Oestrogen deficiency produces oxidative stress (OS) and changes in hippocampal neurons and also reduces the density of dendritic spines (DS). These alterations affect the plastic response of the hippocampus. Oestrogen replacement therapy reverses these effects, but it remains to be seen whether the same changes are produced by tibolone (TB). The aim of this study was to test the neuroprotective effects of long-term oral TB treatment and its ability to reverse DS pruning in pyramidal neurons (PN) of hippocampal area CA1.
MethodsYoung Sprague-Dawley rats were distributed in 3 groups: a control group in proestrus (Pro) and two ovariectomised groups (Ovx), of which one was provided with a daily TB dose (1mg/kg), OvxTB and the other with vehicle (OvxV), for 40 days in both cases. We analysed lipid peroxidation and DS density in 3 segments of apical dendrites from PNs in hippocampal area CA1.
ResultsTB did not reduce lipid peroxidation but it did reverse the spine pruning in CA1 pyramidal neurons of the hippocampus which had been caused by ovariectomy.
ConclusionsOestrogen replacement therapy for ovariectomy-induced oestrogen deficiency has a protective effect on synaptic plasticity in the hippocampus.
El hipoestrogenismo produce estrés oxidativo (EO) y cambios en las neuronas del hipocampo (H) y reduce la densidad de las espinas dendríticas (ED). Estas alteraciones repercuten en la respuesta plástica del H. La terapia de sustitución intraperitoneal con estrógenos revierte estos efectos, pero no se sabe si ocurre lo mismo con la tibolona (TB). El objetivo fue comprobar los efectos neuroprotectivos de la TB administrada por vía oral a largo plazo y su capacidad para revertir la poda de ED de las neuronas piramidales (NP) del CA1 del H.
MétodosRatas Sprague-Dawley jóvenes: distribuidas en 3 grupos: control en proestro (Pro) y 2 grupos ovariectomizados (Ovx), uno suplementado con dosis diaria de TB (1mg/kg), OvxTB, y otro con vehículo (OvxV), por 40 días. Se analizaron la peroxidación de lípidos y la densidad de las ED en 3 segmentos de la dendrita apical de las NP del CA1 del H.
ResultadosLa TB no redujo la peroxidación de lípidos en el H, pero recuperó la poda de espinas en las NP del CA1 del H, producida por la ovariectomía.
ConclusionesLa terapia de sustitución estrogénica en el hipoestrogenismo por ovariectomía tiene un efecto protector.
Increased levels of free radicals, mitochondrial dysfunction, and oxidative stress (OS) are interdependent mechanisms that have been proposed as the main factors contributing to brain damage. They promote chain reactions in the presence of catalytic metal ions, a process that impairs membrane phospholipids, proteins, and most importantly, DNA within cells.1–3 OS, inflammation, innate immune function, and neuronal-glial communication increase with age. These processes are crucial not only in brain ageing but also in neurodegenerative diseases such as Alzheimer disease.4,5 Ageing in humans and other primates leads to morphological changes in both hippocampal and cortical neurons, manifesting as reductions in dendritic tree complexity, dendritic length, postsynaptic sites, and dendritic spines (DS).6 DS are morphologically complex specialisations for synaptic interaction. In vitro studies have found that number and shape of spines are highly mutable on time scales ranging from seconds to days due to a number of intrinsic mechanisms that exert dynamic control over spines.7 In situ, these changes in shape and density of DS occur under both physiological and pathological conditions that have been linked to memory loss and functional changes in neuronal circuits.8,9 In neurons, DS make up the postsynaptic area that receives excitatory input, and they react to stimulation by changing their density and morphology. Those changes occur with the activation of primary genes combined with the effect of growth factors; both processes modify the membrane cytoarchitecture, resulting in either generation of new spines or maturation of existing spines.10
