Increased blood homocysteine levels are a known cardiovascular risk factor. Epileptic patients on long-term treatment with antiepileptic drugs may present higher homocysteine levels and, consequently, a potential increase in cardiovascular risk.
Material and methodsWe conducted an observational case-control study to compare plasma levels of homocysteine, folic acid, and vitamin B12.
ResultsOur study included a total of 88 subjects: 52 patients with epilepsy and 36 controls. Epileptic patients showed higher homocysteine levels (P=.084) and lower levels of folic acid (P<.05).
ConclusionHomocysteine levels should be monitored in epileptic patients on long-term treatment with antiepileptic drugs. We suggest starting specific treatment in patients with high homocysteine levels.
El aumento de homocisteína en sangre constituye un conocido factor de riesgo cardiovascular. Los pacientes epilépticos en tratamiento crónico con fármacos antiepilépticos pueden presentar niveles más elevados de homocisteína y, en consecuencia, un potencial aumento del riesgo cardiovascular.
Material y métodosEstudio observacional de casos y controles para la comparación de los niveles plasmáticos de homocisteína, ácido fólico y vitamina B12.
ResultadosSe reclutó a un total de 88 sujetos, 52 de ellos epilépticos y 36 controles. Se observó una tendencia a niveles de homocisteína más elevados (p=0,084) en los pacientes epilépticos y unos valores de ácido fólico más bajos (p<0,05).
ConclusionesPor su potencial efecto como factor de riesgo cardiovascular, es importante prestar atención a los niveles de homocisteína en los pacientes epilépticos en tratamiento crónico con fármacos antiepilépticos y en caso de encontrar niveles elevados sugerimos la instauración de tratamiento específico.
Epilepsy is one of the most prevalent chronic neurological diseases, with about 5 to 10 cases per 1000 population in Spain.1 Treatment is fundamentally based on long-term use of antiepileptic drugs (AED), achieving seizure control (no seizures) in 70% of cases. However, seizures are not controlled in up to 30% of patients; this type of epilepsy is known as drug-resistant epilepsy.2 The precise causes leading to AED resistance remain unknown today and represent a significant challenge for neurologists.3
According to some studies, epileptic patients receiving long-term treatment with AEDs present an increased risk of cardiovascular disease, especially myocardial infarction, stroke, and cardiovascular death.4 Different lines of research have reported increased plasma levels of cardiovascular risk markers, such as homocysteine, in these patients.
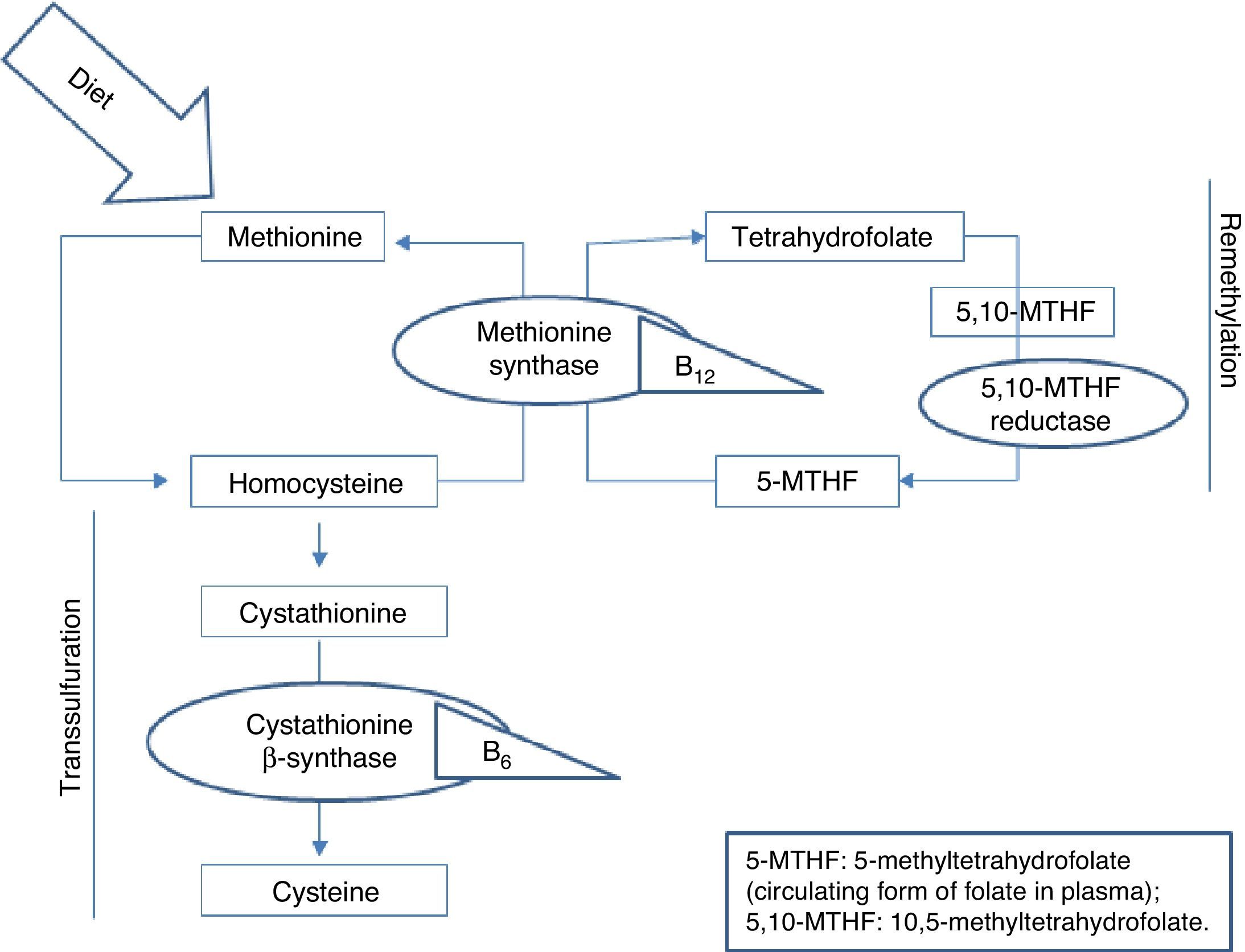
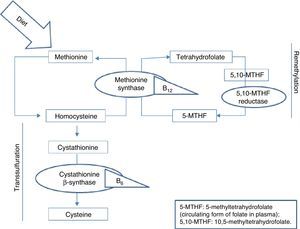
