Spontaneous spinal epidural haematoma (SSEH) has an estimated incidence of one per million population. It is classified as spontaneous when no identifiable cause can be linked to its onset.
ObjectiveTo describe a sample of patients with SSEH and analyse variables related to its functional prognosis.
Patients and methodsRetrospective study carried out in patients diagnosed with SSEH between 2001 and 2013 in our hospital.
ResultsWe included 13 subjects (7 men) with a mean age of 71 years. Of the total, 62% had hypertension and 54% were treated with oral anticoagulants; of the latter, 57% had an International Normalised Ratio above 3. The most frequent manifestation was spinal column pain (85%). Nearly all subjects presented an associated neurological deficit, whether sensory-motor (70%), pure motor (15%), or pure sensory (7%). Five patients underwent surgical treatment and 8 had conservative treatment. After one year, 3 of the patients treated surgically and 4 of those on conservative treatment had a score of 2 or lower on the modified Rankin Scale. Poorer prognosis was observed in patients with anticoagulant therapy, large haematomas, location in the lumbar region, and more pronounced motor disability at onset.
ConclusionsOld age, hypertension, and anticoagulant therapy are the main risk factors for SSEH. The typical presentation consists of back pain with subsequent motor deficit. In patients with established motor symptoms, surgical treatment within the first 24hours seems to be the best option.
El hematoma espinal epidural espontáneo (HEEE) tiene una incidencia estimada de un caso por millón de habitantes al año. Se considera espontáneo cuando no se logra relacionar ninguna causa de forma directa con su aparición.
ObjetivoDescribir una muestra de pacientes con HEEE y analizar las variables relacionadas con el pronóstico funcional del mismo.
Pacientes y métodosEstudio retrospectivo de pacientes diagnosticados de HEEE en nuestro centro entre 2001 y 2013.
ResultadosTrece pacientes, 7 varones, con edad media de 71 años. El 62% presentaba hipertensión arterial (HTA) y el 54% utilizaba anticoagulantes orales, teniendo una razón normalizada internacional >3 el 57% de ellos. La presentación clínica más frecuente fue dolor en columna vertebral (85%). El 92% asoció déficit neurológico en forma de síndrome sensitivo-motor (70%), motor puro (15%) o sensitivo puro (7%). Cinco pacientes recibieron tratamiento quirúrgico y 8 fueron tratados de forma conservadora. Al año, 3 de los pacientes tratados de forma quirúrgica y 4 de los de manejo conservador tenían una puntuación igual o menor de 2 en la Escala Rankin Modificada. Se observó peor pronóstico en pacientes anticoagulados, en hematomas de mayor extensión, en hematomas localizados en región lumbar y cuando el compromiso motor inicial era mayor.
ConclusionesLa edad avanzada, la HTA y la anticoagulación son los principales factores asociados con el HEEE. La presentación típica consiste en dolor en la columna vertebral seguido de déficit motor. En pacientes con déficits motores establecidos, el tratamiento quirúrgico dentro de las primeras 24h parece ser la mejor opción terapéutica.
Spontaneous spinal epidural haematoma (SSEH) is a rare disease that may lead to rapid and irreversible neurological impairment. Early diagnosis and treatment are therefore essential.1–4 Haematoma is considered to be spontaneous when no causes can be directly associated with its onset, as occurs in 40% to 50% of cases.5–7 However, there are some well-known predisposing factors, including blood dyscrasia, use of anticoagulant or antiplatelet drugs, tumours (ependymomas, neurinomas, or spinal cord gliomas), pregnancy, etc.1–18 Its estimated incidence rate is 1 new case per million population per year, accounting for 0.3% to 0.9% of all space-occupying spinal cord lesions.4,5,8,11–16,19–21 The most common clinical presentation features pain in the spinal column with a radicular component; this may be accompanied or followed by clinical signs of acute myelopathy.3,4,6–8,11–13,15,17,19,20,22–24 Early clinical diagnosis and confirmation with an imaging study (preferably an MRI scan) are of vital importance.7,9,10,19,25 SSEH is considered a surgical emergency since early haematoma evacuation is associated with better functional outcomes.1,4–6,9,11,21,23,26,27 Conservative treatment is a reasonable option when neurological impairment is minimal or when patients experience significant spontaneous improvement during the first hours after onset.1
ObjectiveThe purpose of the present study is to describe a sample of patients diagnosed with SSEH in our hospital, and to identify and analyse the variables that may affect their functional prognosis.
