Neurocutaneous melanosis (NCM) is an infrequent non-hereditary congenital syndrome characterised by the presence of giant congenital nevi or multiple satellite nevi and both benign and malignant melanocytic tumours of the leptomeninges (Table 1). It is believed to be caused by phacomatosis, probably secondary to the abnormal development of melanin-containing cells derived from the neural crest.2 The most frequent age of onset is 2 years; the syndrome is extremely infrequent in adults.3 We present a case of NCM with onset at 19 years. This work analyses the diagnostic and therapeutic approach used.
Diagnostic criteria.
| 1. Presence of a giant congenital nevus (>20cm diameter in adults) or multiple (3 or more) congenital nevi associated with meningeal melanosis or melanoma |
| 2. No evidence of cutaneous melanoma except in those patients with histologically benign meningeal lesions |
| 3. No evidence of meningeal melanoma except in those patients with histologically benign cutaneous lesions |
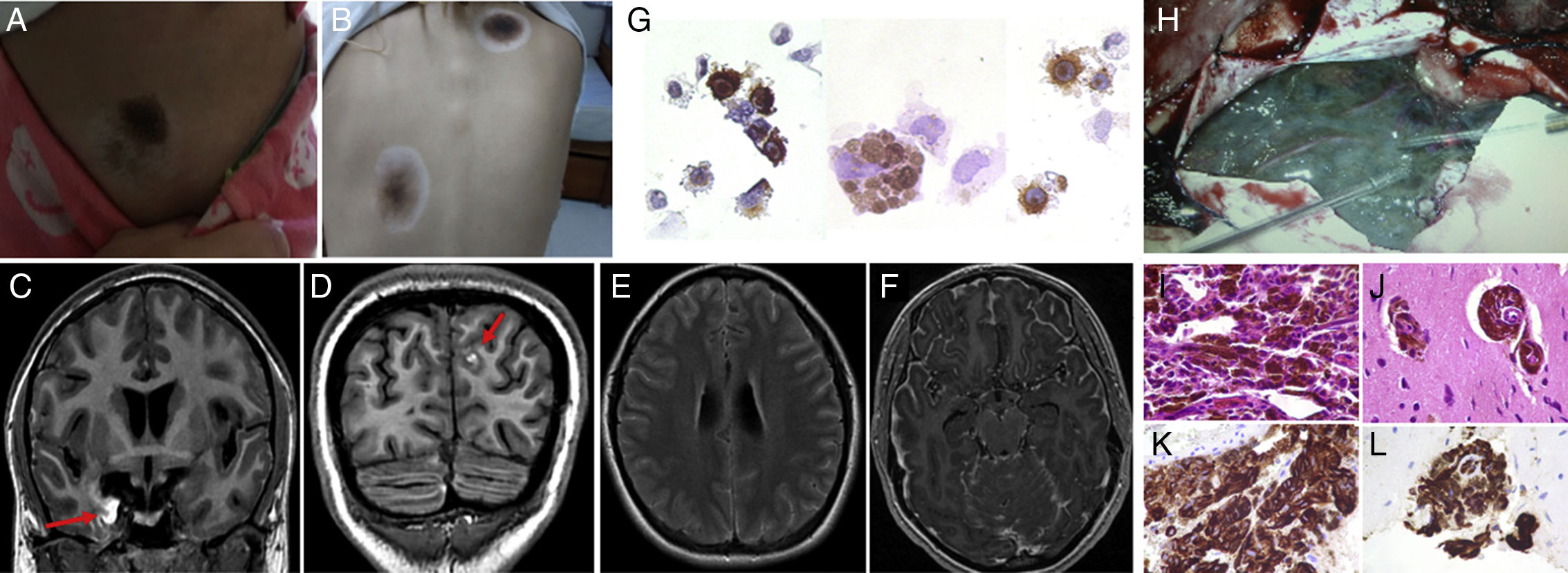
Our patient was a 20-year-old woman with a history of vitiligo and multiple congenital nevi, who consulted due to headache, vomiting, photophobia, blurred vision, diplopia, and secondarily generalised seizures. Eighteen months before, she had presented uncinate seizures, monitored by another hospital without diagnosis or treatment. Physical examination at admission showed bilateral papilloedema, paralysis of the left sixth cranial nerve, generalised hyperreflexia, Hoffman syndrome, ankle clonus, bilateral Babinski sign, neck stiffness, and Kernig and Brudzinski signs. Furthermore, a skin examination revealed acral nevi on all 4 limbs and 3 halo nevi in the right lumbar region and trunk (Fig. 1A and B). Due to the intracranial hypertension syndrome associated with the reported meningeal syndrome, a brain MRI scan was requested to rule out a structural lesion with risk of brain herniation; the scan revealed diffuse leptomeningeal thickening, predominantly involving the right temporal lobe, with hyperintensities on T1-weighted images (Fig. 1C-F). Given the extensive