Competency means the capacity to make responsible and balanced decisions. This may be performed in clinical settings (decision-making abilities on treatment or risky diagnostic procedures) and also in daily-life activities (financial matters, nursing home admittance, contracts, etc.). Competency is linked to the ethical principle of autonomy and to a horizontal doctor–patient interaction, far from ancient paternalistic relationships. It is contemplated in the Spanish law as the patient's right to be informed and to make free choices, particularly in cases of dementia.
DevelopmentThe competency that we assess is the so-called natural or working capacity. It is specific for an action or task. The level of required capacity depends on the decision: higher for critical ones, lower for low-risk decisions. The assessment process requires noting the patient's capacity to understand, analyse, self-refer and apply the information. There are some guides available that may be useful in competency assessments, but nevertheless the final statement must be defined by the physician in charge of the patient and clinical judgment.
Capacity is directly related to the level of cognitive deterioration. Nevertheless, specific cognitive tests such as MMSE (mini-mental) have a low predictive value. The loss of competency is more associated with the so-called legal standards of incapacity (LS). These encompass a range of five steps (LS1–LS5), which may detect the incapacity from the mild levels of dementia. The cortical functions that are the best predictors of incapacity are language and executive dysfunctions. These explain the incapacity in the cases of Alzheimer's and Parkinson's diseases, and have been studied more.
ConclusionsIncapacity is common and it influences the clinical decision-making process. We must be particularly cautious with clinical trials of dementia. It also involves other areas of daily life, particularly financially related ones, where limitations are present from the mild cognitive impairment (MCI) level. The neurological community has already produced specific and invaluable documents like the one from Sitges, although in our opinion this community has to increase its awareness, and also its involvement as much in the clinical as in the research sides of this field.
Competencia significa capacidad o aptitud para tomar decisiones responsables y razonadas. Puede verse comprometida en acciones de la vida diaria (asuntos económicos, de residencia, contratos, etc.) o en escenarios clínicos (decisiones sobre tratamientos o pruebas diagnósticas de riesgo). Emana del principio de autonomía y de una relación médico-enfermo horizontal y no paternalista. Reconocida en la legislación española como parte del derecho a la información y libre elección del paciente, estamos obligados a determinarla, particularmente en demencias.
DesarrolloLa competencia que evaluamos es la natural o de obrar. Es específica de tarea. El nivel de capacidad exigido varía con la decisión, muy alto en las críticas, bajo en las de escaso riesgo. El proceso requiere perfilar las capacidades de comprensión, de análisis y de aplicación de la información. Existen guías que facilitan la valoración, aunque la decisión final recae en el médico responsable y depende de su juicio clínico.
La capacidad se relaciona directamente con el nivel de deterioro cognitivo. No obstante, baterías específicas o test como el Minimental tiene muy escaso valor predictivo. La pérdida de competencia se correlaciona mejor con medidores como los llamados niveles legales de competencia (LS), que con 5 grados (LS1-LS5) detectan incapacidades desde estadios leves de demencia. Las funciones corticales mejor predictoras de incapacidad son el lenguaje y sobre todo las disfunciones ejecutivas. Estas explican la incapacidad de muchos casos de Alzheimer y Parkinson, los más estudiados.
ConclusionesLa incapacidad es frecuente y tiene implicaciones en decisiones clínicas. Se debe ser especialmente cauteloso con los ensayos clínicos en demencia. La demanda alcanza múltiples esferas de vida diaria, sobre todo las económicas, donde se detectan limitaciones desde el deterioro cognitivo leve. La comunidad neurológica ha elaborado documentos específicos de alto valor como el de Sitges, aunque creemos ineludible aumentar su concienciación y participación—asistencial e investigadora—en este campo.
Competency refers to a person's ability to make responsible decisions on matters that affect different areas of his or her life and involve assuming risks. The individual must make an informed decision freely and without being influenced. While this influence is understood to be external, it can also be internal and result from the person's mental state; a weakened mental state is a factor limiting the individual's ability to make decisions entailing risk. It is from this perspective that we will be examining competency in dementia. Cognitive impairment, and the emotional state which frequently accompanies it, involve a type of internal duress which limits a patient's ability to make decisions entailing risks, including health care decisions having to do with accepting certain diagnostic procedures or treatments.