Patients and methodsWe conducted a retrospective descriptive study of the patients attended and diagnosed with SSEH at Hospital General Universitario Gregorio Marañón, Madrid, between 2001 and 2013. Patients were gathered from the hospital database, which follows the coding criteria of the 8th edition of the International Classification of Diseases (ICD-9, January 2012) and its data corresponds to the Spanish health system's Minimum Data Set. We searched for the following diagnoses: ‘non-traumatic spinal haemorrhage’, ‘non-traumatic spinal haematoma’, and ‘non-traumatic spinal epidural haemorrhage’ (ICD-9 code 336.1 in all cases). Our centre is a public hospital in the Madrid health district providing care to a local population of 317940. More than 230000 people are attended at the emergency department every year.28 Data analysed here were gathered from patients’ medical histories, including discharge reports, clinical reports, and reports from the intensive care unit and surgery departments. We analysed demographic data, risk factors, clinical characteristics, neuroimaging findings, treatment approach, clinical progress, and long-term prognosis. The study only included patients with spinal epidural haematomas and no known direct cause of the bleed. Patients presenting haematomas related to any type of trauma (including indirect and mild trauma, and therapy-induced trauma) were excluded. All MRI studies were performed with a 1.5T MRI scanner (Achieva, Philips) using T1-weighted turbo spin echo (TSE), T2-weighted TSE, Short-Time Inversion Recovery (STIR), and T2*-weighted (T2-star) gradient echo (GE) sequences. Presence of SSEH was confirmed if there was a space-occupying lesion in the spinal canal (epidural space) which was hyperintense in T1-weighted and T2-weighted TSE sequences, not suppressed on STIR sequences, and possibly presenting heterogeneous signal intensities corresponding to haemoglobin degradation products on T2*-weighted GE sequences. Presence of myelopathy was determined when spinal cord hyperintensities were found on T2-weighted TSE and STIR sequences. The patients’ functional status was assessed one year after SSEH onset using the modified Rankin Scale (mRS), whose scores range from 0 to 6. Patients scoring≤2 were considered to have a good functional status. When mRS scores were not available, they were calculated based on data from the patient's medical history.
Categorical variables are given as percentages and numerical variables are expressed as mean and interquartile range (IQR), except for age, which is expressed as either the mean±SD or the median.
ResultsDemographic dataWe included a total of 13 patients aged 71±20.3 years (median, 79 years; IQR, 57.75-85.75). Mean age for 70% of the patients was 81 years. Women accounted for 46% of the patients. The most frequent comorbidities were vascular risk factors and antiplatelet drugs or oral anticoagulants (OAC). Hypertension was present in 8 patients (62%), OAC treatment in 7 (54%), dyslipidaemia in 5 (38%), diabetes mellitus in 5, obesity in 4 (31%), antiplatelet drugs in 2 (15%), and ischaemic heart disease in 2. Forty-two percent of the patients presented 2 or more vascular risk factors. In addition, 4 patients had some type of rheumatic disease (rheumatoid arthritis, polymyalgia rheumatica, or spondyloarthropathy). One of the patients was in the postpartum period. The international normalised ratio (INR) was determined in patients treated with anticoagulants upon arrival at the emergency department. One patient (14%) presented an INR<2, 2 patients (28%) had INRs between 2 and 3, and 4 patients (57%) presented INR>3. The main demographic data are summarised in Table 1.