Homocysteine, a sulfur-containing amino acid not present in dietary proteins, is synthesised in the body exclusively via methionine metabolism. Methionine is an essential amino acid that can only be obtained from the diet, mainly from animal proteins. It fulfils important functions in DNA maintenance and plays an essential role in DNA methylation. Once ingested, methionine is metabolised in the liver to form homocysteine. At this point, it can follow 2 pathways: remethylation or transsulfuration.5 B vitamins and folic acid are necessary for homocysteine metabolism in both pathways, as can be seen in Fig. 1.
Increased plasma levels of homocysteine have been associated with increased cardiovascular risk.6 Several epidemiological case-control studies have shown that hyperhomocysteinaemia is associated with a higher risk of developing coronary, cerebrovascular, and peripheral vascular disease.7,8 Hyperhomocysteinaemia is therefore an independent cardiovascular risk factor with a multiplier effect on such other risk factors as arterial hypertension or smoking. Although there is still controversy regarding the level at which homocysteine should be considered pathological, we currently know that the atherothrombotic risk of homocysteine increases at concentrations above 10μmol/L.9,10
Another interesting, though less studied, aspect of increased plasma homocysteine is its potential involvement in the pathogenesis of drug-resistant epilepsy. This potential association has been suggested in studies of experimental animals which demonstrate the proconvulsant potential of homocysteine.11,12 Baldelli et al.12 induced epileptic seizures in rats and observed greater resistance to antiepileptic treatment in those animals which had high homocysteine levels. Furthermore, these rats were found to have greater cortical atrophy. The study concluded by suggesting that a similar phenomenon may occur in epileptic patients.
The causes leading to increased homocysteine levels in epileptic patients following long-term treatment with AEDs are yet to be understood. It has been suggested that the increased levels may be due to alterations in folic acid and vitamin B12 levels; both vitamins are essential for homocysteine metabolism, as mentioned previously. AEDs are believed to interfere with intestinal absorption of folate, metabolism of folate coenzymes, and vitamin B12 metabolism, increasing homocysteine levels.13,14 This hypothesis is supported by the fact that homocysteine levels decrease in epileptic patients treated with AEDs when they receive vitamin B12 and folic acid supplements.15,16
Nevertheless, studies determining folic acid and vitamin B12 levels in epileptic patients following long-term treatment with AED have yielded heterogeneous results. For example, Linnebank et al.17 found low folate and vitamin B12 levels in these patients. In contrast, other studies such as the one published by Mintzer et al.18 found no alterations in these parameters.