Informed consent and evaluating capacity or competencyOnly an informed and capable individual, meaning one fully aware of the risks involved in making a decision and able to understand the gravity of its consequences, is able to choose responsibly. Therefore, the first step in evaluating competency is assessing informed decision-making. In these matters, we are guided by the principle of autonomy: the rule states that the patient may freely accept or reject a medical option, and this principle only applies in practice if the patient has sufficient information about the procedure, whether diagnostic or therapeutic.1 The doctor responsible for the patient must be the one to provide that information so that the patient will be able to accept or refuse the proposed option or options, that is, make use of the power of choice implicit to the autonomy principle. The relationship between one party providing information and the other party's logical decision based on the information is what precisely defines informed consent. We will therefore address this specific aspect of the doctor–patient relationship, seen from the perspective of recognising the patient's own ability to make decisions. The doctor's position will now be diametrically opposed the paternalistic attitude which was dominant only few years ago. According to the former paradigm, the doctor was responsible for making decisions which the patient would then directly accept as the most appropriate and beneficial.2
The history of informed consent is long and convoluted.3 The successive events spanning the last 5 centuries have brought us into the modern era. Relationships between individuals are now horizontal, and people in positions of power no longer impose their will on others. Vertical relationships, those in which one party was considered superior based on his medical knowledge, meant that the doctor decided what was best for the patient, who was viewed as a tutored, passive subject. Horizontal relationships, characterised by transmitting information, dialogue, and decision-making by the patient, constitute a leap forward.4–6
In Spain, these ideas only began to arrive in the last 20 years, and they were accepted grudgingly due to their being perceived as a challenge to the doctor's knowledge and decision-making capacity. This mentality gave rise to a concept of informed consent that is more a legal formality than true transmission of information. This process should be part of the underlying structure of a doctor–patient relationship, which was and still is essentially based on the spoken word: medical history, examinations, information on diagnostic and treatment procedures, discussion of pros and cons, and the patient's own reasoned decisions. An informed consent document cannot replace this entire process, even if it might seem faster or more convenient from the standpoint of legal concerns, and even if it is easier to hand over a pamphlet instead of using our limited time to speak with the patient. The fact is that the consent document should only be one part in the process. It could be seen as an outline that summarises and organises the information which the patient should already have heard during direct conversations with the doctor.6
The entry of these ideas into our healthcare system was formalised in our codes of ethics7–9 and in Spanish legislation,10–12 especially Law 21/2000 on patients’ right to information and Law 41/2002 on patient autonomy. The latter contains 2 chapters specific to the topic which concerns us. The first covers the right to information and the second, respect for the patient's autonomy. The second chapter states that the more uncertain the result of the intervention, the greater the doctor's obligation to obtain the patient's written consent.
Legislation also addresses the concept of ability or competency from the patient's point of view. This brings us to the next basic concept: the decision-making capacity of a patient who has been informed. This ability refers to natural or functional capacity, and it must be established before a medical procedure can take place. Legal competence, on the other hand, may only be declared by a judicial authority.
The topic we will be addressing here is therefore natural capacity, which affects daily life events and has consequences for the patient and his/her immediate family, social, employment or economic situation. In fact, a patient's natural or functional capacity may affect every event of his or her daily life, from signing contracts, marriage/adoption certificates, testaments, etc., to accepting or refusing treatment or institutionalisation. However, functional capacity as described here is task-specific. This means we must judge whether or not a patient is competent to make a specific decision. Given the limitations caused by the patient's disease, meaning dementia or cognitive impairment in our case, can he or she understand treatment risks and benefits, refuse institutionalisation, or even vote? These are direct questions, but we will rarely have recourse to a standard tool to provide us with answers.
Declaring legal competence—and its opposite, legal incompetence—are the processes in which several judicial bodies intervene, almost always at the request of the patient's family, in order to protect the patient's life and property. A legally incompetent person must have a legal guardian who reports periodically to a judicial authority.13 Doctors and medical professionals in general do not have the power to declare legal competence. The Office of the Public Prosecutor and the judge normally request medical evaluations and reports upon which to base their decision. In these cases, rulings are all-encompassing, unlike pronouncements on natural or functional capacity, which are task-specific.
The doctor responsible for the patient should be the one to evaluate functional capability. The medical personnel caring for the patient must determine his or her capacity, with recourse to additional evaluations by other colleagues as needed.1 Strangely enough, neurologists are absent from this list of consulting colleagues, even during the process of evaluating capacity in dementia patients.14–16 We believe that the neurological community should be involved in this process.
Evaluating capacity: general principlesEvaluating capacity or competency is a task that entails considerable responsibility, and not just in a strictly clinical sense. Declaring a person incapable, even for specific tasks, means depriving him or her of basic rights and liberties. These rights and liberties are assumed by a third party in order to protect the patient. The task of incapacitating a patient is based on the principle of “first, do no harm”. According to Diego Gracia, this principle plus the fairness or justice principle should guide our work as a whole, and that of healthcare administrators as well.17 The primary goal is to protect the patient from all harmful activity, while guaranteeing equitable and efficient use of healthcare resources. Applying these principles to determining competency or capacity entails, firstly, preventing harm to the patient. Examples include preventing the patient from making risky decisions beyond his or her capacity, and not depriving the patient of rights through unnecessary incapacitation.15
Spanish law states that the doctor responsible for the patient must be the one to define his or her capacity or competency.12 Legal dispositions and codes of ethics establish three types of requirements: cognitive, affective, and conative or volitional. In fact, this threefold model is rooted in Hippocratic medicine and the classical schools, which defined three different capabilities of the mind: knowledge, will, and emotion or preference.18 Unfortunately, the law neither establishes normal parameters for competency nor defines methods or tools for measuring it.