Main clinical, diagnostic, therapeutic, and prognostic characteristics for the 13 patients.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex/age | F86 | F76 | F76 | F84 | M40 | M82 | M85 | F27 | F88 | M75 | M52 | M79 | M59 |
| Comorbidities | HT, DMPulmonary embolism | HT, DL, DMHT heart diseaseCOPD, AF | HTAF, DVT, autoimmune thrombocytopenia | DVT, PEPolymyalgia rheumatica | None | DLIschaemic heart disease | HT, DLAF, COPD, OSA | None | HTAF, cerebral infarct, heart disease, RA | HT, DM | HT, DL, DMHeart disease, terminal CKD, retinopathy | HT, DMIschaemic heart disease, pemphigus, SAH, epilepsy | DLObesity |
| Anticoagulant/antiplatelet drugs | AcenocoumarolINR 2.4 | AcenocoumarolINR 4.19 | AcenocoumarolINR 2.73 | AcenocoumarolINR 4.33 | None | ASA | AcenocoumarolINR 5.83 | None | AcenocoumarolINR 3.07 | AcenocoumarolINR 1.16 | None | ASA | None |
| Pain | Yes (thoracic) | Yes (cervical) | Yes (cervical) | Yes (cervical) | Yes (thoracic) | Yes (cervical) | Yes (cervical) | Yes (cervical) | No | No | Yes (thoracic) | Yes | Yes (cervical) |
| Radiating pain | No | Yes (right-sided) | No | Yes (interscapular region) | No | Yes (lumbar region) | Yes (shoulders) | No | No | No | No | No | Yes (upper limbs) |
| Type of deficit | Paraplegia | Right-sided hemiparesis | Left-sided hemiparesis | Quadriplegia | Paraplegia | Left-sided hemiparesis | Right-sided hemiplegia | Paraparesis | Paraplegia | Paraparesis | None | Paraplegia | None |
| Other spinal cord symptoms | T8 sensory level, urinary retention | None | Right lower limb hypoaesthesia | C7 sensory level, urinary retention | Sensory alterations | Right-sided T1 sensory level | None | T6 sensory level | T6 sensory level | L4 sensory level, loss of sphincter control | None | T5 sensory level | C2 sensory level |
| Time (h) | |||||||||||||
| Emergency dept. | 48 | 12 | 18 | 3 | No data | 1.5 | 1 | 0 (Ad) | 0 (Ad) | 48 | 72 | 6.5 | 240 |
| Neurology dept. | 96 | 14 | 20 | 5 | 4.5 | 1 | 8 | 72 | 48 | – | 7.5 | 240 | |
| Neurosurgery dept. | 96 | 24.5 | 23 | 10 | 7.5 | 21 | 20 | 74 | 65 | 264 | 13 | No data | |
| MRI | 101 | 34 | 22 | 10 | 6.5 | 21 | 20 | 74 | 65 | 264 | 9.5 | 288 | |
| Treatment | 101 | 44 | 23 | 10 | 10 | 22 | 21 | No specific treatment | 65 | No specific treatment | 14 | No specific treatment | |
| Diagnostic tool | MRIOther: lumbar Rx, cervical column CT | MRIOther: cervical Rx, cranial CT | MRIOther: cranial CT, cervical CT | MRI | MRI | MRI | MRIOther: cranial CT | MRI | MRI | MRI | MRI | MRIOther: thoracic and lumbosacral Rx | MRIOther: thoracolumbar CT |
| Treatment | Conservative: dexamethasone 8mg IV and 4mg/8 hours in decreasing doses | Surgical:C4-C5 hemilaminectomy | Conservative:dexamethasone | Conservative:methylprednisolone 1g for 5 days | Conservative | Surgical:C3-C6 laminectomy | Surgical:C3-C6 laminectomy | Surgical:C6-T1 laminectomy | Conservative | Conservative:dexamethasone | Conservative | Surgical:laminectomy | Conservative |
| mRS score at 1 year | 6 | 3 | 0 | 4 | 0 | 2 | 1 | 1 | 4 | 4 | 0 | 4 | 0 |
RA: rheumatoid arthritis; ASA: acetylsalicylic acid; C: cervical; T: thoracic; DL: dyslipidaemia; DM: diabetes mellitus; mRS: modified Rankin Scale; COPD: chronic obstructive pulmonary disease; AF: atrial fibrillation; SAH: subarachnoid haemorrhage; HT: hypertension; Ad; admitted at symptom onset; INR: international normalised ratio; CKD: chronic kidney disease; IV: intravenous; F: female; mg: milligrams; MRI: magnetic resonance imaging; Rx: radiography; OSA: obstructive sleep apnoea; CT: computed tomography; PE: pulmonary embolism; DVT: deep vein thrombosis; M: male.
