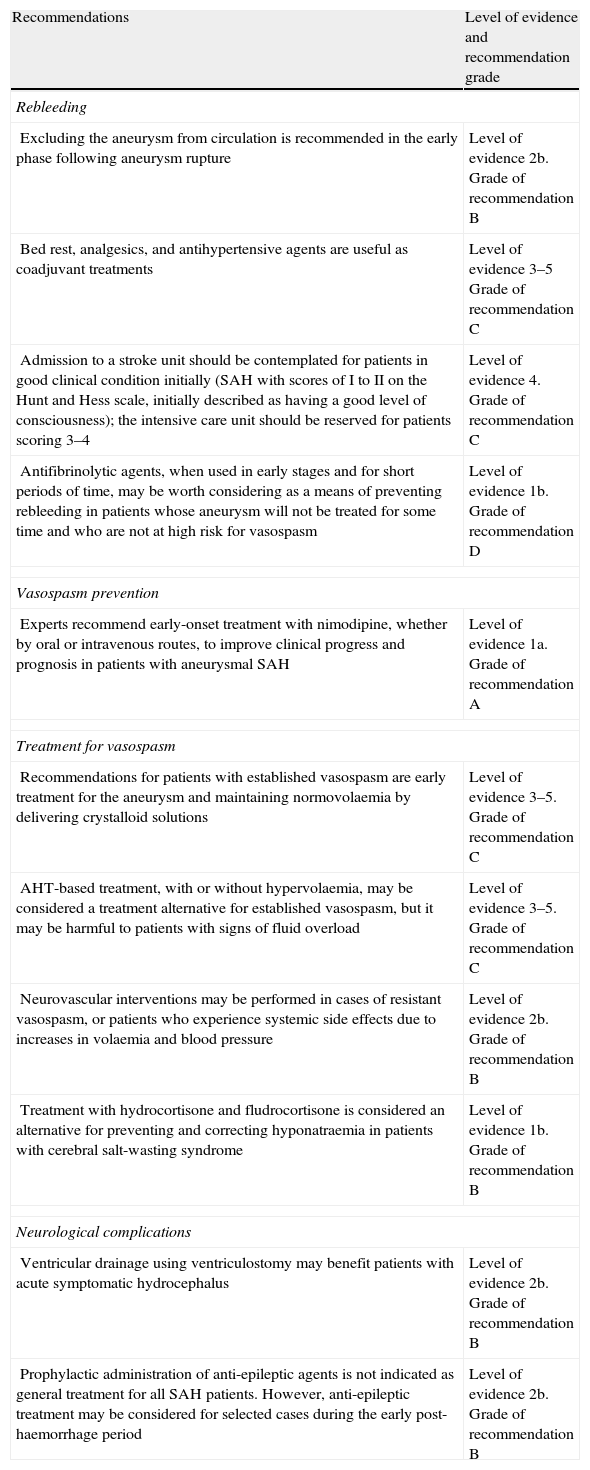
Recommendations for treating aneurysmal subarachnoid haemorrhage. Levels of evidence and recommendations.
| Recommendations | Level of evidence and recommendation grade |
| Rebleeding | |
| Excluding the aneurysm from circulation is recommended in the early phase following aneurysm rupture | Level of evidence 2b. Grade of recommendation B |
| Bed rest, analgesics, and antihypertensive agents are useful as coadjuvant treatments | Level of evidence 3–5 Grade of recommendation C |
| Admission to a stroke unit should be contemplated for patients in good clinical condition initially (SAH with scores of I to II on the Hunt and Hess scale, initially described as having a good level of consciousness); the intensive care unit should be reserved for patients scoring 3–4 | Level of evidence 4. Grade of recommendation C |
| Antifibrinolytic agents, when used in early stages and for short periods of time, may be worth considering as a means of preventing rebleeding in patients whose aneurysm will not be treated for some time and who are not at high risk for vasospasm | Level of evidence 1b. Grade of recommendation D |
| Vasospasm prevention | |
| Experts recommend early-onset treatment with nimodipine, whether by oral or intravenous routes, to improve clinical progress and prognosis in patients with aneurysmal SAH | Level of evidence 1a. Grade of recommendation A |
| Treatment for vasospasm | |
| Recommendations for patients with established vasospasm are early treatment for the aneurysm and maintaining normovolaemia by delivering crystalloid solutions | Level of evidence 3–5. Grade of recommendation C |
| AHT-based treatment, with or without hypervolaemia, may be considered a treatment alternative for established vasospasm, but it may be harmful to patients with signs of fluid overload | Level of evidence 3–5. Grade of recommendation C |
| Neurovascular interventions may be performed in cases of resistant vasospasm, or patients who experience systemic side effects due to increases in volaemia and blood pressure | Level of evidence 2b. Grade of recommendation B |
| Treatment with hydrocortisone and fludrocortisone is considered an alternative for preventing and correcting hyponatraemia in patients with cerebral salt-wasting syndrome | Level of evidence 1b. Grade of recommendation B |
| Neurological complications | |
| Ventricular drainage using ventriculostomy may benefit patients with acute symptomatic hydrocephalus | Level of evidence 2b. Grade of recommendation B |
| Prophylactic administration of anti-epileptic agents is not indicated as general treatment for all SAH patients. However, anti-epileptic treatment may be considered for selected cases during the early post-haemorrhage period | Level of evidence 2b. Grade of recommendation B |
The main objective of medical treatment for SAH is to place the patient in the best clinical situation in order to exclude the ruptured aneurysm from the circulation as safely as possible. Therefore, in such cases and in those without aneurysmal aetiology, doctors aim to prevent the appearance of two of the main neurological complications: rebleeding and vasospasm. Vasospasm must also be treated if it occurs. Likewise, doctors will employ strategies for managing other problems linked to this disease, such as headache, cerebral oedema, potential appearance of seizures, and systemic manifestations. The latter may include ion imbalances (hyponatraemia due to cerebral salt-wasting or to syndrome of inadequate secretion of antidiuretic hormone and hypernatraemia due to diabetes insipidus); heart complications (arrhythmias, acute myocardial infarction, or takotsubo cardiomyopathy); gastrointestinal complications (digestive tract haemorrhage), and respiratory complications (respiratory distress syndrome, neurogenic pulmonary oedema, or pulmonary thromboembolism).