In women, several situations may contribute to changes in DS, including ageing, memory loss, redox homeostasis, and decreased circulating levels of estradiol. Numerous replacement therapies have been used to mitigate alterations related to decreased circulating levels of hormones during menopause. These include replacement therapies with oestrogenic compounds (phytoestrogens, 17-β-estradiol, progestogens, medroxyprogesterone acetate)11,12 and oestrogenic derivatives such as tibolone (TB), the precursor of hydroxylated metabolites. In vivo, hydroxylated metabolites act as oestrogens in the liver and endometrium. They may also function as progestogens and exert an androgenic effect (which lowers cholesterol and triglyceride levels), and they protect against bone loss.13 In 2008, De Aguiar et al.14 described the effects of estradiol valerate (EV; 0.3mg/kg) and 2 concentrations of TB (0.5 and 1.0mg/kg) on brain OS and blood biochemistry in juvenile, adult, and senile ovariectomised female rats. The authors found decreased lipid hydroperoxide levels in the brain cortex of juvenile and senile rats, and both TB concentrations increased total antioxidant capacity compared to that in adult female rats treated with EV. For all treatments, ovariectomised rats showed lower levels of total antioxidant capacity in the hippocampus than controls. OS was higher in senile rats than in juveniles or ovariectomised rats treated with TB (1.0mg/kg). The latter group showed higher LDL levels than the control and EV treatment groups. The purpose of our study is to evaluate the antioxidant and neuroprotective effects contributing to the pharmacological activity of long-term oral TB treatment, and to determine whether this treatment can revert OS and DS pruning in pyramidal neurons in hippocampal area CA1.
MethodsAnimalsThe study protocol follows international standards for the handling and use of experimental animals as established by National Institutes of Health (NIH) and the National Academy of Science. It was presented to and approved by the Bioethics Committee of the Institute of Neurobiology (INB) at Universidad Nacional Autónoma, Mexico. A total of 30 female Sprague-Dawley rats (weight range, 200-300g) were used in the study. Rats were housed individually in polycarbonate cages (45×24×21cm) containing sterile sawdust bedding. Standard rat chow (Purina® Rodent Lab Chow 5001) and purified water were available ad libitum throughout the experiment. The animals were kept at a temperature of 22°C to 23°C, 40% to 50% humidity, with a 12:12 light/darkness cycle and lights on at 7.00h.
Study design and sample preparationWe conducted an experimental randomised double-blind placebo-controlled study with a total of 30 female Sprague-Dawley rats weighing 200 to 250g. They were divided in 3 groups: group 1 included control rats in the proestrus phase (Pro); group 2 consisted of ovariectomised (Ovx) rats treated with vehicle (saline solution) every 24hours for 40 days (OvxV); and group 3 included Ovx rats treated with TB at a dose of 1mg/kg every 24hours for 40 days (OvxTB). After 40 days, once rats had completed their treatment, all 30 rats were killed by decapitation. Their brains were collected, placed into a metal brain matrix, and the 2 hemispheres were separated and randomly allocated to biochemical or morphometric analysis.
Quantification of lipid peroxidationWe used the method described by Triggs and Willmore15 to quantify end products of lipid peroxidation (LP). The hippocampus was removed from one of the hemispheres of each brain and homogenised in saline solution (3mL, 0.9% NaCl). An aliquot of 1mL was taken and mixed with 4mL of chloroform/methanol (2:1, v/v). Samples were incubated on ice for 30minutes to allow chloroform phase separation; this phase contains fluorescent end products of LP. An aliquot of 1mL was subsequently taken and read with a luminescence spectrophotometer (Perkin-Elmer 5500) at 370nm excitation and 430nm emission wavelengths. Spectrophotometer sensitivity was set at 330 fluorescence units with a standard solution of quinine (0.1μg/mL). Results were expressed in fluorescence units/g fresh tissue.