The most frequent clinical presentation (85%) was sudden-onset pain in the spinal column, coinciding with the location of the haematoma. Five participants (38%) experienced pain radiating with a non-metameric distribution. Most patients (92%) presented neurological impairment appearing simultaneously with or after pain onset, whether as a sensorimotor (70%), pure motor (15%), or pure sensory syndrome (7%). Only one patient experienced isolated spinal cord pain with no other accompanying symptoms. Motor deficit manifested as paraplegia (4 patients), hemiparesis (3), paraparesis (2), and quadriplegia (1). Seven patients had a sensory level and 2 experienced loss of sphincter control. Progression times from pain onset to appearance of neurological deficits were available for 9 patients (75%); mean progression time was 3.5hours (IQR, 0-7). The main clinical findings of our study are shown in Table 1.
Diagnostic testsThe median time elapsed between symptom onset and medical attention at the emergency department was 6.5hours (IQR, 1-48). The median time between symptom onset and being examined by the on-call neurologist was 14hours (IQR, 5-72). All patients underwent a spinal cord MRI scan. The median time elapsed from symptom onset to the MRI scan was 28hours (IQR, 10-101). Seven patients underwent other imaging studies before having the spinal cord MRI: vertebral column simple radiography (3), cranial CT (2), and vertebral column CT (3). Two out of the 3 patients undergoing vertebral column CT displayed a hyperdense extramedullary mass suggestive of spinal epidural haematoma. Two patients underwent angiography after the MRI study showed no pathological findings. Mean time between arriving at the emergency department and completing the MRI scan was similar for patients undergoing MRI only (20hours; IQR, 7-74) and in those who had previously completed other diagnostic tests (20hours; IQR, 4-48). The main radiological findings are summarised in Table 2. Fig. 1 shows examples of spinal cord MR images.
Main MRI findings in the study patients.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Affected segment | T4-L2 | C4-C7 | C3-C7 | C4-T7 | T10-T11 | C3-C7 | C2-C7 | C6-C7 | T4-conus medullaris | T10-S1 | T12-L1 | T1-L3 | C7 |
| Location | Anterior | Posterior | Posterior | Posterior | Anterior | Anterior | Posterior | Posterior | Posterior | Posterior | Posterior | Posterior | Posterior |
| Signs of myelopathy | Yes | Yes | No | Yes | No | Yes | No | Yes | Yes | No | No | No | Yes |
| Associated vertebral column disease | Degenerative changes | Degenerative changes | Degenerative changes | Degenerative changes | None | Degenerative changes | Degenerative changes, cervical canal stenosis at C3-C4 | None | None | Spondylolisthesis degenerative changes, cervical canal stenosis at L4-L5, vertebral angiomas | Degenerative changes | None | Degenerative changes, spondylolisthesis at C7-T1, medullary stenosisC3-C7 |
Degenerative changes: signs of spondyloarthropathy and disc degeneration (osteophytes, decreased intervertebral disc space, subchondral sclerosis).
C: cervical; T: thoracic; L: lumbar; MRI: magnetic resonance imaging.