If there is a clinical suspicion of SAH, the patient must be referred to a specialist centre immediately in order to receive the best management and treatment. All patients with SAH should ideally be treated in hospitals that include a neurologist, neurosurgeon, neurointerventionist, CT scanner, MRI scanner, digital angiograph, stroke unit, and an intensive care unit. Hospitals caring for low volumes of patients (for example, <10 cases of aneurysmal SAH per year) should consider early referral of these patients to centres with larger volumes (for example, >35 cases of aneurysmal SAH yearly).66 We recommend early exclusion of the aneurysm from the circulation using endovascular or surgical techniques to prevent rebleeding,44 and the best management practices for potential complications. A recent study recommends excluding the aneurysm within 24hours of stroke onset.67 The patient's clinical condition will determine whether he is admitted to the stroke unit or the intensive care unit. In one tertiary-level hospital registry of patients hospitalised with spontaneous SAH, 25% of the total were admitted to the stroke unit only. The profile of these patients reflected their good initial clinical condition, evidenced by the fact that they maintained their level of consciousness and were graded I and II on the Hunt and Hess scale. Most were candidates for aneurysm embolisation; surgical intervention was generally not necessary. Patients’ functional status progressed favourably and mortality rates were low.12 General treatment measures included resting in a bed angled at 30°, a quiet room with restricted visits, vital sign monitoring, nil by mouth diet, antiemetic drugs, fluid therapy avoiding where possible hypo-osmolar solutions (such as dextrose solutions), analgesia (metamizole, paracetamol, and opiates), laxatives, stomach protectors, and where indicated, prophylactic anticonvulsants and agents preventing deep vein thrombosis.
RebleedingRebleeding is an extremely serious complication with mortality rates ranging from 50% to 70%. For patients presenting with SAH, preventing rebleeding from the ruptured aneurysm is crucial once they have been stabilised. The 24-hour period after the event carries the greatest risk of rebleeding, and this occurs in 4% of all patients. In the subsequent 14 days, cumulative risk of rebleeding remains between 15% and 25%. It will later drop by 0.5%/day between days 15 and 30. Patients are exposed to less risk the earlier the aneurysm is treated, and likewise, other complications can be managed with a greater margin of confidence (for example, treating vasospasm or arterial hypotension). Risk factors for rebleeding are as follows: delays in hospital admission and treatment onset; SBP>160mmHg (although events are more closely related to changes in blood pressure than to specific readings), and poor neurological outlook at admission. We recommend monitoring patients and providing antihypertensives with a short half-life (for example, labetalol) in cases of blood pressure spikes. Likewise, doctors must work to prevent arterial hypotension that may promote ischaemic complications; this may be favoured by the presence of vasospasm.44
Excluding aneurysms from cerebral circulationAneurysms may be excluded from cerebral circulation by either endovascular or surgical treatment. The International Subarachnoid Aneurysm Trial (ISAT) is a study that compared surgical and endovascular treatments for ruptured aneurysms.68 The study provides the following data on endovascular treatment compared to surgical treatment: mortality rate of 8.1% vs 10.1%, disability rate of 15.6% vs 21.6%, and morbidity/mortality rate of 23.5% vs 30.9%. The endovascular group had higher rebleeding rates and lower rates of complete occlusion, and the surgical group had a higher epilepsy rate. Endovascular treatment is the first-choice option provided that the aneurysm can be reached using that technique. If not, the aneurysm may be treated by clipping, or by a combination of the techniques. Patients who have undergone embolisation will require periodic check-ups (at 6 months, 1 year, and 2 years); the procedure must be repeated in cases in which the neck of the aneurysm reopens. Another set of guidelines will provide specific details about techniques used to treat cerebral aneurysms.
