One of the main symptoms of Parkinson's disease is the high incidence of falls occurring due to the decline of both static and dynamic balance. The aim of this study is to determine the effect of an Ai Chi programme designed to prevent falls in patients with Parkinson's disease by improving both functional independence and perception of physical pain.
MethodsFifteen patients diagnosed with Parkinson's disease (Hoehn and Yahr stages 1–3) participated in a 10-week Ai Chi programme consisting of 30–45-minute aquatic exercise sessions twice a week. The assessment measures used in this study were the pain visual analogue scale (VAS), the Tinetti gait and balance assessment tool, and the Timed Get Up and Go test.
ResultsThe results were calculated by applying the Friedman test to 3 related measurements: patients at baseline, at post-treatment (at the end of the 10 week programme) and after one month of follow-up. The data obtained showed a significant improvement (P<.001) in scores for pain perception, balance, and gait function after the treatment programme. Furthermore, patients continued to show significant improvements and the benefits remained at the one-month follow-up visit.
ConclusionAi Chi is a promising and feasible aquatic treatment for improving pain perception, balance, and functional capacity in patients diagnosed with mild or moderate Parkinson's disease.
Un síntoma principal de la enfermedad de Parkinson es la alta incidencia en caídas y deterioro en el equilibrio, tanto estático como dinámico. El objetivo de este estudio es determinar el efecto de un programa de entrenamiento de Ai Chi acuático sobre la prevención de caídas en pacientes con Parkinson, mejorando su autonomía funcional y su percepción del dolor físico.
MétodoQuince pacientes diagnosticados de Parkinson (Hoehn y Yahr, rango: 1-3) participaron en un programa de intervención de Ai Chi acuático de 10 semanas de duración, con sesiones 2 veces por semana. Se emplearon las escalas EVA de dolor, Tinetti de equilibrio y marcha y el test Get up and Go.
ResultadosLos resultados se obtuvieron aplicando la prueba de Friedman para 3 muestras relacionadas, en los pacientes antes de la terapia, después de la terapia y al mes de la terapia. Los datos obtenidos mostraron una mejoría significativa (P<0,001) al comparar los resultados mostrados en las 3 mediciones, donde los valores de percepción de dolor, equilibrio y funcionalidad en marcha han disminuido significativamente, incluso al mes de finalizar las mismas los pacientes seguían mostrando mejoría y mantenimiento en sus resultados.
ConclusiónUn programa de Ai Chi en el agua parece ser un tratamiento factible para los pacientes diagnosticados con EP en un grado leve y moderado en el tratamiento del dolor, equilibrio y capacidad funcional.
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease in the world. In Spain, approximately 120000 people have PD. It affects both sexes roughly equally, being slightly more common in men. Its estimated prevalence, ranging from 150 to 200 cases per 100000 people, increases exponentially between the ages of 65 and 90. Around 10% of patients develop symptoms prematurely (before the age of 40).1 Gait disorders are a major therapeutic challenge in PD2 since they involve a high risk of falls, disability, and physical decline.3 Treatment for gait disorders, especially in the field of pharmacotherapy, has improved, and surgery has emerged as a therapeutic alternative. However, current treatments do not definitively solve gait and balance disorders.4,5
Pain, another incapacitating symptom of PD, is very frequently undiagnosed in clinical practice.6 According to the literature, patients in early stages of the disease may experience back and neck pain due to shoulder girdle stiffness, and leg pain resulting from restless leg syndrome or dystonia. In advanced stages, pain may be caused by dyskinesia, akathisia, or off-state dystonia (40%), and may be musculoskeletal, articular, or radicular (20%).6 Other types of complex pain described by Moreno et al. include deep burning or stabbing pains with tingling or itching sensations in poorly-defined areas of the body, generally on the side with the most motor impairment. Patients experience a diffuse