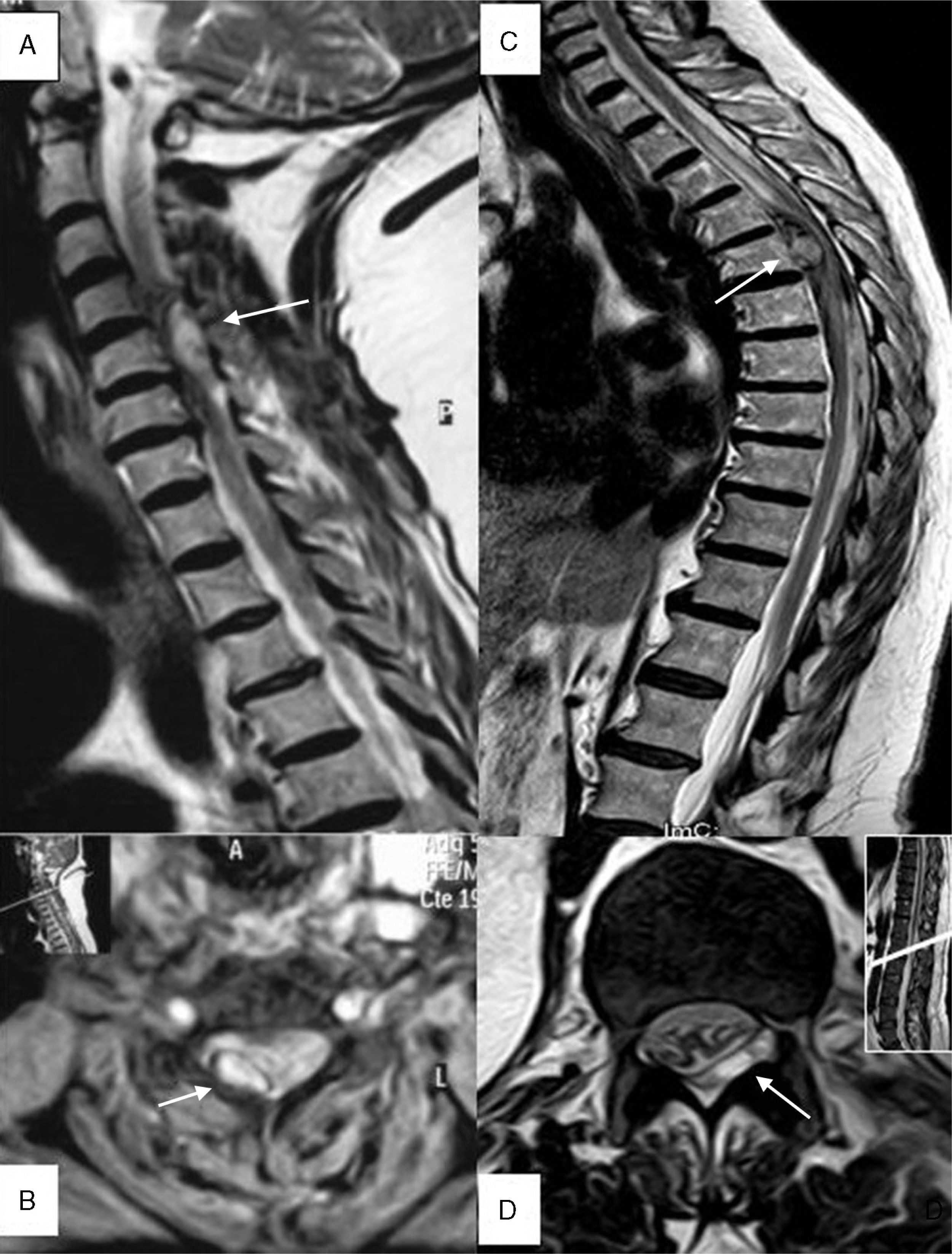
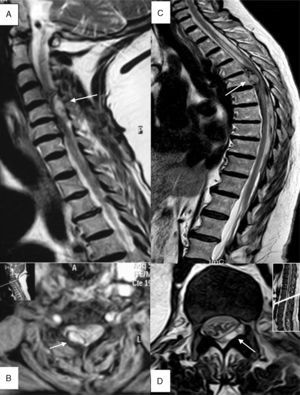
(A) Patient 4, sagittal section, T2-weighted TSE sequence. Epidural haematoma (arrow) in right posterior region located between segments C4 and C6 with a compressive effect on the spinal cord that causes myelopathy. (B) Patient 4, axial section at C4, T2*-weighted GE sequence. A haematoma can be seen in the right posterior region (arrow). (C) Patient 1, sagittal section, T2-weighted TSE sequence. Predominantly anterior epidural haematoma (arrow) between segments T6 and L2, causing medullary compression and associated signs of myelopathy. (D) Patient 11, axial section at level of T12-L1, T2-weighted TSE sequence. Epidural haematoma (arrow) in the lateral posterior region, displacing and compressing the thecal sac.