Bed restAccording to the Cooperative Study on aneurysms, bed rest alone was inferior to surgical treatment for preventing rebleeding in the global analysis. It was also inferior to antihypertensive treatment, surgery, and carotid ligation in groups that completed treatment.69 Although bed rest is currently contemplated by all SAH protocols, it must be combined with other treatment measures that are more effective for preventing rebleeding. Patients should remain in quiet rooms with few visitors, with the head of the bed elevated to 30° to facilitate venous drainage. It is important that patients avoid efforts that increase intracranial pressure (any symptoms of cough, nausea and vomiting, and constipation must be treated).
Antihypertensive treatmentTreating AHT to prevent rebleeding is a controversial subject. The resulting hypotension may be harmful, especially if the patient experiences vasospasm or intracranial hypertension, as this will decrease cerebral perfusion.70 In a randomised study of antihypertensive and antifibrinolytic treatment, Nibbelink71 described higher rates of rebleeding in groups treated with antihypertensive drugs. It must be noted, however, that rebleeding among these patients was more closely related to AHT than to treatment for that condition, and furthermore, this study was completed 20 years ago. If it were repeated today, using new antihypertensive agents that are safer and more effective, the results would very likely be different.69 An observational study carried out by Wijdicks72 found a higher percentage of rebleeding among patients not treated with antihypertensive agents than among treated patients, even though blood pressure was lower in the first group. This indicates that rebleeding may be more closely linked to abrupt changes in pressure than to specific blood pressure values. Experts recommend using the precise dose of analgesics necessary to achieve good control over the patient's headache and agitation in order to prevent abrupt blood pressure spikes. In theory, metamizole or paracetamol may be administered orally or intravenously if they are not contraindicated. If these drugs are not sufficient, doctors may administer opiates but must guard against hypotension.
Antifibrinolytic drugsStudies published in the last few years have shown antifibrinolytics to be a poor treatment alternative for preventing rebleeding since they are associated with a high rate of ischaemia-related adverse effects.73 Recent studies, which have followed short courses of medication initiated early on, have obtained better results. In a randomised study published in 2002, patients received 1g of intravenous tranexamic acid at time of diagnosis with SAH. Following that, they took 1g every 6hours until aneurysm occlusion. These patients showed significantly lower rebleeding rates and better clinical outcomes than other groups.74 Along similar lines, a prospective study in which ¿-aminocaproic acid was administered during a maximum of 72hours after bleeding onset found a decrease in rebleeding rates without any major ischaemic complications. Researchers observed a higher rate of deep vein thrombosis, but not of pulmonary embolism. Mortality was similar in the treatment and placebo groups, but the prognosis showed a non-significant tendency towards improvement in the treatment group.75
VasospasmIn general, vasospasm will appear between days 4 and 12 after onset. There have also been cases in which vasospasm did not appear for several weeks after the initial bleed, or of onset in the first 48hours. Angiographic vasospasm is present in 66% of all patients, but symptomatic vasospasm (delayed cerebral ischaemia) only occurs in about 30%. This complication is considered responsible for 20% of all morbidity and mortality in SAH, and it is the main cause of delayed morbidity and mortality. Vasospasm intensity is directly related to the initial amount of extravasated blood. Typical presentation involves neurological impairment, with or without associated neurological focal