Such classic AEDs as phenytoin, phenobarbital, valproic acid, primidone, and carbamazepine are the AEDs most likely to increase homocysteine levels.19,20 However, recent studies have shown that increased homocysteine levels may also be observed in patients treated with such new-generation AEDs as oxcarbazepine and topiramate, among others.21
We decided to conduct the present study in view of the contradictory data on the influence of AEDs on homocysteine, vitamin B12, and folic acid levels and the potential involvement of these biochemical alterations in drug resistance.
Patients and methodsWe conducted an observational study of cases and controls who were recruited from the neurology department at Complejo Hospitalario de Navarra, which belongs to the Instituto de Investigación Sanitaria de Navarra (IdiSNA). The recruitment period lasted 12 months.
Cases were patients older than 18 years with a diagnosis of epilepsy who were receiving long-term treatment with AEDs (minimum 2 years). Controls were patients older than 18 years without a diagnosis of epilepsy who were not taking AEDs.
Exclusion criteria for both groups were as follows: taking vitamin supplements including B vitamins or folic acid, and a diagnosis of a disorder of or a genetic defect in folic acid metabolism.
This study was approved by the Clinical Research Ethics Committee at Complejo Hospitalario de Navarra; all participants signed informed consent forms after being duly informed about the study. We gathered blood samples and collected clinical information from all participants.
Clinical variables were gathered by interviewing the study participants. For all patients, we collected data on demographic variables, medical history, presence of cardiovascular risk factors (arterial hypertension, diabetes mellitus, dyslipidaemia, and tobacco use), and current treatment. In the case of epileptic patients, we also collected data on the following variables: disease progression, seizure frequency, and number of AEDs being taken at the time of the study. The Complejo Universitario de Navarra laboratories analysed the biochemical variables; vitamin B12, homocysteine, and folate levels were measured by chemiluminescence immunoassay.