Five (38%) of the 13 study patients underwent urgent surgical treatment (hemilaminectomy in 1, bilateral laminectomy in 4). Eight (61%) were treated conservatively (corticosteroids in 4, symptomatic treatment in 4). Furthermore, 3 of the 5 patients undergoing surgical treatment scored≤2 in the mRS at one year (60%) vs 4 out of the 8 patients who were treated conservatively (50%). All patients underwent rehabilitation, except for one patient who died and 2 patients who experienced no motor deficits.
DiscussionSSEH pathophysiology is not yet completely understood and the exact pathogenesis of epidural haematomas is still subject to debate.11 Some authors state that epidural haematomas are venous and postulate that haemorrhages are caused by an increase in intrathoracic or intra-abdominal pressure that is transmitted directly to the veins in the epidural venous plexus, resulting in vessel rupture.2–5,7,9,11,14,15,17,29 This theory has been questioned since the epidural venous plexus lacks valves and has a low pressure. The implication is that venous haemorrhage would not be able to progress and cause compression of the intradural content.4 Some authors suggest that epidural haematomas are arterial in origin3–5,8,9 and are caused by rupture of the radicular arteries that run along the nerve roots in the epidural space. Rupture may be a consequence of trauma or abrupt movements, especially in patients with spondyloarthropathy.3,5,9 In most published cases, however, no arterial ruptures can be observed at the time of surgery, whereas patients do show epidural venous bleeding. As a result, SSEH is widely accepted to be venous in origin.3,4
SSEH is most frequently reported in patients between the ages of 40 and 80.8,9,11 The mean age of patients in our series was 71 years. Most patients were aged between 75 and 88 years. The male/female ratio is 1.5:1.4,9,11 According to some series, there are no age- or sex-related differences in outcomes.1,30 In our series, however, better mRS scores at 1 year were observed in male patients and in patients younger than 75 years. Our results coincide with those reported in previous studies in which the main predisposing factors are HT and use of OACs with an INR above the therapeutic range.14,22,25 Patients not treated with OACs or antiplatelet drugs, and those with an INR<3, had a better long-term prognosis.
The initial level of neurological impairment has traditionally been considered one of the most important prognostic factors. Patients presenting complete sensorimotor deficits at onset tend to have poorer outcomes.1,5–7,15,19 In our series, as in previous series, patients with partial motor deficits (hemiparesis, paraparesis) displayed better outcomes. Patients experiencing no pain were the last to seek medical attention and had the highest mRS scores at one year even though their neurological impairment was the least severe at first.
Previous studies point to early treatment as one of the main prognostic factors: results are optimal when treatment is administered within the first 12 to 36hours after symptom onset.1,5,6,10,19,31,32 Early diagnosis is therefore essential. In our study, a definitive diagnosis of SSEH was determined by different variables on a case-by-case basis, including time elapsed from symptom onset to medical assistance, delays in ordering a neurological assessment, and delays in performing an urgent MRI scan.