signs, in a patient whose symptoms cannot be explained by hydrocephalus or rebleeding, and whose baseline cranial CT shows no relevant changes in early phases. The patient may also experience fever and confusion. Near the cisterns, in the proximal cerebral circulation where the large arteries of the circle of Willis are located, vasospasm may be detected by angiography or ultrasonography. However, vasospasm may not be identified by these tests if it affects microcirculation in distal vessels exclusively. In such cases, it may be detected by functional tests, such as CT perfusion scanning or diffusion/perfusion MR imaging.76
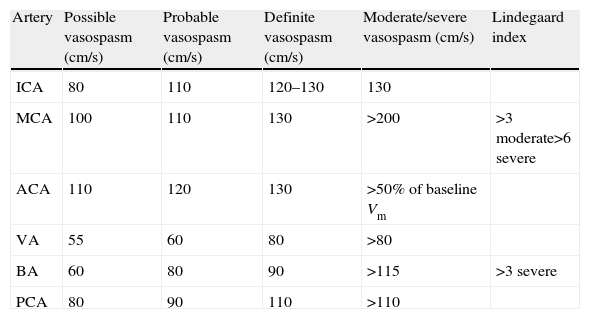
Diagnosing vasospasmTranscranial Doppler ultrasonography. This non-invasive technique is a very useful diagnostic tool because it is largely accessible. Its sensitivity for detecting vasospasm in large arteries of the circle of Willis, especially the middle cerebral artery,76 is similar to that achieved by angiography. However, it is less useful for monitoring the anterior cerebral artery.77 The technique may be performed every 24 to 48hours as a monitoring method, or when changes in the patient's clinical state are indicative of vasospasm. Taking a baseline reading in the first few days is recommendable. The disadvantages of this technique are that up to 10% of all patients have a poor echographic window, and that results depend on the operator. Transcranial Doppler ultrasonography shows a high sensitivity (about 80%) for diagnosing large-calibre vessels (those proximal to the circle of Willis). The increase in mean velocities allows us to establish the degree of vasospasm (Table 8). Reliability is improved by using the Lindegaard indexes, which compare velocity in the intracranial artery with that in the cervical artery. This prevents assigning a diagnosis of vasospasm to increases in velocity secondary to increased flow or hyperaemia. The relative increases in velocity in intracranial arteries compared to cervical arteries are what provides data on frank vasospasm. An index>3 corresponds to the presence of angiographic vasospasm; scores of 5 or 6 denote severe vasospasm.
Criteria for using transcranial Doppler to diagnose vasospasm in different arteries following a subarachnoid haemorrhage (SAH).133
| Artery | Possible vasospasm (cm/s) | Probable vasospasm (cm/s) | Definite vasospasm (cm/s) | Moderate/severe vasospasm (cm/s) | Lindegaard index |
| ICA | 80 | 110 | 120–130 | 130 | |
| MCA | 100 | 110 | 130 | >200 | >3 moderate>6 severe |
| ACA | 110 | 120 | 130 | >50% of baseline Vm | |
| VA | 55 | 60 | 80 | >80 | |
| BA | 60 | 80 | 90 | >115 | >3 severe |
| PCA | 80 | 90 | 110 | >110 |
SAH, subarachnoid haemorrhage; ICA, internal carotid artery; MCA, middle cerebral artery; ACA, anterior cerebral artery; VA, vertebral artery; BA, basilar artery; PCA, posterior cerebral artery.
Note: Velocity figures in the table refer to mean flow velocity: (Vm)=Vd+1/3 [peak systolic velocity (Vs)−end diastolic velocity (Vd)].
Perfusion computed tomography. Perfusion computed tomography is becoming increasingly widespread. It provides a functional diagnosis of the overall circulatory status and evaluates ischaemia resulting from either large vessels or microcirculation. Severe vasospasm, as identified by this technique, is associated with absolute cerebral flows of less than 25mg/100g/min and mean transit times greater than 6.5s or 20% higher than average; the latter cut-off point has a negative predictive value of 98.7% compared to angiography.76 Other authors identify time to peak as the best parameter for estimating the presence of delayed cerebral ischaemia.78
Other diagnostic techniques. While arteriography is considered the test of reference, other complementary diagnostic tests are also used. These include multimodal MRI with MR angiography and perfusion/diffusion weighted sequences that let us check for mismatch (delimiting the ischaemic penumbra), monitor jugular oxygen saturation, and assess tissue oxygen pressure.