Doctors in general, and neurologists in particular, routinely establish competency or functional capacity based on a mixture of experience, intuition, and common sense. We have been doing this for some time now, and it would seem that the procedure works, judging by how little we hear about cases requiring a second opinion or generating conflicts. However, more careful analysis reveals that this effect is really a reporting bias. Only cases in which patients refuse highly effective treatments or procedures attract attention and create conflict. The example of Jehovah's Witnesses who refuse blood transfusions is the most striking. But many other situations also occur in which treatment or diagnostic procedures are initiated without the patient having sufficient competency or capacity to make a decision. When the patient does not refuse the procedure and there are no significant risks or severe consequences, lack of competency goes unnoticed. The fact that sensitivity to lack of competency is low, and that this lack only becomes an issue in grave cases, goes against respect for the principle of autonomy. This provides food for thought, and should lead us to improve our ability to diagnose functional incompetence. Healthcare administrators must be aware of this as well, since this task will require more dedication and more time, which is our scarcest resource due to its high cost.
If we do not accept and provide case-by-case evaluations of competency, we will come face to face with reality. In fact, cases in which there is a request for assessment of the financial decision-making capacity of a patient with cognitive impairment are increasingly common. Cognitive impairment is not always severe enough for us to be able to state that the patient is obviously incapable. In other cases, patients who refuse to live in a residence may require evaluation. Other patients may accept or refuse risky diagnostic or treatment procedures, and our well-informed society requires that these procedures be used cautiously, in keeping with the patient's decision-making capability. For these purposes, the general principles used to guide any competency assessment process are listed in Table 1. Tools specific to evaluating dementia are listed in the final sections of this study.
General principles in evaluating capacity.
| 1. Incapacity is not pronounced based on diagnosis of a specific pathology (drug addiction, dementia, psychosis, etc.), but rather from individual evaluation of each case. |
| 2. Evaluation must always be task-specific, since some tasks may be affected while others remain completely unaltered. |
| 3. Competency may fluctuate, and evaluations must therefore be continuous. |
| 4. The seriousness of the decision determines the required level of competence, which must be higher if the choice may entail severe consequences (Drane's sliding scale model, see text). |
| 5. The decision itself constitutes no basis for declaring a patient incapable, even if its seems absurd. Autonomy includes the right to refuse life-saving treatment (if the patient is informed, capable and under no duress). |
| 6. Assessing capacity is based on the decision-making process (receiving and understanding information, implications for the patient, reasoned decision, communicating the decision), and not on the choice itself. |
| 7. Cognitive evaluation tools (MMSE) must be used in conjunction with specific tests of capacity (see “Tests” section). |
At this point, we should reiterate the important fact that the required degree of capacity or competence is directly proportionate to the level of risk associated with a decision. With this in mind, making a decision entailing great risk (undergoing an emergency carotid endarterectomy) or in turn, refusing a manifestly beneficial procedure or treatment (antibiotics for meningitis) requires a high degree of capacity on the part of the patient. Meanwhile, other clinical situations entail a very low degree of risk, whether the patient opts to refuse a procedure (cranial CT as dementia follow-up) or accept it (vitamins for a non-deficiency-related neuropathy). Aside from evidence-based benefit considerations, risk in such clinical situations is very low, and the patient's requisite degree of capability is also low. There are also situations ranging between the two extremes, in which risks or benefits may be significant, but not vitally important. It is likely that most clinical situations fall into this last category. In such cases, the potential benefit of accepting diagnostic tests or treatments, and the resulting risk of refusing them, is moderate. In practical terms, this means that the procedures are neither vital nor urgent, although by refusing them a patient's quality of life or even life span may be lessened. Examples of decisions regarding treatment include refusing statins used in secondary prevention of cardiovascular disease or drugs used to treat Parkinson disease, dementia, or multiple sclerosis. Examples of decisions regarding diagnostic procedures include accepting non-emergency conventional angiography, refusing a DaTscan to evaluate parkinsonism, or refusing lumbar puncture for a demyelinating disease. Potential risks and lost potential benefits are not negligible, even if they are not of vital importance. This is why we must be more rigorous regarding the patient's capacity in intermediate cases, while this would be a lesser concern in critical, life-or-death situations. This array of situations is shown in the Drane sliding scale (Table 2).2,20,21
Drane's sliding scale of competency.
| Decisions | Competence | |
| Standard 1 | 1. Consent where risk/benefit balance is favourable | Low |
| 2. Refusal where risk/benefit balance is unfavourable | ||
| Standard 2 | Consent or refusal where risk/benefit balance is unclear | Moderate |
| Standard 3 | 1. Acceptance where risk/benefit balance is unfavourable | High |
| 2. Refusal where risk/benefit balance is favourable |
From a purely cognitive viewpoint, the 3 levels for situations also entail different requirements regarding the patient's understanding and decision-making capacity. For standard 1 situations, it is only necessary for a patient to be aware of the current situation and agree, either explicitly or implicitly, to the procedure. For standard 2 situations, the patient must understand the information and make a choice based on thorough cognitive and emotional evaluation of the options. Lastly, for standard 3, the competent patient must be able to take a reflective and critical view of the disease and available options, and make a logical, reasoned decision based on evaluation of its consequences and his or her personal beliefs.