sensation of tension and unease and respond poorly to dopaminergic treatment.6,7 This central neuropathic pain is a primary symptom and is regarded as a direct result of the illness rather than being due to any musculoskeletal changes.6 The physiopathological mechanisms of this type of pain in PD patients have not been established. It has been suggested that the cause is dysfunctional nociceptive processing in the central nervous system. This is supported by the decreased threshold for pain produced by heat stimuli in some of these patients. It could also be a change in pain modulation due to the dopaminergic deficit in the basal ganglia-thalamocortical circuits.6
Physical exercise is recommended for patients with PD regardless of their state of health or disease progression.8 Studies evaluating several types of physical exercise have proved the benefits of exercising compared to other interventions that do not include physical exercise.9 Some researchers have suggested that the neuroprotective effects of exercise may decrease the risk of developing PD or slow down neurodegeneration.10,11 Although physical exercise is receiving increasing attention as a therapy for PD patients, few studies on clinical practice recommendations have been published.12 Several types of exercises, including tai chi, yoga, and Pilates, have been indicated for these patients.8,13
Hydrotherapy is a physiotherapy technique used as a complementary treatment for several neurological and musculoskeletal disorders in both adults and children. Although several studies have confirmed the effectiveness of a number of gait rehabilitation programmes in PD,14,15 few data on the effects of water exercise are available.16,17 However, the scientific evidence provided by these studies has several methodological limitations, especially the lack of objective assessment techniques.18 One therapeutic option is Ai Chi, which was created by Jun Konno in Japan in 1996 based on tai chi and qi qong. In this technique, the physiotherapist explains and demonstrates a slow, coordinated combination of movements of the limbs and trunk which patients must repeat while standing in a pool.19,20
The purpose of this study is to evaluate the effects of a 10-week Ai Chi programme on pain, balance, and functional capacity in patients diagnosed with PD.
Patients and methodsOur study lasted 10 weeks and was conducted at an aquatics centre where patients attended Ai Chi sessions.
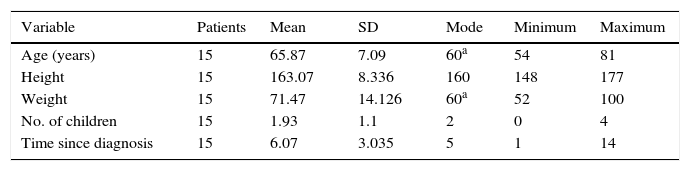
We included 15 patients from the PD patient association in our town. Inclusion criteria were as follows: patients diagnosed with stage 1–3 PD (Hoehn and Yahr scale) who were assessed at the local health service, time since diagnosis >6 months, aged over 40, without dementia, presenting independent walking and unaltered postural reflexes, not presenting any medical contraindications for this therapy, and receiving stable antiparkinsonian treatment during the study period. All patients were required to sign an informed consent form and accept the rules for participating (regular attendance and active participation). Exclusion criteria were: lack of commitment to attending sessions; having a neurological disorder that may prevent patients from understanding verbal orders, or any other disorder that may affect the results; cardiac, gynaecological, or dermatological diseases; bladder or bowel incontinence; and joint and/or muscle lesions in the lower limbs affecting independent walking.
Our study was approved by the Human Research Ethics Committee at the hospital of the lead researcher, and all subjects signed informed consent forms in compliance with the Declaration of Helsinki before the study started.
MethodsThis clinical study was aimed at treating patients and therefore lacks a control group. Patients attended a preliminary meeting, where they were assessed out of the water for 30–45minutes by an independent physiotherapist. We established schedules and informed the participants regarding appropriate clothing for the activity. Patients were assessed before starting therapy, after completing all sessions, and a month after therapy. All participants were evaluated during the on-phase (60–90minutes after receiving their antiparkinsonian drugs).