PreventionCalcium channel blockers. Nimodipine has been shown to improve functional prognosis, but there is no evidence of it reducing the radiological appearance of vasospasm.79 This benefit is believed to be due to its protective effect on the neurovascular unit. In contrast, nicardipine has been shown to decrease vasospasm figures by 30%, although this does not deliver any functional benefits to patients.80 Although many centres make use of intravenous nimodipine, a recent randomised study of 106 patients found no differences between intravenous and oral treatment with regard to either prognosis or complications. Doctors may therefore opt for intravenous perfusion dosed at 0.2mg/mL at 10mL/h, or oral therapy with 2 tablets of 30mg/4h.81 Nevertheless, a randomised pilot study of 17 patients that measured their nimodipine levels in the acute phase of SAH and compared oral vs intravenous treatment found that levels were lower in patients on oral or enteral treatment. This was especially true if they presented severe SAH with a low level of consciousness (grade IV or V on the Hunt and Hess scale), as that condition may interfere with proper absorption of the drug.82
Statins. Since statin drugs produce multiple effects, including anti-inflammatory, antiplatelet, antioxidant, and vasomotor effects, researchers have designed studies to evaluate the effectiveness of these agents for preventing the vasospasm and delayed cerebral ischaemia associated with aneurysmal SAH. The first such study to demonstrate beneficial results employed 40mg of pravastatin. Patients began taking the drug a mean of 1.8 days after haemorrhage onset; vasospasm was identified by TCD. The study, which included 80 patients, showed significantly lower vasospasm incidence rates in the treatment group, corresponding to a 32% reduction in vasospasm and a 42% reduction in severe vasospasm. Likewise, it recorded an 83% decrease in delayed cerebral ischaemia associated with vasospasm in addition to a 75% decrease in mortality.83 Later studies of the same patient group demonstrated additional benefits in patients treated with pravastatin; a multivariate analysis showed that they had less need for triple-H therapy and a better functional condition at discharge. These benefits remained at the 6-month mark.84 Other study groups have run randomised trials of the effects of 80mg simvastatin on patients with aneurysmal SAH in which vasospasm was measured using TCD and angiography. The first of these studies, including 39 patients and published in 2005, reported a significant reduction in vasospasm in the treatment group, as well as decreased incidence of certain serum markers associated with brain damage, such as von Willebrand factor and S100β protein.85 The second trial, which also included 39 patients, demonstrated the safety of the drug. However, despite there being a tendency towards lower vasospasm and mortality rates in the treatment group, efficacy results were not statistically significant.86 Although one published meta-analysis recommends statin treatment for aneurysmal SAH,87 findings from several recent studies call into question those good results with statins. One of these is a randomised study including 32 patients who were also treated with 80mg simvastatin daily. The study did not find differences between the treatment and placebo groups with regard to decreases in vasospasm or in serum inflammatory markers.88 A more recent meta-analysis that criticises the methods used in the earlier meta-analysis contradicts the benefits attributed to statins.89 As a result, current guidelines are waiting for more solid evidence supporting generalised use of statins. STASH, a multicentre study testing the administration of 40mg of simvastatin, is currently underway and will attempt to clarify whether that drug should be indicated.
Magnesium. Multiple articles have reported on magnesium as a preventive agent for vasospasm associated with aneurysmal SAH. Drug preparation is based on magnesium sulphate, and the drug is delivered intravenously during a 10 to 14-day period, generally in combination therapy with nimodipine. Although one study showed that magnesium and nimodipine have a similar efficacy, the treatment goal is for both agents to exert synergic action to prevent delayed cerebral ischaemia.90 The rationale for administering this drug is based on its vasodilator and cerebroprotective properties. These properties are explained by its antagonist action on both calcium and N-methyl-d-aspartate (NMDA) receptors. Patients taking this drug must remain hospitalised in an intensive care or intermediate care unit to permit monitoring for potential adverse effects, principally hypotension and hypocalcaemia.91 Multiple randomised pilot studies92–94 report different benefits that were not subsequently corroborated by the phase III trial IMASH (Intravenous magnesium sulphate for aneurysmal subarachnoid haemorrhage).95 Neutral results from the MASH-II study were announced very recently but have not yet been published. The purpose of this study is to re-evaluate whether subjects treated with intravenous magnesium demonstrate any differences in disability as measured by the modified Rankin scale (mRS).
Other agents studied. Additional agents under study for their vasospasm-suppressing properties include endothelin receptor antagonists. The CONSCIOUS-1 study (Clazosentan to overcome neurological ischaemia and infarction occurring after subarachnoid haemorrhage), was designed to measure clazosentan efficacy as the primary endpoint. This study showed that the drug was able to reduce the incidence of angiographic vasospasm significantly; furthermore, researchers also observed differences in functional progress between the treatment and placebo groups, which was a secondary endpoint.96 A meta-analysis of 3 published studies found the same result: endothelin antagonists were able to reduce radiological vasospasm, but did not observe any differences in patient progress.97 The CONSCIOUS-2 clinical study, conducted in patients with SAH who had undergone aneurysm clipping, reported that doses of 5mg/h did not deliver benefits.98 CONSCIOUS-3, a randomised clinical trial in patients with SAH who had undergone aneurysm embolisation, found that clazosentan