Instruments for evaluating capacity or competencyWe will now focus on evaluating competency specifically. In the most common clinical situation, a doctor transmits information on a diagnostic or therapeutic procedure which implicitly entails potential risks as well as benefits. Table 3 shows the aspects of the procedure which the patient should understand. The same aspects, broken down step by step, also usually appear in the informed consent documents which we manage on a daily basis. The first aspect to be assessed is the patient's awareness of his or her disease. If the patient does not accept that he/she is ill, as frequently occurs in Alzheimer disease, the patient will probably be unable to evaluate the real risks of the procedure, or will underestimate those risks. It is important for the patient to be aware of and understand particularly serious or critical risks, and these should be explained using direct everyday language without too many suppositions. All other aspects listed in the table should be explained in the same way.22
As stated previously, neither the law nor other disciplines having to do with the study of competency have provided universally accepted tools for assessing competency. Competency criteria and the minimum degree of competence required to make each decision remain undefined. It was the subject of considerable research in the 1980s and early 1990s, particularly among legal experts, bioethicists and psychiatrists. This research resulted in an array of instruments for assessing competency. These tools first evaluate subjects’ ability or capacity to receive, understand and process information. In the next step, they measure the ability to make an appropriate, reasoned decision based on available information. Lastly, they consider a patient's ability to communicate the decision in an organised and comprehensible way.
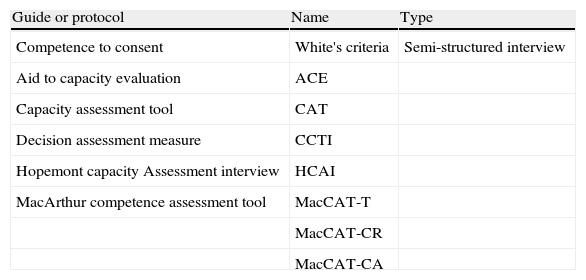
Table 4 provides a summary of the most systematic and widely-used scales and evaluation methods. The first of those methods is White's list of criteria for competence (Table 5),23 a good example of the systematic method to follow when assessing competency.
Guides for evaluating competence.
| Guide or protocol | Name | Type |
| Competence to consent | White's criteria | Semi-structured interview |
| Aid to capacity evaluation | ACE | |
| Capacity assessment tool | CAT | |
| Decision assessment measure | CCTI | |
| Hopemont capacity Assessment interview | HCAI | |
| MacArthur competence assessment tool | MacCAT-T | |
| MacCAT-CR | ||
| MacCAT-CA |
White's competence criteria.
| A. Informability |
| 1. Capacity to receive information. |
| 2. Capacity to recognise relevant information as information. |
| 3. Capacity to remember information. |
| B. Cognitive and affective capabilities |
| 1. Capacity to relate situations to oneself. |
| 2. Capacity to reason about alternatives. |
| 3. Capacity to rank alternatives. |
| C. Ability to choose |
| 1. Capacity to select an option. |
| 2. Capacity to resign oneself to the choice. |
| D. Critical review of the process |
| 1. Ability to recount one's decision-making process. |
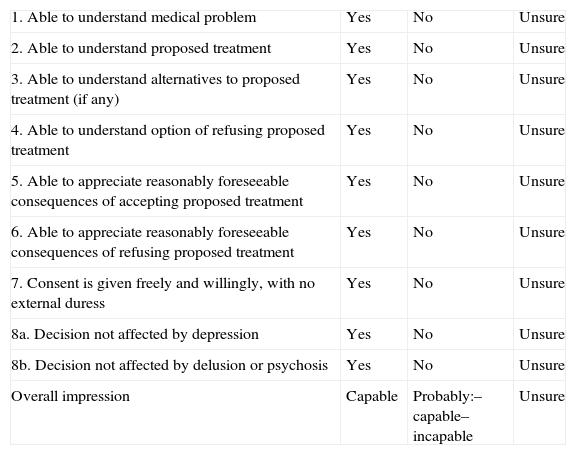
The ACE Guide (aid to capacity evaluation) prepared by the University of Toronto is a free-use, free-access resource.24 It lists a series of questions intended to evaluate aspects that are decisive when defining functional capacity (Table 6). It includes 8 groups of questions, which must be asked sequentially. Specific tips and guidance are offered for each section containing 3 or 4 questions. These questions may also be substituted by equivalents drawn up when the test is administered. Lastly, the doctor checks “yes” (the patient understands), “no” (the patient does not understand) or “unsure” after finishing each group of questions, rather than relying on intuition. The final score establishes whether the patient is definitely capable, probably capable, probably incapable or definitely incapable. The test has no cut-off points. Once again, we must recall that the seriousness of the decision at hand will determine the patient's score requirements. This guide is a good first assessment before proceeding to the final decision.