AssessmentPatients were initially asked to rate their pain using the visual analogue scale (VAS)21 (“I would like you to score your pain on a 10-point scale, with 0 meaning no pain and 10 meaning worst imaginable pain, by calculating the average score between the days with the least and the most pain”). Other variables associated with pain were subsequently scored from 1 to 5 points on a Likert-type scale. These variables were obtained by asking patients open questions about the characteristics of pain they deemed most relevant.18,19
The Tinetti Test is a very useful tool for detecting gait and balance disturbances in the elderly; higher scores indicate greater risk of falls or suspicion of gait disorders. Frequently used for functional assessment, it evaluates balance and gait based on direct observation and uses numerical qualifiers to rate the ability to perform the specific actions that make up the functional activity tested.22,23
For the Get-Up and Go Test, we recorded the time it took each patient to rise from a chair, walk 3 metres at their normal pace, turn around, walk back to the chair, and sit down again.24 This test evaluates basic functional mobility and locomotor ability (dynamic balance) in elderly patients. It is very useful for assessing physical mobility in clinical practice as it is simple, quick, easy-to-administer, and requires no special equipment.
For aquatic therapy, all the patients attended 2 sessions per week for 10 weeks (20 sessions in total). The group-based 45-minute sessions were led by an independent physiotherapist specialising in aquatic therapy.
Therapy took place in a swimming pool measuring 20×6 m and 110cm deep. Water temperature was 30°C (±0.5°C) and room temperature was 27.5°C (±1°C). The programme was conducted in a swimming pool adapted for groups.
Sessions, with gradually increasing difficulty, were planned in advance. At the beginning of each session, patients warmed up with a game, and at the end they cooled down with a relaxing activity. Ai Chi was practised for 30minutes each session, and consisted of 19 exercises in a specific order.
The safety measures taken were similar to those for any aquatic activity.
In addition to improving balance and reducing fall risk, Ai Chi also has relaxing and analgesic effects and is therefore indicated for the general population.
In our study, Ai Chi was the independent variable and pain, quality of life, and level of dependence were the dependent variables (qualitative variables).
An independent researcher performed the statistical analysis. We calculated means and standard deviations to describe the characteristics of the study sample. The Kolmogorov–Smirnov test was applied to determine whether the sample followed a normal distribution. We employed the t-test for paired samples to determine the effects of Ai Chi on each variable. The statistical analysis was conducted using SPSS statistical software version 19. Since all data were normally distributed, the study was completed using statistical parametric tests. Fisher's exact test was used, and P-values<.05 were considered statistically significant.
ResultsOur sample included 15 patients: 6 men (40%) and 9 women (60%). Table 1 shows the sociodemographic characteristics of the study population.
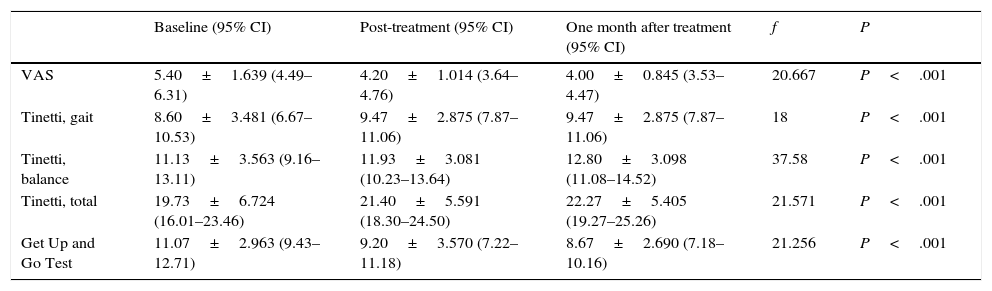
All participants adhered satisfactorily to treatment and completed all sessions. No incidents that might have potentially produced an unwanted effect on patients occurred. According to our results, the intervention led to changes in the study variables (Table 2).
Study results.