dosed at 15mg/h reduced morbidity associated with vasospasm in SAH but did not affect the functional prognosis.99 Regarding antioxidant agents, numerous free radical scavengers have been studied to evaluate their effectiveness for vasospasm prevention. Tirilazad and nicaraven are key examples. Two different meta-analyses reviewing the results obtained with tirilazad, an aminosteroid that reduces lipid peroxidation, observed that the drug is able to decrease the incidence of symptomatic vasospasm but does not improve patient outcomes.100,101 A recent randomised trial evaluated edaravone, a neuroprotective drug available in other countries. Results showed a non-significant tendency towards a decrease in the incidence of delayed cerebral ischaemia and poor prognosis in treated subjects.102 Erythropoietin is another substance to have been studied in recent years because of its potentially neuroprotective effects that promote self-regulation of the neurovascular unit. A phase II randomised pilot study in 80 patients with aneurysmal SAH showed that the group treated with intravenous erythropoietin during the acute phase presented lower rates of severe vasospasm and delayed cerebral ischaemia.103 Fasudil hydrochloride, a vasodilator that acts by inhibiting protein kinase, was evaluated in a randomised trial of 72 patients in which the control group was treated with nimodipine. Results from this study showed similar vasospasm prevention capacities for the two drugs, while fasudil was more effective for improving motor deficit.104 Other studies have observed that intra-arterial treatment with that drug may also be effective against established vasospasm.105 A randomised trial of 96 patients treated with methylprednisolone, dosed at 16mg/kg and administered intravenously within 6hours of the diagnosis of ruptured aneurysm, found a better functional outlook in the treatment group even though the drug did not affect vasospasm incidence.106 Acetylsalicylic acid has been studied for the purpose of halting potential thromboembolic mechanisms related to delayed cerebral ischaemia. According to some studies, it offers promising results as a drug able to decrease the incidence of delayed cerebral ischaemia; however, the randomised MASH trial, designed to corroborate the drug's efficacy, found no significant differences between treatment and placebo groups.107 A Cochrane review of use of antiplatelet drugs reached similar conclusions.108 Cilostazol, which has antiplatelet and vasodilator properties, acts by selectively inhibiting phosphodiesterase III and thereby elevating levels of cyclic adenosine monophosphate (cAMP). A randomised prospective trial carried out in patients with spontaneous SAH found a better prognosis in the group treated with cilostazol.109 Other antithrombotic agents, including low molecular weight heparins, have also been studied. A randomised trial in 120 patients pointed to benefits from 20mg enoxaparin administered within 3 days of bleeding onset and over the 3 following weeks. The treatment group had lower rates of vasospasm and hydrocephalus, and a better prognosis.110 Other means of decreasing vasospasm rates that have been investigated include combined medical and surgical therapies. Intracisternal irrigation with low doses of fibrinolytic drugs to dissolve clots in SAH patients who have undergone aneurysm clipping is yet another treatment strategy to have been evaluated as a means of preventing vasospasm. A randomised study of tisokinase, a tissue plasminogen activator, found that the drug decreased incidence of vasospasm and increased functional prognosis in the treatment group of a sample of 60 patients.111 In another randomised double-blind study of 32 patients with severe SAH, individuals in the treatment group were fitted with nicardipine prolonged-release implants near the basal cistern during the surgical clipping procedure. This group displayed lower incidence of angiographic vasospasm (7% vs 73% in controls) and delayed cerebral ischaemia viewed with cranial CT (14% vs 47%). Furthermore, this treatment strategy was also associated with better functional outcomes as measured by the modified Rankin scale, better clinical condition according to the NIHSS score, and lower mortality (38% vs 6%).112 However, despite the above list of benefits, a newer study by the same authors carried out to analyse quality of life in patients one year after the SAH did not find better QoL in the treatment group; that parameter was more closely related to the severity of the haemorrhage itself.113
Treatment for established vasospasmTreating established vasospasm requires excluding the aneurysm from circulation as early as possible. This will lower the risk of rebleeding in the case that the patient's volaemia and blood pressure must be increased. Although classic treatment uses triple-H therapy (hypertensive hypervolaemic haemodilution) as the first line of treatment when vasospasm is already established and symptomatic, the efficacy of that treatment has not been proved in clinical trials. Consequently, it cannot be recommended as a general treatment for patients with SAH. Triple-H is currently regarded as a reasonable treatment alternative. The aim of this treatment in SAH is to prevent a hypovolaemic state, to which end doctors attempt to achieve a normovolaemic state with a neutral fluid balance. Achieving hypervolaemia is associated with undesirable effects that include fluid overload, pulmonary oedema, and others. Furthermore, triple-H therapy is associated with a risk of causing rebleeding at the aneurysm site if it has not been closed. Hypervolaemia or normovolaemia may be achieved by administering saline bolus dosed at 15mL/kg in 1hour. Synthetic colloids and blood transfusions, unlike crystalloid solutions, may be associated with a poorer functional prognosis in patients with SAH.114 AHT may be induced with vasopressor agents such as dopamine and dobutamine; vasopressin is not recommended due to the risk of triggering hyponatraemia. Haemodilution has not been found effective in treating vasospasm.115 If another unruptured cerebral aneurysm is present, measures to achieve hypertension and hypervolaemia should be used with caution. If the patient is resistant to treatment measures