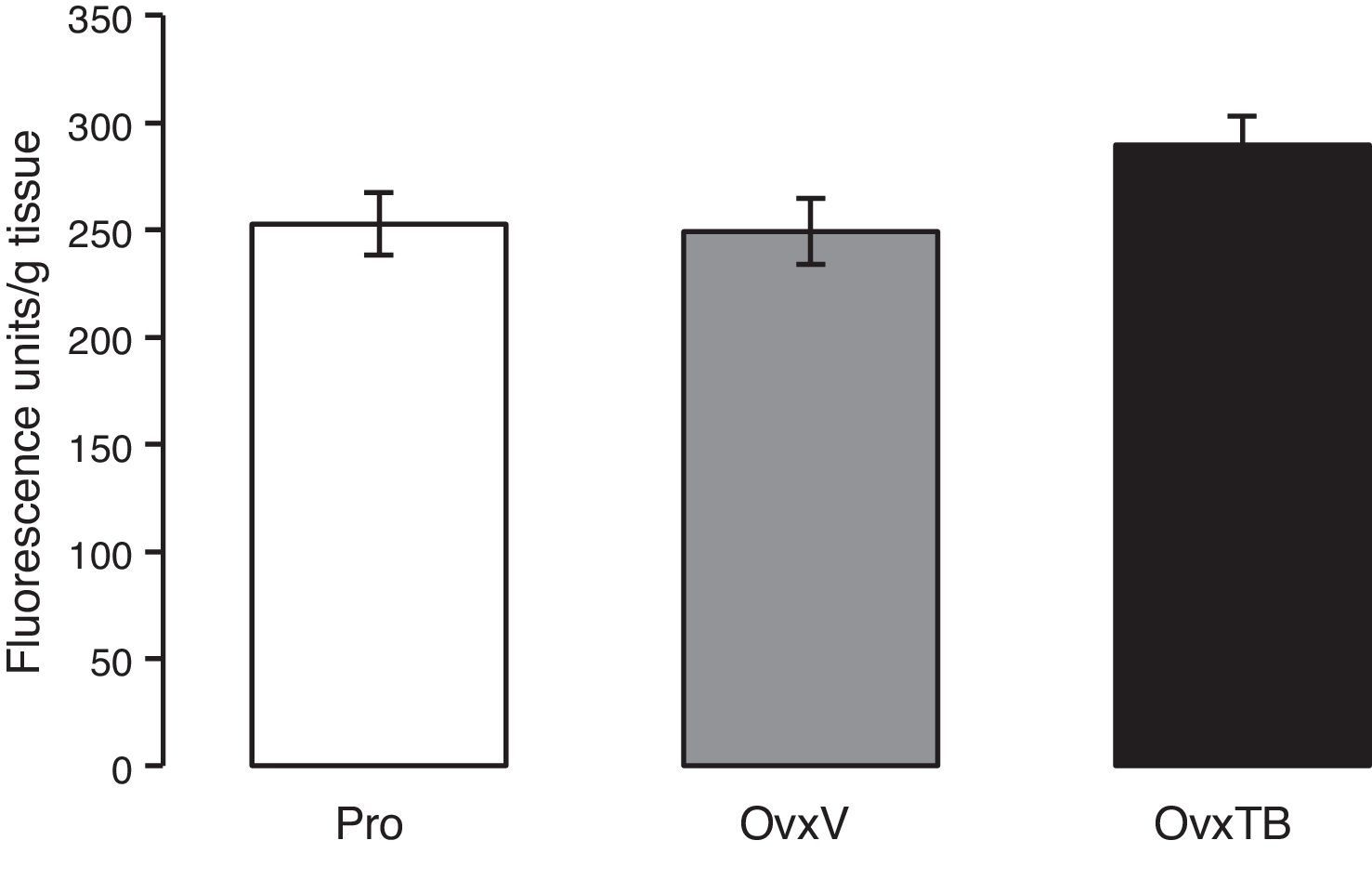
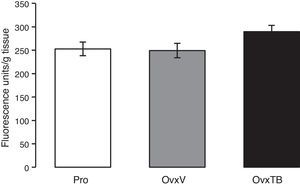
Graph representing mean values±standard value for each group (n=10 rats per group). Results are expressed in fluorescence units/g of fresh tissue. OvxV: ovariectomised rats treated with vehicle; OvxTB, ovariectomised rats treated with tibolone (1mg/kg); Pro: rats in the proestrus phase.