| Baseline (95% CI) | Post-treatment (95% CI) | One month after treatment (95% CI) | f | P | |
|---|---|---|---|---|---|
| VAS | 5.40±1.639 (4.49–6.31) | 4.20±1.014 (3.64–4.76) | 4.00±0.845 (3.53–4.47) | 20.667 | P<.001 |
| Tinetti, gait | 8.60±3.481 (6.67–10.53) | 9.47±2.875 (7.87–11.06) | 9.47±2.875 (7.87–11.06) | 18 | P<.001 |
| Tinetti, balance | 11.13±3.563 (9.16–13.11) | 11.93±3.081 (10.23–13.64) | 12.80±3.098 (11.08–14.52) | 37.58 | P<.001 |
| Tinetti, total | 19.73±6.724 (16.01–23.46) | 21.40±5.591 (18.30–24.50) | 22.27±5.405 (19.27–25.26) | 21.571 | P<.001 |
| Get Up and Go Test | 11.07±2.963 (9.43–12.71) | 9.20±3.570 (7.22–11.18) | 8.67±2.690 (7.18–10.16) | 21.256 | P<.001 |
We show the results obtained after using the Friedman test for 3 related samples: patients at baseline, patients after therapy, and patients after one month of follow-up. Distributions before, immediately after, and one month after therapy were different (significance level: 5%).
DiscussionWe aimed to analyse the effects of a programme of aquatic therapy in a population of patients diagnosed with mild to moderate PD (Hoehn and Yahr stages 1–3). After completing the intervention, patients displayed evident improvements in functional mobility and pain, as shown by the results obtained from the different dimensions that we assessed.
Pain, a common symptom in PD, is associated with depression and represents a predictor of poorer quality of life and less autonomy. However, despite being a common symptom and the increasing number of studies addressing pain diagnosis and management in PD,25–33 pain is still underestimated, underdiagnosed, and undertreated.32,33
Chronic pain may be a distracting factor interfering with the cognitive functions required to prevent falls. Tinetti et al.34 and Stubbs et al.35 suggest an association between pain and falls. Mickle et al.36 found that the prevalence of foot pain was significantly higher in elderly patients showing greater fall rates. Their results are similar to those reported by Stubbs35: foot pain and chronic pain are 2 crucial risk factors that should be considered when assessing these patients.
Symptoms of depression certainly coexist with pain in patients with PD28,37,38 and previous studies have shown that depression is directly associated with higher scores on pain assessment scales.38–40
Lower scores on the EuroQol-5D VAS indicate lower risk of falls.40 This conforms with the mean scores on the VAS obtained by our sample, which are slightly lower than those of the general population according to previous studies conducted in Spain.41 We found statistically significant differences between patients at baseline and patients at post-treatment (f=−1000, P=.006) and between patients at baseline and patients at one month of follow-up (f=−1200, P=.001). Improvements were more marked one month after the intervention. Therapy did not help 4 of the patients, and the results at one month after treatment were non-significant. Scores have been shown to decrease with time, which makes this type of therapy an adequate intervention for pain control as it has an immediate and long-lasting effect. The average difference between the initial and final assessments was 1.2 points. This difference is not as marked as in other studies, which additionally state that changes of 2–3 points are necessary to produce a clinically relevant effect. Furthermore, we cannot state that this decrease is due to a reduction in lower limb pain or pain at other locations.
To assess balance, we applied the two-way ANOVA. The factors were the group of 15 patients and their scores (at baseline, at post-treatment, and at one month). The Tinetti test for static balance showed significant differences in all patients, meaning that therapy affected each patient differently (f=37.58; P<.001). We also found significant differences in scores: therapy had a positive influence on score changes (f=12.98; P<.001).
The Tinetti test is widely recommended for assessing motor abilities in the elderly, since it evaluates balance and gait on motor tasks used in activities of daily living. Losing the ability to perform these motor actions may lead to loss of autonomy and dependence.42,43 The extent of the problem, its severity (quality of life, mortality rate, comorbidities, etc.), initial symptom, social and economic impact, and progression reflect the importance of research and intervention in these patients. Further studies are needed.
Hartley44 described a patient with PD whose scores rose from 10 to 17. However, this case does not provide any relevant information since the patient presented several comorbidities and the HY stage is not indicated. Park45 used the Tinetti test in stroke patients and found similar differences in total scores. This author implemented a programme of balance exercises that was comparable to Ai Chi. We found significant differences in our sample, which suggests that this type of therapy is useful and effective for balance and can therefore be evaluated in randomised controlled studies.