or presents any contraindications (cerebral oedema, established cerebral infarct, pulmonary oedema, haemoglobin<10, intracranial hypertension, ischaemic heart disease, non-excluded aneurysm), doctors may resort to neurovascular interventions involving local infusions with local dilators such as intra-arterial nimodipine or verapamil. Another option is angioplasty, which is more effective and long-lasting, but which includes a 5% risk of artery rupture. Papaverine, another local vasodilator, is no longer used because its side effects include intracranial hypertension. One event that can limit the effective action of hypervolaemic treatment is the onset of cerebral salt-wasting syndrome that produces hyponatraemia. Osmotic water loss due to excessive natriuresis is a risk factor for developing vasospasm. A randomised study of 72 patients found that hydrocortisone treatment can maintain natraemia levels; it also found a non-significant tendency towards reducing vasospasm with an effect on patient prognosis in the treatment group.116 Two randomised studies have also found that fludrocortisone can prevent hyponatraemia by decreasing natriuresis.117,118 Milrinone, a phophodiesterase III inhibitor with a positive inotropic effect that is administered intravenously, has a good safety profile as a treatment for vasospasm.119,120
HydrocephalusHydrocephalus is an early-onset complication which may arise in the first hours after the event. Symptomatic hydrocephalus affects 20% of SAH patients. Risk factors for hydrocephalus are late hospitalisation and treatment onset, and poor neurological condition at admission (Hunt and Hess score of 3–5). When hydrocephalus manifests clinically with an altered level of consciousness, we can resort to different treatment measures that include placing a temporary ventricular drain or a permanent ventriculoperitoneal or ventriculoatrial shunt. These techniques increase the risk of ventriculitis and rebleeding. A randomised trial with 84 patients observed lower rates of hydrocephalus in patients who underwent early CSF drainage in the acute phase at the time the aneurysm was embolised.121 A series of lumbar punctures may also be performed, preferably once the aneurysm in question has been treated to lower the risk of rebleeding. In exceptional circumstances, and even when the aneurysm has been excluded from circulation, doctors may resort to intraventricular fibrinolysis in resistant cases of hydrocephalus that drain poorly when the catheter becomes obstructed by blood cells.122
Epileptic seizuresTo date, no studies have demonstrated the benefits of prophylactic anti-seizure drugs in patients with aneurysmal SAH. In fact, one study observed that this treatment was linked to a poorer functional prognosis and a higher rate of in-hospital complications.123 Another randomised study comparing phenytoin treatment to levetiracetam treatment observed equal efficacy for seizure prevention; however, patients treated with levetiracetam presented a better functional prognosis.124 Other recent guidelines conclude that prophylactic administration of anti-seizure drugs may be considered in the early posthaemorrhagic period.44
Other complicationsRegarding intracranial hypertension, a randomised study in 22 SAH patients on mechanical ventilation showed that a 7.2% hypertonic saline hydroxyethyl starch solution was able to reduce intracranial pressure readings and improve cerebral perfusion pressure compared to the effects of placebo.125 These findings were corroborated by other study groups that compared the hyperosmolar solution to 15% mannitol or 10% saline solution.126 Approximately one-fourth of the patients with aneurysmal SAH will experience cerebral infarct. The presence of cerebral infarct is associated with poor clinical progress, and risk factors that have been linked with infarct are advanced age, poor clinical condition at admission, AHT, diabetes, larger aneurysms, inducing prophylactic or therapeutic hypertension, body temperature above 38°C at 8 days after the bleed, and symptomatic vasospasm. Vasospasm is the most significant risk factor that is potentially treatable.127 In SAH, as in other types of stroke, the stress the body experiences with the acute vascular event triggers a release of catecholamines that favour a hyperglycaemic state.128 Furthermore, the tendency towards elevated glucose levels will remain over the following days, and presence of hyperglycaemia increases vasospasm frequency, which worsens the patient's prognosis.129 Methods of maintaining strict glycaemic control with intensive insulin therapy have not been shown to improve vasospasm rates or patients’ final outcomes. Experts recommend maintaining normal glycaemia levels so as to avoid both hyper- and hypoglycaemia; abnormal glucose levels are associated with a poor clinical outcome.44,130 Using low molecular weight heparin decreases the risk of thromboembolic complications in patients with parenchymatous intracerebral haemorrhage without increasing the risk of haemorrhage.131 Nevertheless, in the case of aneurysmal SAH, no clear benefits have been found for using low molecular weight heparin to prevent deep vein thrombosis. Data obtained from prospective registers of patients with aneurysmal SAH indicate that high levels of haemoglobin are associated with a better long-term prognosis. Transfusions of red blood cell concentrates for treating anaemia may be considered for patients at risk for developing delayed cerebral ischaemia, but the optimal haemoglobin level has not yet been determined.66 Patients with SAH may experience heart complications, including arrhythmias, acute myocardial infarction, and takotsubo syndrome. Release of catecholamines due to the increased sympathetic tone present in SAH may cause myocardial alterations. A meta-analysis including 25 studies and 2930 patients concluded that elevated levels of troponin I, creatine kinase MB, and the brain natriuretic peptide, together with presence of tachycardia, ST segment depression, T-wave changes, and contractility changes showed a significant association with higher mortality rates, poorer clinical outcomes, and higher rates of delayed cerebral ischaemia.132
Conflicts of interestThe authors have no conflicts of interest to declare.