The current diagnostic tool of choice is MRI.3,4,7,8,11,12,15,16,19–22,26,33 Performing a spinal column CT is a valid alternative for health centres lacking an MRI scanner.8,11,33 Arteriography enables us to rule out dural fistulas and other malformations that may have caused the haematoma and can go unnoticed in MR images.7
The regions of the spine most frequently affected by haematoma are the cervicothoracic and thoracolumbar regions;4,7,8,11,14–16,20 most lesions are posterior to the spinal cord.3,9,14,15,20 Some studies have analysed the position (anterior or posterior), extension, and degree of compression in MRI, and concluded that there were no significant differences that would allow these measurements to be regarded as prognostic factors.1,30
In our series, performing other imaging studies before the MRI scan did not result in significant diagnostic or therapeutic delays. The most commonly affected areas were the cervical and thoracolumbar regions; in most cases, the haematoma was located posteriorly. Patients with extensive haematomas located in the cervicothoracic or thoracolumbar junctions scored higher on the mRS at 1 year, although differences were not statistically significant.
Most of the published series recommend early surgical treatment, preferably within 12 to 24hours of onset of motor symptoms,1,5,7,15,18,20 or within 48hours in patients with mild motor impairment.4,7,8,19,20,22 In patients with marked motor impairment, surgery is not recommended more than 36hours after symptom onset.1,4,7,8,22 Exceptionally, complete recovery has been reported in patients undergoing surgery 96hours after symptom onset.6
The literature shows that increasing numbers of patients are treated conservatively (corticosteroids or symptomatic treatment).1,7,8 Conservative treatment is indicated for patients with minimal to no symptoms of spinal cord compression, or those demonstrating significant clinical improvements within the first few hours.4,12,16,20,34 Thorough clinical and neuroimaging follow-up is necessary for early detection of any exacerbation that may require surgery.1,3,9,12,33 Conservative treatment is also recommended for patients with bleeding disorders or in those whose general health state contraindicates surgery.4,8,20
In our series, patients undergoing surgery, especially those who were treated surgically within 24hours, achieved better mRS scores at one year. Among the patients treated conservatively, better mRS scores at one year were observed in those experiencing spontaneous improvements in the first few hours and in those with mild deficits who received early treatment (<24hours) with corticosteroids. No improvements were observed in patients with complete deficits.
The limitations of our study derive from the low incidence rate of SSEH, which makes it more difficult to perform a complex statistical analysis and attempt treatment. Since our study was retrospective, we were unable to obtain some of the data for the conservative treatments (type of corticosteroid, dose, administration route, etc.) and rehabilitation programmes. The mRS may not be the most suitable scale for assessing functional status and disability after spinal cord injury. In fact, other scales provide a more precise analysis of sequelae after spinal cord injury, such as the Walking Index for Spinal Cord Injury (WISCI and WISCI II) and the American Spinal Injury Association (ASIA) Impairment Scale. We could not use those scales because they include items that may be difficult to answer retrospectively.
In conclusion, the main predisposing factors for SSEH are HT and use of OACs. SSEH should be suspected in patients with cardiovascular risk factors who experience sudden-onset pain in the vertebral column accompanied by neurological deficits.
The factors associated with poorer prognosis are use of OAC with an INR>3, presence of large haematomas, lumbar location, and severe motor impairment. Early surgical treatment is the most suitable option for patients with motor deficits. Conservative treatment is recommended in patients with mild spinal cord injury experiencing spontaneous improvements in the first few hours, or in patients for whom surgery is contraindicated. In these cases, early treatment with corticosteroids is highly recommended.
FundingThis study received no public or private funding.
Conflict of interestAll authors have given their approval for publication of the manuscript and have no conflicts of interest to declare.
Please cite this article as: Muñoz González A, Cuello JP, Rodríguez Cruz PM, Iglesias Mohedano AM, Domínguez Rubio R, Romero Delgado F, et al. Hematoma espinal epidural espontáneo: estudio retrospectivo de una serie de 13 casos. Neurología. 2015;30:393–400.