Marinho-Buzelli et al.46 conducted a systematic review on the influence of aquatic therapy on the mobility of patients with neurological disorders, including PD, and concluded that it significantly improved dynamic balance and gait speed in adults. Other studies47,48 discuss patients who showed significant improvements in postural stability after implementing an aquatic therapy programme. These patients experienced fewer falls than those undergoing land-based interventions.48
According to Sai et al.,49 the Timed Up and Go Test is the best balance test for predicting recurrent fallers. Whitney et al.50 stated that elderly patients who take longer than 15seconds to complete the Timed Up and Go Test have a high risk of falls. Likewise, poor performance in this same test was significantly correlated with slow gait speed. Other studies51,52 confirm that patients with PD display slower gait speed, shorter stride length, and reduced movement amplitude across all lower limb joints compared to healthy subjects.
In our study, test results showed significant differences between baseline and post-treatment scores (f=1033, P=.005) and between baseline and one month follow-up scores (f=1367, P<.001). In 2 cases, however, therapy had no effect and the results at one month of follow-up were not as relevant. We therefore conclude that this type of intervention is useful for preventing and delaying the appearance of motor symptoms in patients with balance disturbances. Low scores in this test indicate higher gait speed and lower risk of falls.
A cut-off point of 14seconds in conducting the test identifies patients with a high risk of falls. Our patients were able to complete the test in less time; the difference between baseline and post-treatment values was 3.05seconds (minimal detectable change). Ai Chi does not include gait exercises and, as a result, pronounced changes in Get-Up and Go Test scores should not be expected. Nevertheless, score changes in our study were similar to those reported by Volpe48 and differ from those described by Vivas,47 who found no significant changes in Timed Up and Go Test scores or symptoms after the intervention.
In addition, we should consider the potential influence of 2 determinant factors on the effect of gait training programmes in patients with PD: use of rhythmic cues and stimulation of aerobic metabolism.53 The first of these factors is known for significantly improving gait patterns in PD patients, whereas the second results in longer stride length and increased gait speed.54 In our study, movements were demonstrated so that participants were able to see them and perform them correctly. Although session intensity was not objectively assessed, we cannot rule out the presence of an aerobic stimulus that was sufficiently strong to produce the effect previously described, since session duration was considerable and most of the exercises involved large muscle groups. We should point out that a water-based physiotherapy protocol for PD patients found no significant changes in gait kinematics.47 The contents of that programme differed from our own and the intervention time was shorter.
In light of the above, our results suggest that water-based physical exercise has a beneficial effect on some biomechanical components of gait pattern in patients with PD. Neurologists should regard aquatic therapy as a valid rehabilitation method when the necessary resources are available. Further randomised controlled studies with larger samples are needed to confirm these findings.47
The positive progression of motor symptoms after water-based exercises has been associated with the performance of tasks based on a rhythmic pattern and constant postural control, which may indicate that these benefits result from a learning process. In addition, the advantages of water-based therapy (a pleasant and recreational environment, a wider variety of exercises that are easier to perform and low-impact due to hydrostatic pressure and buoyancy) and the opportunity to work in groups may have had a positive impact on patients’ motivation to adhere to the programme.
The warmth of the water used during the Ai Chi sessions may have had a therapeutic effect on such motor symptoms as rigidity and postural instability, as other studies have pointed out.46
The water-based training programme we present here was shown to have a positive impact on adherence compared to other physical exercise programmes designed for patients with PD, as stated by Ellis et al.55
The limitations of the study design (mainly its small sample size and lack of a control group) are a weak point of our investigation, which may diminish the impact of the beneficial effects of this water-based exercise programme. These effects can only be extrapolated to individuals similar to our patients; further studies on this topic should be conducted.
In conclusion, our results indicate that Ai Chi is a viable treatment for patients diagnosed with mild to moderate PD. Our water-based exercise programme improved balance and quality of movement in a group of patients. This programme involved tasks and motor actions that helped increase muscle strength, motion range, and motor function, which are directly involved in functional mobility.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pérez-de la Cruz S, García Luengo AV, Lambeck J. Efectos de un programa de prevención de caídas con Ai Chi acuático en pacientes diagnosticados de parkinson. Neurología. 2016;31:176–182.