meningeal involvement and despite the absence of fever, a lumbar puncture was performed to rule out infectious, inflammatory, and oncological aetiologies; results yielded turbid cerebrospinal fluid (CSF) with an opening pressure of 52cm H2O, low white blood cell count (14cells/mm3; 60% mononuclear), glucose level of 95mg/dL (blood glucose level of 90mg/dL), high protein levels (1415mg/dL), and lactic acid of 1.3mmol/L. A CSF cytology study revealed atypical melanin-containing cells which were positive for HMB-45 (Fig. 1G). Microbiological analysis yielded negative results. Therefore, we requested a full-body CT scan with double contrast which revealed no involvement outside the central nervous system (CNS). In view of the skin and CNS findings and the determination of melanin-containing cells in the CSF, we diagnosed NCM. In order to rule out malignant transformation in the CNS and to enable genetic analysis of melanin-containing cells, which may lead to a targeted oncological treatment, we requested an open meningeal biopsy (Fig. 1H). The meningeal anatomical pathology study (Fig. 1I-L) showed extensive melanocytic infiltration, Ki67 protein at 8%, and negative genetic screening for the BRAFv600E mutation. Given the absence of BRAFv600E, we started treatment with temozolomide and dexamethasone to treat cerebral oedema; the patient progressed poorly and died due to status epilepticus after one month of treatment. Overall survival since symptom onset was 20 months.
(A and B) Nevus lesions surrounded by depigmentation (halo nevi). (C-F) Brain MRI: right temporomesial and left frontal hyperintense lesions on T1-weighted sequences (red arrows) (C and D), with diffuse subarachnoid hyperintensity on FLAIR sequences (E) and leptomeningeal enhancement with gadolinium (F). (G) CSF cytology study: HMB-45-positive melanin-containing cells. (H) Open meningeal biopsy: dark leptomeninges adhered to the brain parenchyma; minimal touch caused bleeding. (I-L) Anatomical pathology study: atypical melanin-containing cells stained with haematoxylin–eosin (I-J) and HMB-45-positive melanin-containing cells (K and L).