Data were analysed using the IBM® SPSS statistical software, version 21. We performed a descriptive analysis of the sample. We used the one-sample Kolmogorov–Smirnov test to check the normality of data and the t test and the chi-squared test to compare means of quantitative and qualitative variables, respectively. P-values <.05 were considered to be statistically significant.
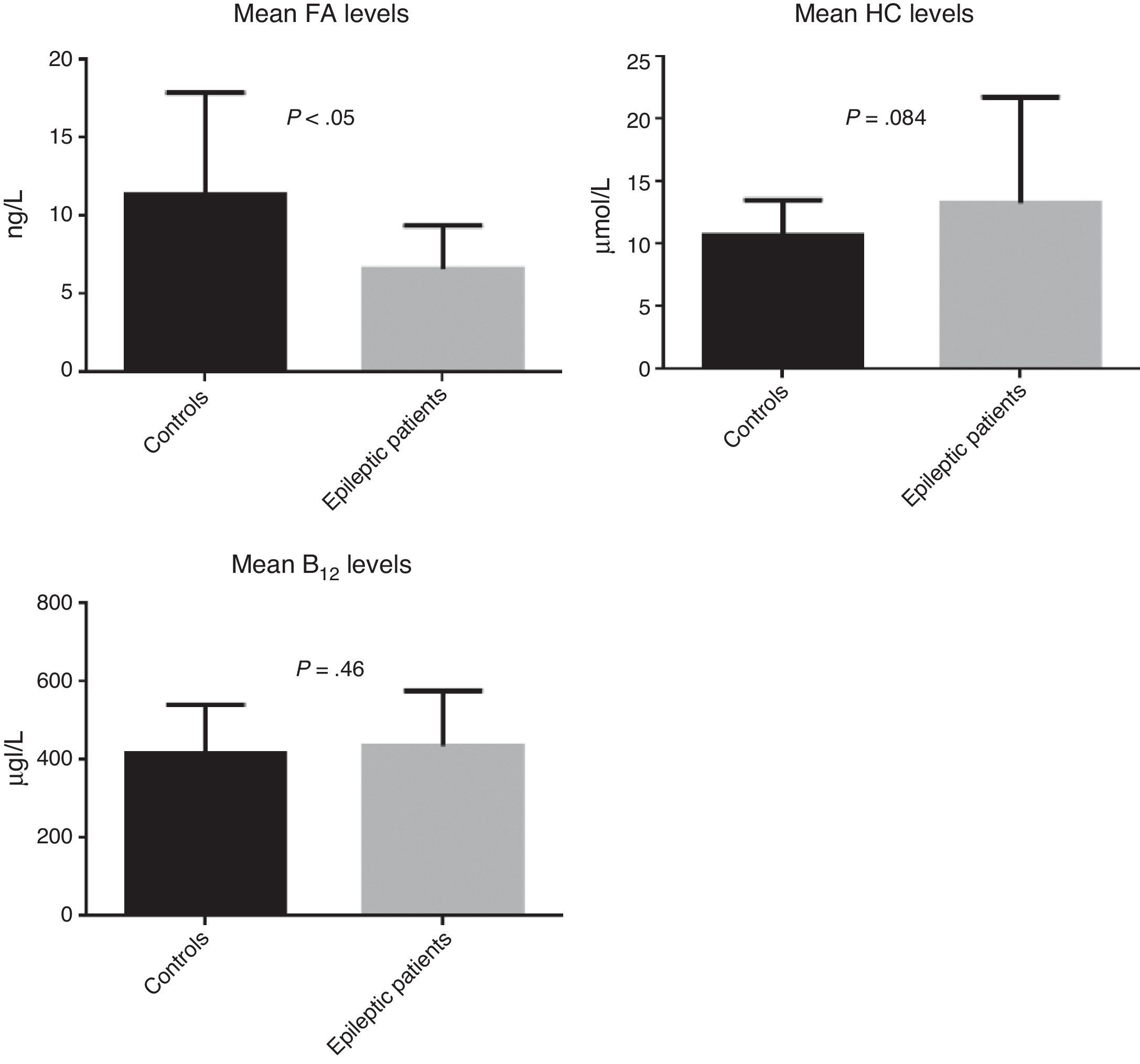
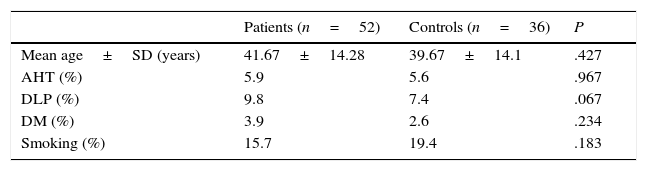
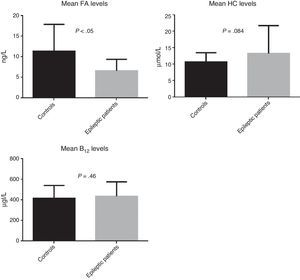
ResultsWe recruited a total of 88 patients: 52 had epilepsy (26 men and 26 women) and 36 were controls (11 men and 25 women). Table 1 describes the clinical characteristics of our sample. Epileptic patients displayed higher mean levels of homocysteine and vitamin B12 than controls. The t test revealed non-significant differences in homocysteine and vitamin B12 levels; however, homocysteine levels were slightly higher in epileptic patients (P=.084). Folic acid levels, in contrast, were slightly lower in epileptic patients than in controls (6.6ng/L vs 11.4ng/L); this difference was statistically significant according to the t test (P<.05) (Fig. 2).
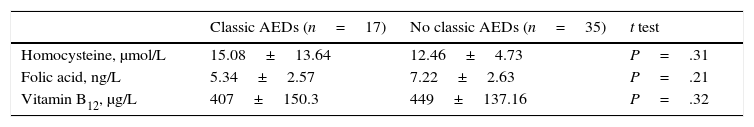
Table 2 describes treatment characteristics in the group of epileptic patients and Table 3 describes the biochemical variables in patients showing adequate response to AEDs and in those with drug-resistant epilepsy. No differences were found in distribution of cardiovascular risk factors and use of classic AEDs between groups. The group of patients with drug-resistant epilepsy displayed higher mean homocysteine levels and lower folic acid and vitamin B12 levels than those achieving good seizure control. However, these differences were not statistically significant.
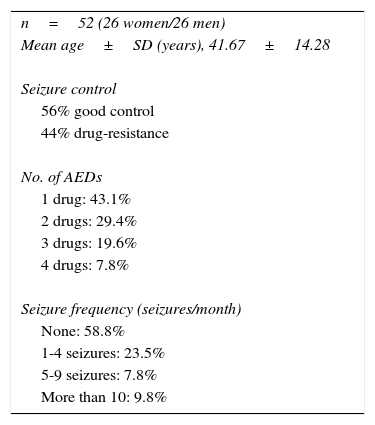
Treatment characteristics in the group of epileptic patients.
| n=52 (26 women/26 men) |
| Mean age±SD (years), 41.67±14.28 |
| Seizure control |
| 56% good control |
| 44% drug-resistance |
| No. of AEDs |
| 1 drug: 43.1% |
| 2 drugs: 29.4% |
| 3 drugs: 19.6% |
| 4 drugs: 7.8% |
| Seizure frequency (seizures/month) |
| None: 58.8% |
| 1-4 seizures: 23.5% |
| 5-9 seizures: 7.8% |
| More than 10: 9.8% |
Mean levels of biochemical variables in patients with and without good seizure control.