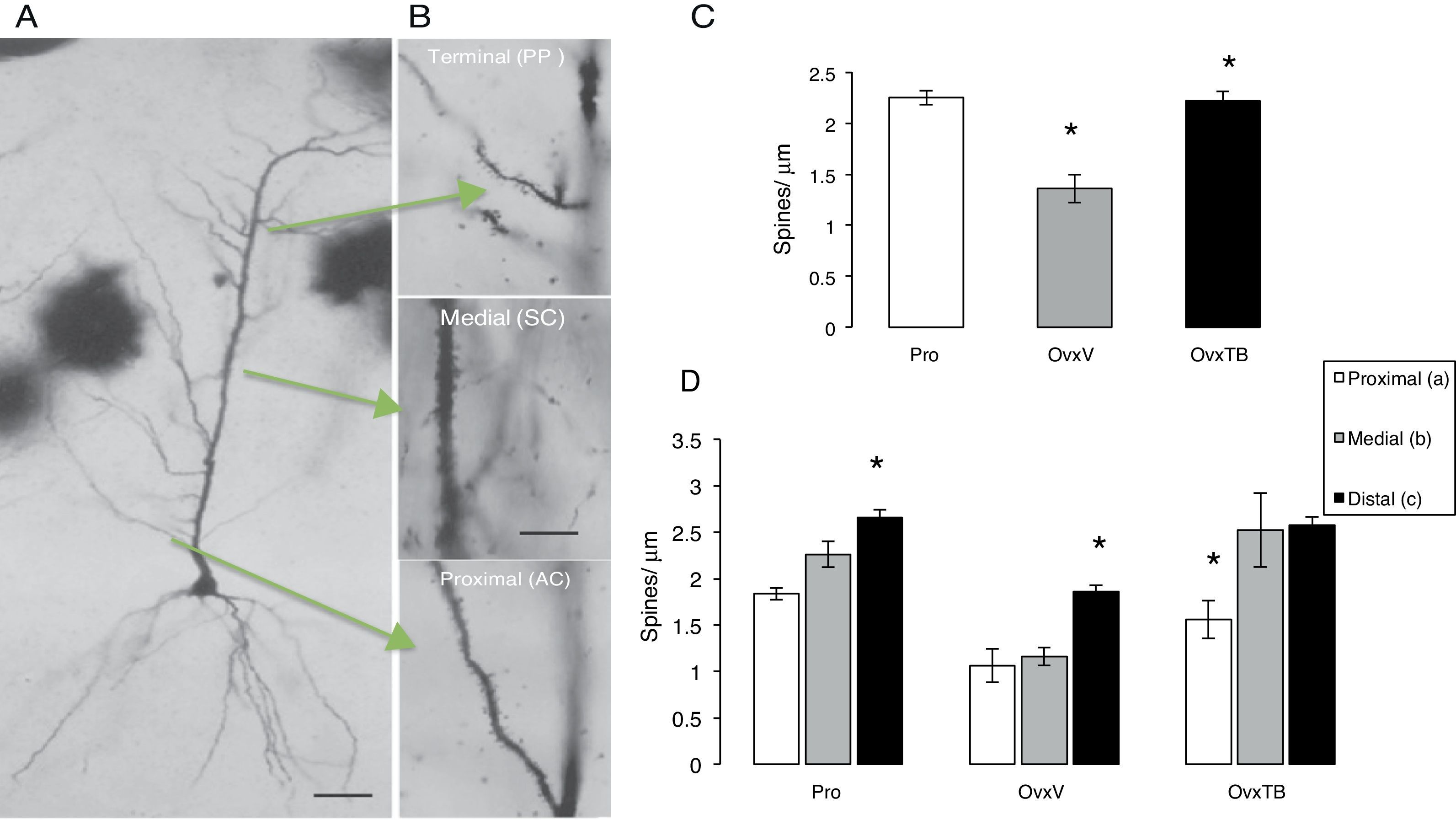
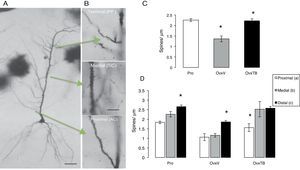
Six hemispheres were processed using the rapid Golgi technique16 and immersion-fixed in 10% formalin in phosphate-buffered saline; 72hours later, we cut rostral blocks 4mm wide including the dorsal hippocampus (2.8-4.2 posterior to bregma), which were fixed in a 4.5% potassium dichromate solution with 1% osmic acid (8:1) for 13 days. Blocks were treated in 0.75% silver nitrate for 24hours, and then dehydrated for 30minutes in alcohol solutions of increasing concentrations (from 50% alcohol to absolute ethyl alcohol and ether). Blocks were embedded in low-viscosity nitrocellulose, and coronal sections of 120μm were subsequently obtained using a sliding microtome (Leitz Wetzlar, model 47160). Sections were dehydrated in graded alcohol solutions, cleared in terpineol and subsequently in xylene, and mounted in Entellan. After completing this process for each experimental subject, we selected the pyramidal neurons (PN) in hippocampal area CA1 showing a complete dendritic tree, the pyramidal soma, and basal dendrites (Fig. 2A). A random number was assigned to each slide so that researchers could not identify the study subgroups to which they belonged. We measured the PNs that could be observed in a focal plane permitting a clear view at 10× magnification. PNs had to be homogeneously impregnated and their apical dendrites had to be complete up to their terminal branches in the stratum lacunosum-moleculare (SLM). PNs in the CA1 typically have 3 to 5 primary basal dendrites and an apical dendritic tree ascending up to the SLM and secondary ramifications in the stratum radiatum. Dendrites were divided in 3 segments representing the main afferences (Fig. 2B): a proximal segment measuring 100μm (commissural and main areas of inhibitory input), a medial segment starting at 150μm from the soma and continuing to 350μm (Schaffer-commissural), and a distal segment at the beginning of the dendrite curvature on the SLM (afferent perforant path).17 We counted the number of spines on each dendrite in 10μm segments with a 100× magnification.
(A) Photomicrographs of a complete pyramidal neuron from hippocampal area CA1. (B) Arrows indicate the examined segments and the abbreviations for information input. Dendritic segments are shown at 40× magnification. (C and D) Mean±standard error of the total number of spines per μm in each group: rats in proestrus phase (Pro), ovariectomised rats treated with vehicle (OvxV), and ovariectomised rats treated with tibolone (OvxTB). Graph C shows a significantly lower number of spines in the OvxV group than in the OvxTB and Pro groups (*P<.005). Graph D shows the 3 segments broken down by group; statistically significant differences (*) in the number of dendritic spines can be observed between segment c and segments a and b in the Pro and OvxV groups, and between segment a and segments b and c in the OvxTB group. AC: associational commissural; PP: perforant path; SC: Schaffer collateral.