Aid to capacity evaluation (ACE).
| 1. Able to understand medical problem | Yes | No | Unsure |
| 2. Able to understand proposed treatment | Yes | No | Unsure |
| 3. Able to understand alternatives to proposed treatment (if any) | Yes | No | Unsure |
| 4. Able to understand option of refusing proposed treatment | Yes | No | Unsure |
| 5. Able to appreciate reasonably foreseeable consequences of accepting proposed treatment | Yes | No | Unsure |
| 6. Able to appreciate reasonably foreseeable consequences of refusing proposed treatment | Yes | No | Unsure |
| 7. Consent is given freely and willingly, with no external duress | Yes | No | Unsure |
| 8a. Decision not affected by depression | Yes | No | Unsure |
| 8b. Decision not affected by delusion or psychosis | Yes | No | Unsure |
| Overall impression | Capable | Probably:– capable– incapable | Unsure |
Another 5 guides, scales and instruments are listed in Table 4. They are cited by their acronyms CAT,25 CCTI,26 DAM,27 HCAI28 and MacCAT.29,30 Of these instruments, the MacArthur Competence Assessment Tool (MacCAT) is the one with the most proven validity.1,14–16,31 Three versions of the test are available: one is for considering treatment decisions (MacCAT-T), another screens clinical research subjects to determine their capacity for consent (MacCAT-CR), and a third is of interest for determining adjudicative competence in criminal cases (MacCAT-CA).
In daily clinical practice, an interview may be sufficient to determine a patient's functional capacity. For this to be true, its structure must take into account the basic steps in the responsible decision-making process: understanding the information, making the decision, and clearly communicating the selected option. Guidelines organise, facilitate and quantify this procedure, although the result of the procedure and the final decision will be based on clinical criteria.22,32
Before moving on to the next section, we should reiterate that all patients must be considered capable by default. If the patient's history or current clinical situation point to decreased capacity, the doctor must consider that possibility prudently, but transparently. The burden of proof and that of the added workload rest on the doctor who will decide if the patient is able to make the decision in question. Information from family members and close friends may be crucial. Opinions of colleagues, nurses, or bioethics committees may also contribute to the final decision to declare the patient capable or incapable. In any case, recourse to a legal evaluation should only be used when a patient may be legally incompetent, or when conflicts arise.
Dementia: key aspects related to capacityDementia is a disorder associated with the ageing population, and its prevalence is increasing. This is the most common disorder in which a patient's capacity may be questioned, particularly in the early and intermediate phases of the course of the disease corresponding to mild and moderate stages of dementia. Disease progression, which is intrinsic to the disorder, determines the healthcare needs and bioethical conflicts typical of each phase. With this in mind, determining patient capacities and writing advance directives are problems for the early stages, while support provided by the carer, devices to prevent wandering, and institutionalising patients are situations that come up in later stages of the disease.
A list of bioethical problems that may arise in dementia appears in Table 7.16,33 Number 4 on that list (consent and competency) is the one examined by our study. The others are not considered within the sphere of this project, although different problems may overlap.
Bioethical problems with dementia care.
| 1. Doctor–patient relationship |
| 2. Explaining the diagnosis |
| 3. Age as a limit on patient care |
| 4. Consent and competence |
| 5. The carer |
| 6. Residence admission and care |
| 7. Advance directives |
| 8. Preventing wandering |
| 9. End-of-life care |
| 10. Research on dementia |
As stated previously, capacity or functional competence is task-specific, and this may apply to any of the patient's life activities. In cases of dementia, the most common situations are decisions regarding treatments and diagnostic tests or other financial or contractual decisions. But many other life activities may be at stake as well; accepting or refusing institutionalisation, the ability to drive and the right to vote are all topics that have been addressed in the literature. With such a wide variety of activities being affected, we run the risk of being asked to assess competence in controversial situations that are not in keeping with our role as dementia experts. To cite an example, the voting ability of Alzheimer patients was called into question during the U.S. presidential election in 2000, in which George Bush's victory depended on the outcome in Florida. That state has a high percentage of elderly residents, meaning that the prevalence of dementia among Florida's voters is also likely to be high. This matter was debated in a number of highly visible journals34–36 that questioned the legality of the election and the practical applicability of screening tests designed by medical societies and expert panels. Were this debate to reach our country, our role would be superseded by the result of the debate, which is more social than scientific in nature. Presumably, we would then be viewed as advisers rather than as evaluators.