The most frequent initial manifestations of NCM are those caused by intracranial hypertension, probably secondary to non-communicating or communicating hydrocephalus (due to aqueductal obstruction or altered CSF reabsorption, respectively), as a result of melanocyte infiltration. Another frequent manifestation is epileptic seizures.1,4 The CSF study generally shows increased opening pressure, pleocytosis, marked high protein levels, variable glucose level, and even hyperglycorrhachia.5,6 The CSF cytology study may reveal melanin-containing cells positive for HMB-45, although results may be negative on occasion.5,7 Brain or spinal MRI scans generally show extensive leptomeningeal infiltration, which prompts us to perform differential diagnosis for different pathologies: oncological (meningeal carcinomatosis secondary to solid tumours, meningeal lymphomatosis, and primitive neuroectodermal tumours), inflammatory (sarcoidosis, systemic lupus erythematosus, rheumatoid arthritis, Sjögren syndrome, and Vogt–Koyanagi–Harada syndrome), and infectious conditions (tuberculosis, schistosomiasis, and systemic mycosis).8 However, the characteristic paramagnetic behaviour on MRI of melanin, which is hyperintense on T1-weighted sequences, suggests a diagnosis of NCM, which is assessed in the clinical context.4–9 Malignant transformation to cutaneous or leptomeningeal melanoma occurs in 40%-60% of cases.1,10,11 From a clinical viewpoint, it is sometimes very difficult to determine the moment of transformation; MRI findings of nodular or thick plaque-like contrast enhancement, perilesional oedema, growth in subsequent follow-up, and haemorrhage suggest malignant transformation,11 although none of these findings is definitive. No standardised treatments are currently available. The existing treatments are based on radiotherapy, chemotherapy, and CSF shunt for hydrocephalus; these have achieved limited success, with survival times of less than 3 years from onset of neurological symptoms.1,3,4 Although the MRI findings mentioned above (some of which were present in our patient) may suggest malignant transformation to melanoma, it is essential to confirm said transformation by leptomeningeal biopsy, since transformation may affect prognosis and modify the treatment approach to be used.1,10 Furthermore, advances in molecular studies enable us to more accurately classify patients with transformation to melanoma by identifying different genetic mutations, such as BRAFv600E.12 Since the approval of targeted therapy with BRAFv600E inhibitors (vemurafenib and dabrafenib) for cases of metastatic melanoma, screening for this mutation should be performed in all candidates for systemic therapy,13 since it improves overall survival time with no progression as compared with other drugs.14 Although there is no evidence of its efficacy in cases of NCM, patients with extensive or unresectable CNS tumour may be candidates for this therapy. Another aspect to be discussed is the selection of the site of biopsy to confirm diagnosis, determine whether there is transformation to melanoma, and perform genetic typification: biopsy of the skin lesions may be considered, as they are more accessible than leptomeningeal lesions. However, halo nevi often test negative due to their pronounced cytotoxic reaction to melanocytes. Furthermore, prognosis is dictated by malignant transformation involving the CNS; therefore, demonstrating this transformation together with the genetic analysis (BRAFv600E), which on occasion may be different to that of the skin, determines the oncological approach. In our case, screening for the BRAFv600E mutation returned negative results, and therefore treatment with temozolomide as an oral alternative was started. Due to the presence of intracranial hypertension syndrome, we considered a ventricular shunt as a palliative measure. Considering the possibility of remote metastasis of these tumours via the shunt,15 and given the significant inflammatory characteristics, with markedly increased CSF protein levels, possibly entailing a CSF flow obstruction, we decided to prioritise treatment with dexamethasone. Despite this treatment, the patient progressed unfavourably and finally died, with a total survival time of 20 months.
In conclusion, NCM should be suspected not only in patients in the first or second decade of life, but also in young adults. In addition to neurological signs (mainly intracranial hypertension and epileptic seizures), skin examination is essential for diagnosis. Extensive leptomeningeal involvement on MRI with hyperintense regions on T1-weighted images is highly suggestive of NCM. Biopsy and anatomical pathology study of meningeal tissue is of both diagnostic and potentially therapeutic value, since it may be used to identify genetic targets in order to establish a more appropriate treatment; this may improve the currently poor prognosis.
Conflicts of interestThe authors have no conflicts of interest to declare.
Dr. Naomi Arakaki for the anatomical pathology images. Anatomical Pathology department, Raúl Carrea Neurological Research Institute (FLENI), Buenos Aires, Argentina.
Dr. Ernesto Castellani for the meningeal biopsy images. Neurosurgery department, Raúl Carrea Neurological Research Institute (FLENI), Buenos Aires, Argentina.
Please cite this article as: Alessandro L, Blaquier JB, Bártoli J, Diez B. Melanosis neurocutánea en paciente adulto joven: abordaje diagnóstico y terapéutico. Neurología. 2019;34:336–338.