Ad hoc committee of the SEN Study Group for Cerebrovascular Diseases formed to draw up clinical practice guidelines for stroke.
Coordinator: Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid.
Exuperio Díez-Tejedor (Coordinator), Hospital Universitario La Paz, Madrid; Blanca Fuentes (Secretary), Hospital Universitario La Paz, Madrid; María Alonso de Leciñana, Hospital Universitario Ramón y Cajal, Madrid; José Álvarez-Sabin, Hospital Universitari Vall d’Hebron, Barcelona; Juan Arenillas, Hospital Universitario Clínico de Valladolid; Sergio Calleja, Hospital Universitario Central de Asturias, Oviedo; Ignacio Casado, Hospital San Pedro, Cáceres; Mar Castellanos, Hospital Josep Trueta, Gerona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Antonio Dávalos, Hospital Universitario German Trias i Pujol, Badalona; Fernando Díaz-Otero, Hospital Universitario Gregorio Marañón, Madrid; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; José Antonio Egido, Hospital Clínico Universitario San Carlos, Madrid; Juan Carlos López Fernández, Hospital Universitario Dr. Negrín, Las Palmas; Mar Freijo, Hospital Universitario de Basurto, Bilbao; Blanca Fuentes, Hospital Universitario La Paz, Madrid; Jaime Gállego, Hospital General de Navarra, Pamplona; Andrés García Pastor, Hospital Universitario Gregorio Marañón, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; Francisco Gilo, Hospital Universitario La Princesa, Madrid; Pablo Irimia, Clínica Universitaria de Navarra, Pamplona; Aida Lago, Hospital Universitario La Fe, Valencia; José Maestre, Hospital Universitario Virgen de las Nieves, Granada; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Joan Martí-Fábregas, Hospital de la Santa Cruz y San Pablo, Barcelona; Patricia Martínez-Sánchez, Hospital Universitario La Paz, Madrid; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Carlos Molina, Hospital Universitario Vall d’Hebron, Barcelona; Ana Morales, Hospital Universitario Virgen de la Arrixaca, Murcia; Florentino Nombela, Hospital Universitario La Princesa, Madrid; Francisco Purroy, Hospital Universitario Arnau de Vilanova, Lérida; Marc Ribó, Hospital Universitari Vall d’Hebron, Barcelona; Manuel Rodríguez-Yáñez, Hospital Clínico Universitario, Santiago de Compostela; Jaime Roquer, Hospital del Mar, Barcelona; Francisco Rubio, Hospital Universitari de Bellvitge, Barcelona; Tomás Segura, Hospital Universitario de Albacete, Albacete; Joaquín Serena, Hospital Joseph Trueta, Gerona; Patricia Simal, Hospital Clínico Universitario San Carlos, Madrid; Javier Tejada, Hospital Universitario de León, León; José Vivancos, Hospital Universitario La Princesa, Madrid.
José Álvarez-Sabín, Hospital Universitari Vall d’Hebron, Barcelona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; José Larracoechea, Hospital de Cruces, Bilbao; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Jorge Matías-Guiu, Hospital Clínico Universitario San Carlos, Madrid; Francisco Rubio, Hospital de Bellvitge, Barcelona.
Elisa Correas Callero, Neurology Department, Hospital Universitario La Paz, Madrid; Remedios Frutos, Radiology Department, Hospital Universitario La Paz, Madrid; Fernando Quintana, Radiology Department, Hospital Universitario Marqués de Valdecilla, Santander; José María Roda, Neurosurgery Department, Hospital Universitario La Paz, Madrid; Álvaro Ximénez-Carrillo, Neurology Department, Hospital Universitario La Princesa, Madrid.
The affiliations of the authors and the composition of the Committee ad hoc group's study of diseases stroke of the society Spanish of Neurology are listed in Addendum.
Please cite this article as: Vivancos J, Gilo F, Frutos R, Maestre J, García-Pastor A, Quintana F, et al. Guía de actuación clínica en la hemorragia subaracnoidea. Sistemática diagnóstica y tratamiento. Neurología. 2014;29:353–370.