Advance directives are especially relevant in cases of dementia. Where present, they can facilitate care and decision-making in critical moments. The instructions they contain serve to orient the patient's end-of-life care. They may serve to prevent futile treatments or discontinue enteral feeding, if these approaches are listed. We should be aware that discontinuing feeding does not produce additional suffering. Furthermore, it is coherent with the patient's own decision, provided that treatments, including feeding and hydration by artificial means, are no longer effective.2,32,33 Patient participation in studies or clinical trials also should be mentioned. Specific protection must be extended to dementia patients. This means that guarantees must exist that the patient is both informed and capable. With the dual objective of meeting the above requirements on the one hand, and avoiding investigator manipulation and conflicts of interest on the other,37 research and clinical trial committees should make an effort to supervise studies of dementia patients. If the patients have advance directives stating their specific wishes in this regard, this is particularly useful.
Spanish law and healthcare administrations now encourage the preparation of advance directives. However, several unforeseen conflicts have arisen in recent years. Specifically, there have been cases in which the patient's initial wishes expressed in the advance directive are at odds with the wishes the patient is expressing at the current time. Using advanced dementia as an example, we find cases of patients who had previously expressed the wish to have their feeding tubes removed if they ever reached severe stages of dementia. However, once they do reach that situation, patients may countermand previous instructions and ask to be fed. In such cases, which orders are to be respected? Dworkin states that the initial instructions, written when the patient was still competent, are a better reflection of that person's identity, values, and convictions, and should therefore be followed.38 However, other authors maintain that identity and being are present even in obviously incompetent patients with severe cognitive decline, and their wishes in that stage should be respected.39 Our own opinion is that changing circumstances give rise to different requests that are appropriate and proportional to the patient's condition and time of life. The patient's decreased needs make the wishes expressed at this moment of life plausible and respectable. On a practical level, we recommend revising all advance directives periodically, a step which is not stressed enough. It is understandable that a young, healthy individual would be appalled by the perspective of devastating loss entailed by dementia, and would opt to dictate the end of his or her life upon reaching a severe stage of cognitive decline and dependency. At the same time, it is understandable that an elderly person with limited capacities would present radically different, less stringent requirements for his or her well-being and happiness. This individual's perspective on the same disease might then be more tolerant and relaxed in the end.
Results from studies of capacity in dementia (Alzheimer)The most widely-studied capacity in dementia patients is competence for making decisions regarding medications. This issue is highly relevant, since a wide array of studies have been devoted to developing treatments, especially for Alzheimer disease, for more than a decade. The appearance of new drugs is accompanied by risks which have not necessarily been foreseen. As an example, we could cite tacrine-induced liver damage, which led to recall of the drug, or even more severe cases of meningoencephalitis during the trial phase of immunisation with Abeta42 peptide.40 The patient must understand these risks, be able to relate them to his or her own condition, decide whether or not to undergo treatment, and explain that choice logically. Essentially, the patient must demonstrate his or her decision-making capacity for a situation entailing risk.
Drugs approved for use in Alzheimer have more precise and quantifiable risks, but they must still be explained. Different tools have been developed for evaluating this type of capacity. The MacCAT-T scale for treatment is the most widely-used example.29,30 With this aim in mind, a simple design study41 used that tool to evaluate 48 patients with mild or moderate Alzheimer and 102 carers as controls. Only 40% of the patients were competent. Lack of understanding of their own limitations and the disease itself—a frequent symptom in these patients—was linked to the presence of incapacity. The Mini-Mental State Examination (MMSE) had a limited discriminatory ability since it does not distinguish between patients scoring between 19 and 23. Patients with mild dementia fall into this precise interval, and decisions about their competence tend to be the most urgent.
The ability to detect incapacity has also been studied for other tools apart from the MMSE that measure cognitive decline. One study of 88 patients with mild or moderate dementia employed 11 neuropsychological tests (auditory, visuospatial, attention, memory, abstraction, language, and executive function tests). These tests were correlated with other tests specifically measuring capacity, including MacCAT-T, White's criteria and the HCAI (see Tables 4 and 5). They found good predictive ability (77.8) for the initial phase of competence, referring to simple comprehension of information. However, predictive ability was lower for more complicated phases of competence: self-reference (39.4%), reasoned decision-making (24.6%) and appropriate communication of the choice (10.2%). These results support the general hypothesis of there being a direct correlation between the degree of cognitive decline and capacity for treatment decision-making. Unfortunately, inter-individual variability is high and results have no direct clinical applicability. On the other hand, the same study shows that verbal evocation ability was the variable with the greatest correlation to competency, in all stages of competence.42 This only reflects the fact that most of the information used in the tests was verbal.