All values were analysed using the Levene test in order to assess variance homogeneity as a precondition for parametric statistical tests. We subsequently performed a statistical analysis of the results of LP using a one-way analysis of variance test (ANOVA) and a post hoc Tukey test. Values were considered statistically significant for P≤.05. To analyse the total number of spines in the different subgroups, we used the one-way ANOVA method in which the independent variable was the treatment and the dependent variable was the mean total number of spines on each of the examined segments. This step was followed by a post hoc Tukey test (P≤.05).
ResultsLipid peroxidationFig. 1 shows the results of the effects of TB on LP levels in the hippocampi of Ovx rats. Results are given as mean value±standard error of each group, and are expressed in fluorescence units/g fresh tissue. We observe that differences in mean values between the 3 groups (Pro, OvxV, OvxTB) are not statistically significant 40 days after treatment administration. One-way ANOVA yielded: F2,25=2.429 (P=.107).
Effects on total dendritic spine densityOne-way ANOVA for total spine density, which included all segments (Fig. 2C), showed statistically significant differences between treatment groups (F2,9=2323.306; P<.003). According to the post hoc Fisher exact test, the OvxV group showed a lower dendritic spine density than the OvxTB and control groups in the proestrus phase (P=.003 and P=.002, respectively).
Effects on dendritic spine densityTwo-way ANOVA of dendritic spine density in each segment revealed statistically significant differences between treatment groups (F2,27=22946; P<.0001). Fig. 2D shows statistically significant differences between each segment (F2,27=17.39; P<.001); however, intergroup differences were not significant. The post hoc Fisher exact test showed increased spine density in proximal, medial, and distal segments in the Pro group, while OvxTB rats displayed significant differences in spine density between the proximal and distal segments. Furthermore, significant differences in dendritic spine density between the distal and proximal or medial segments were observed in Ovx rats (P<.02). We could therefore argue that the distal segment is either not affected by treatment with oestrogenic derivatives, or that it is more resistant to hormonal changes.