| Good seizure control (n=29) | Drug-resistance (n=23) | t test | |
|---|---|---|---|
| Homocysteine, μmol/L | 11.98±3.31 | 15±12.3 | P=.21 |
| Folic acid, ng/L | 6.85±2.24 | 6.35±3.31 | P=.52 |
| Vitamin B12, μg/L | 459±152.4 | 405.1±121.5 | P=.17 |
Data are expressed as means±standard deviation.
We also studied the differences in mean homocysteine, folic acid, and vitamin B12 levels between patients receiving classic AEDs, either in monotherapy or in combination therapy, and patients not receiving treatment with these drugs. Again, no statistically significant differences were found in mean levels of any of the biochemical parameters between groups; however, we did observe higher mean homocysteine levels and lower folic acid and vitamin B12 levels in the patients receiving classic AEDs (Table 4).
DiscussionOur study revealed significantly lower mean folic acid levels in epileptic patients on long-term treatment with AEDs than in controls. Furthermore, patients receiving long-term treatment with AEDs presented higher mean homocysteine levels; this difference was not statistically significant, however (P=.084).
Previous studies have also reported higher homocysteine levels in epileptic patients receiving AEDs, with statistically significant differences in some of these studies. The lack of statistical significance in our study may be due to the small size of the sample compared to those of other similar studies: the study by Linnebank et al.,17 for example, included 2730 patients, and the study by Belcastro et al.21 had a sample of 480 patients.
However, we did find statistically significant differences in folic acid levels, which were lower in patients receiving long-term treatment with AEDs. This is consistent with the hypothesis that increased homocysteine levels in these patients result from AEDs interfering with homocysteine metabolism, in which folic acid is essential.
Decreased folic acid levels in epileptic patients on long-term treatment with AEDs have been reported in several studies.22,23 Some of these studies suggest administering folic acid supplements to correct homocysteine levels, based on the innocuity of folic acid supplementation and the potential cardiovascular risk of hyperhomocysteinaemia.24,25
However, the extent to which cardiovascular risk decreases with folic acid supplementation is yet to be determined. Further prospective studies are necessary to gather more data on this topic. While we await further evidence, folic acid supplementation seems to be a good treatment option when these patients display low folic acid levels.
Lastly, according to our literature search, no studies have compared the levels of these biochemical parameters in epileptic patients receiving and not receiving classic AEDs and in those showing good response to treatment vs those with drug-resistant epilepsy. Our study revealed higher mean homocysteine levels in patients receiving classic AEDs and in those with drug-resistant epilepsy, although this was not statistically significant; these findings are noteworthy despite the lack of statistical significance.
Based on the trend observed in our study (patients receiving classic AEDs displayed higher homocysteine levels), epileptic patients with other cardiovascular risk factors may be advised to discontinue use of classic AEDs. However, these results should be confirmed by future studies with larger sample sizes.
In theory, by doing this we would avoid increasing patients’ risk of cardiovascular disease.
Our study also reported higher homocysteine levels in patients with drug-resistant epilepsy; this finding is interesting considering that a recent study by Bochynska et al.16 commented on the potential relationship between high homocysteine levels and poorer outcomes. Although it is true that these researchers did not further analyse such an association, the proconvulsive potential of high homocysteine levels has been demonstrated in studies with experimental animals.
Our data do not allow us to make further conclusions on this issue, but do point towards a promising line of research, especially considering that folic acid supplementation helps decrease homocysteine levels with practically no adverse effects.
In conclusion, despite the small size of our sample, our results underscore the importance of monitoring homocysteine and folic acid levels in epileptic patients, especially in those with drug-resistant epilepsy and a high cardiovascular risk. Doctors should focus on avoiding factors that favour an increase in homocysteine levels, and when necessary, implementing therapeutic measures to alleviate the potential adverse effects of hyperhomocysteinaemia.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pulido Fontes L, Pulido Fontes M, Quesada Jiménez P, Muruzabal Pérez J, Mendioroz Iriarte M. Estudio comparativo de los niveles plasmáticos de homocisteína, vitamina B12 y ácido fólico en pacientes epilépticos frente a controles. Neurología. 2017;32:440–445.
Part of this study was presented in poster format in the epilepsy section during the 67th Annual Meeting of the Spanish Society of Neurology (November 2015).