In stark contrast to the complexity of the study described above, analysis in another study was limited to detecting the most basic risks in treatment decisions for cases of dementia. To do so, it used a battery of 10 true/false questions. The cut-off point was set at 8 correct responses. Analysis of 250 patients with differing levels of dementia and 165 controls showed that 95% of patients with mild dementia were above the competence threshold, compared to only 67% of patients with moderate Alzheimer.43 This test battery has direct clinical value due to its simplicity and high predictive ability. There is no available data referring to its use in the Spanish-speaking world.
Complex qualitative analyses have also been published analysing capacity in Alzheimer patients with regard to different levels of competence. These have been established as legal standards of competence (LS). Ranging from LS1 to LS5, these levels range from simply understanding that treatment is being offered (LS1) to making a reasoned decision and explaining it appropriately and coherently (LS5). These legal standards have been researched in studies that blur the lines between neurology and legal medicine.44,45 The test presents two vignettes. The subject is asked to envision a case of cancer with two treatment alternatives, and a case of heart disease with two treatment options. The subject listens as information written in a simple style is read aloud. The purpose is to measure capacity for decision-making according to each of the 5 legal standards of competence. The vignettes are good simulations of a clinical situation in which a patient must make rational decisions regarding risk-filled diagnostic and treatment options. In the first study,44 these vignettes were described to 15 elderly control subjects and 29 Alzheimer patients (14 in mild stages and 15 in moderate stages). For less stringent standards, such as those measuring simple understanding of the choice and making the reasonable choice (LS1 and LS2), there were no differences between the control and Alzheimer groups. However, those in mild stages were already limited with regard to appreciating consequences of the choice (LS3). Subjects in moderate stages of Alzheimer showed a marked lack of competence upon trying to justify choices (LS4) or understand the significance of each option (LS5). These vignettes are comparable to clinical situations involving withdrawing treatment, do-not-resuscitate orders, and participation in clinical trials.
The level of cognitive decline is directly related to loss of competency. However, we have already mentioned that test batteries such as the MMSE provide little specific information. For that reason, experts have attempted to correlate lack of competency with other markers of cognitive decline. For example, the legal standards of competence have been compared to neuropsychological test batteries evaluating a range of different capacities: language, attention, memory, reasoning, visuospatial functions, and executive functions.45 The same authors conducted the study, presumably with the same 44 subjects used in the study described above44 since distribution was the same (control, 15; mild Alzheimer, 15; moderate Alzheimer, 14). They found that only those subjects with severe receptive aphasia or anomia had difficulty with the LS1 decision-making level (understanding that options exist). On the other hand, executive dysfunction predicted incompetence on the level of understanding consequences of a choice (LS3), while other frontal functions (semantic memory, conceptualisation, and verbal evocation) had to be preserved for the LS5 level of maximum competence.45 Executive dysfunction is therefore a key factor in determining competency.
In another study using qualitative methodology, doctors analysed the consequences of errors in LS decisions. They designed a test battery measuring 16 qualitative errors in the areas of language, executive dysfunction, affective dysfunction, and compensatory responses. Answers indicative of loss of task, non-response and loss of detachment were key predictors of executive dysfunction and declining competence.46
One simple competency model categorised lack of competency as either operational or general.47 Operational incompetence results from an alteration in a single cognitive sphere, usually in the area of language. General incompetence, on the other hand, would affect more than one cognitive domain or area among the seven listed here: attention, memory, language, orientation, perception, reasoning and emotion. Reasoning capacity, affective dysfunction, memory disorders and attention disorder, in that order, were the most commonly affected areas in the sample of 92 patients assessed over 18 months in one neurobehavioural unit. This study's methodology is imprecise considering the tests used to evaluate each cognitive sphere and the definitions of dysfunction in those spheres. Its strength lies in its clinical applicability and suitability for widespread use.
Lastly, before declaring a patient incompetent, we should analyse any possibility of his having preserved intellectual areas that may compensate for other defective areas.48 To this end, another new simple cognitive model is available that analyses attention, language, memory and frontal function (consciousness of the disease and decision-making ability). The evaluator should be aware of the area responsible for the patient's specific limitation or lack of competence and check if other preserved areas could compensate when making the decision in question. The most typical example is that of a patient with a language disorder who may try to compensate by means of gestures, drawings, etc.
Other types of dementiaThe above results are potentially applicable to other types of dementias, given that the cognitive decline determining incompetence depends on a lesion's location, not on its cause or type.