DiscussionOur results show that treatment with TB had no effect on LP in any of the 3 study groups and support those reported by de Aguilar et al.14 Those authors administered 2 doses (0.5mg/kg and 1mg/kg) of TB to Ovx adult rats for 12 weeks and found no effect on LP levels, and therefore concluded that TB has no impact on OS secondary to ovariectomy. In our study, however, the dose of TB (1mg/kg) administered to the Ovx group for 40 days reverted DS pruning secondary to ovariectomy. A histological study of the proximal, medial, and distal segments of pyramidal neurons from hippocampal area CA1 revealed a lower number of DS in Ovx rats, which suggests that DS as postsynaptic sites are highly sensitive to lack of oestrogens. Previous studies conducted by several research groups, including our own,18–20 have shown that the distribution of DS is heterogeneous and depends on the segment in question. We know that the distal segment displays a greater number of spines than the proximal segment; this tendency was also observed in the present study. Furthermore, the Ovx group was shown to have fewer DS overall, although they preserved the normal pattern of distribution: a greater reduction in the numbers of spines in the distal segment (the segment typically showing the highest numbers) and a less marked reduction in the proximal segment (normally featuring fewer spines). In addition, oral supplementation with TB reversed spine pruning in this highly plastic brain area that regulates learning and memory. The mechanism underlying this effect is not understood; this is in fact the first study describing how TB can inhibit spine pruning in the pyramidal neurons of hippocampal area CA1. The effect of TB has only been addressed by Espinosa-Raya et al.,21 who show that treatment with TB improved learning in ovariectomised rats. According to these authors, the mechanism may be linked to modulation of the cholinergic and serotonergic systems, since they observed an increase in tryptophan hydroxylase content after treatment with TB. With this in mind, TB is likely to exert a neuroprotective effect due to both the regulation of synthesis of neurotransmitters involved in memory and learning, and by means of signalling pathways linked to DS.
In humans, on the other hand, treatment with synthetic oestrogens is believed to modify several physiological activities involving different organs (brain, bones, etc.). However, the benefits of these compounds (for example, TB) are debatable due to their many secondary effects10 after menopause; the potential association between steroid levels in brain tissues and brain functions; and the distribution of mono- and trisulphated metabolites.22
Although treatment with TB did not lead to an antioxidant response in our study, we did observe reversed dendritic spine pruning secondary to ovarectomy along the apical dendrite of pyramidal neurons of CA1. Hippocampal plasticity has been addressed in studies employing exogenous oestrogen treatment and concluding that plastic changes are fluctuations inherent to changes in plasma hormone concentrations throughout the oestrous cycle.18,19 It is interesting that ovariectomy affects all 3 segments (proximal, medial, and distal), whereas treatment with TB has a differential effect on the proximal segment. At this location, dendritic spine density fails to return to normal levels after treatment with synthetic TB over 40 days. These findings may help us understand the specific neuroprotective mechanisms of TB that favour brain plasticity and the links between the proximal segment of dendrites and the oestrogen receptors expressed in that segment. The proximal segment forms the commissural pathway receiving input from the Schaffer collateral pathway and affects the circuit of the hippocampus, which transforms short-term memory information into long-term memory information.
FundingThis study was partially funded by CONACYT (grants nos. 173299 and 17276), Biomedical Sciences Doctoral Programme (PDCB), DGPA-UNAM, and PROMEPUGTO-PTC-324. It was also partially funded by PROMEP (programme for continuing professor education) at Universidad de Guanajuato, Mexico.
Conflicts of interestThe authors have no conflicts of interest to declare.
We wish to thank A. Aguilar Vázquez, M. García Servín, and L. López Villanueva for their help in caring for the animals.
Please cite this article as: Beltrán-Campos V, Díaz-Ruiz A, Padilla-Gómez E, Aguilar Zavala H, Ríos C, Díaz Cintra S. Efecto de la tibolona en la densidad de las espinas dendríticas en el hipocampo de la rata. Neurología. 2015;30:401–406.