Dysexecutive syndromes are the distinguishing feature of frontal lobe lesions, and their presence in cognitive decline is a strong predictor of incapacity or incompetence, as stated previously. Executive functions comprise all capabilities permitting us to manage simple ideas and actions in order to transform them into complex activities, referring to our own goals and daily life activities. To achieve these ends, we require motivation or drive, emotional control, and planning and abstract thought. If any of these 3 components is altered, an executive dysfunction appears, with its own specific frontal lesion profile. Apathy and lack of initiative or drive appear with anterior cingulate and medial frontal cortex lesions; changes in personality, erratic behaviour and loss of emotional control with orbitofrontal lesions; and lastly, direct alterations in abstract thinking with dorsolateral prefrontal lesions. Translating this schema to the field of functional competence obliges us to restate the basic idea that being competent to make a decision means understanding information, weighing the pros and cons and making a final reasoned decision. If executive dysfunction is present, the patient is likely to lack initiative, or even insight or awareness of his or her disease and its limitations. In addition, it is likely that the patient will have lost planning and organisational capacity. This translates to rigid behaviours and extreme difficulty adapting to change or to unforeseen situations that disturb daily routines. Making decisions, whether related to healthcare and its risks, financial matters, or a simple change of residence, becomes a challenge, as the complex planning processes these decisions entail will probably exceed these patients’ abilities. This occurs in patients whose other cortex functions, such as language, visuospatial abilities and reasoning skills, remain intact. It creates a loss of autonomy. This may be a loss of independent decision-making capacity, based on simple conceptual and emotional understanding, or a loss of executive autonomy,49 which reflects the ability to act upon deliberately prepared plans.
Executive dysfunction, which is common in Alzheimer disease, is even more typical in other types of dementia. Parkinson disease is a typical example of functional incompetence resulting from executive dysfunction. This was demonstrated by a study of 20 patients with Parkinson and cognitive decline and 20 control subjects. Capacity was measured using CCTI (capacity to consent to treatment instrument; see Table 4) and segmented according to intervals LS1 to LS5. We found significant alterations at all levels, from legal standard LS1 to LS5. This finding was directly correlated to executive dysfunction and difficulties with abstract reasoning.50
We have not found specific literature on capacity in other common forms of dementia, such as dementia with Lewy bodies or vascular dementia. However, there are recently published articles on the topic of MCI.19,51 Since this diagnosis is very common, and up to 25% of all cases progress to dementia in the first 3 years, these patients are involved in a number of clinical trials. For this reason, there is considerable interest in verifying their competence for understanding healthcare-related risks and choosing treatment alternatives. One study has been published on this topic.51 It analyses medical decision-making ability in 60 patients diagnosed with MCI, compared to 31 patients with mild Alzheimer and 56 control subjects. The study also makes use of the CCTI and legal standards. Findings were that the group of MCI patients had better results than those with mild Alzheimer for all legal standards. However, for LS3 (risk comprehension), LS4 (reasoned assessment of risks) and LS5 (making a reasoned choice), the MCI patient group's results were significantly poorer than those of the control group. Participation of these patients in clinical trials should therefore be considered and evaluated in a framework that considers the possibility of their being incapable of making treatment decisions entailing risk.
Financial capacity is a basic skill in daily life, which makes it an important research topic in studies on capacity and dementia. One study19 analysed financial capacity over 1 year in 66 control subjects, 25 patients with amnesic MCI that progressed to dementia in the follow-up period, and 62 cases of non-progressive MCI. The Financial Capacity Instrument is a tool that evaluates different financial activities ranked in order of increasing complexity, including basic monetary skills, bank statement management and paying bills. The study found that the progressive MCI group scored significantly lower from the start of the study. The loss of financial capacity was more evident for more complex activities (managing bank statements), and it was fundamentally caused by procedural or arithmetic errors rather than conceptual limitations. As a result, MCI patients may show decreased capacity for financial matters. In addition, if this symptom is present, it is an indicator of unfavourable disease progression.
ConclusionsCompetency in dementia is a hot topic with implications for daily clinical practice. Deciding that a patient is competent or capable involves making decisions, which may be critical, regarding multiple facets of that patient's daily life. Neurologists have made important contributions to this research, and the Spanish medical community in particular has produced a valuable multidisciplinary informative guide (Documento de Sitges, 2009).52 Unfortunately, neurologists are not listed by the international literature as specialists of reference or expert advisers in this field. We are convinced that this should change. To this end, we must raise awareness throughout our specialist community, and follow up on that step by updating and distributing the available tools. Only in this way will we join the ranks of the psychiatrists, clinical psychologists and legal doctors who evaluate capacity. This will be true both in clinical practice, in which we are often not called upon to evaluate competency, and in the field of research, in which many discoveries must still be made.
The present article touches on all of these topics. It presents basic concepts such as the link between competency/capacity and bioethics through the principle of autonomy, and the direct relationship between level of competence required and the seriousness of the decision to be made. The article describes several easily accessible tools used in this area. In conclusion, it describes basic research on Alzheimer disease and other forms of dementia, mentioning critical cortex functions and useful methodological constructs such as the legal standards or LS.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Álvaro LC. Competencia: conceptos generales y aplicación en la demencia. Neurología. 2012;27:290–300.