Brief cognitive tests (BCT) may help detect cognitive impairment (CI) in the clinical setting. Several BCT have been developed and/or validated in our country, but we lack specific recommendations for use.
DevelopmentReview of studies on the diagnostic accuracy of BCT for CI, using studies conducted in Spain with BCT which take less than 20minutes. We provide recommendations of use based on expert consensus and established on the basis of BCT characteristics and study results.
ConclusionThe Fototest, the Memory Impairment Screen (MIS) and the Mini-Mental State Examination (MMSE) are the preferred options in primary care; other BCT (Clock Drawing Test [CDT], test of verbal fluency [TVF]) may also be administered in cases of negative results with persistent suspected CI or concern (stepwise approach). In the specialised care setting, a systematic assessment of the different cognitive domains should be conducted using the Montreal Cognitive Assessment, the MMSE, the Rowland Universal Dementia Assessment, the Addenbrooke's Cognitive Examination, or by means of a stepwise or combined approach involving more simple tests (CDT, TVF, Fototest, MIS, Memory Alteration Test, Eurotest). Associating an informant questionnaire (IQ) with the BCT is superior to the BCT alone for the detection of CI.
The choice of instruments will depend on the patient's characteristics, the clinician's experience, and available time. The BCT and IQ must reinforce—but never substitute—clinical judgement, patient–doctor communication, and inter-professional dialogue.
Los test cognitivos breves (TCB) pueden ayudar a detectar el deterioro cognitivo (DC) en el ámbito asistencial. Se han desarrollado y/o validado varios TCB en nuestro país, pero no existen recomendaciones específicas para su uso.
DesarrolloRevisión de estudios sobre el rendimiento diagnóstico en la detección del DC llevados a cabo en España con TCB que requieran menos de 20min y recomendaciones de uso consensuadas por expertos, sobre la base de las características de los TCB y de los estudios disponibles.
ConclusiónEl Fototest, el Memory Impairment Screen (MIS) y el Mini-Mental State Examination (MMSE) son las opciones más recomendables para el primer nivel asistencial, pudiendo añadirse otros test (Test del Reloj [TR] y test de fluidez verbal [TFV]) en caso de resultado negativo y queja o sospecha persistente (aproximación escalonada). En el segundo nivel asistencial es conveniente una evaluación sistemática de las distintas áreas cognitivas, que puede llevarse a cabo con instrumentos como el Montreal Cognitive Assessment, el MMSE, el Rowland Universal Dementia Assessment o el Addenbrooke's Cognitive Examination, o bien mediante el uso escalonado o combinado de herramientas más simples (TR, TFV, Fototest, MIS, Test de Alteración de la Memoria y Eurotest). El uso asociado de cuestionarios cumplimentados por un informador (CCI) aporta valor añadido a los TCB en la detección del DC.
La elección de los instrumentos vendrá condicionada por las características del paciente, la experiencia del clínico y el tiempo disponible. Los TCB y los CCI deben reforzar —pero nunca suplantar— el juicio clínico, la comunicación con el paciente y el diálogo interprofesional.
Population ageing has increased the prevalence of cognitive impairment (CI) and dementia associated with neurodegenerative, vascular, psychiatric, or other medical conditions, many of which are modifiable.1,2 Early diagnosis is vital, as professionals, governments, and groups involved advocate, since there are both preventive and palliative pharmacological and non-pharmacological treatments which can significantly reduce the personal, family, and social burden.3–6
Most of the cases of dementia are preceded by an objectively measured cognitive dysfunction called mild CI (MCI).7–9 This syndromic entity, although aetiologically heterogeneous, involves a high risk of developing dementia,10 but it also offers a great opportunity to assess possible preventive interventions.2 In normal clinical practice, patients who visit our consultation are in different stages of CI, and the line separating MCI and dementia is not clear. Therefore, it seems logical to focus diagnostic efforts towards the identification of the complete CI spectrum, both the earliest variants (MCI) and the more advanced ones (dementia).
A recent population-based study conducted in patients older than 65 in Spain reported a prevalence of MCI of 20%.11 Despite this high prevalence, many of these cases are still to be diagnosed12 or properly registered,13 in contrast to the high frequency of memory loss complaints reported in medical consultations.14 The reasons for this underdiagnosis could be: lack of access or time during consultations, limited training, confidence or diagnostic certainty of professionals, lack of efficient detection tools, or inappropriate use of these tools.15
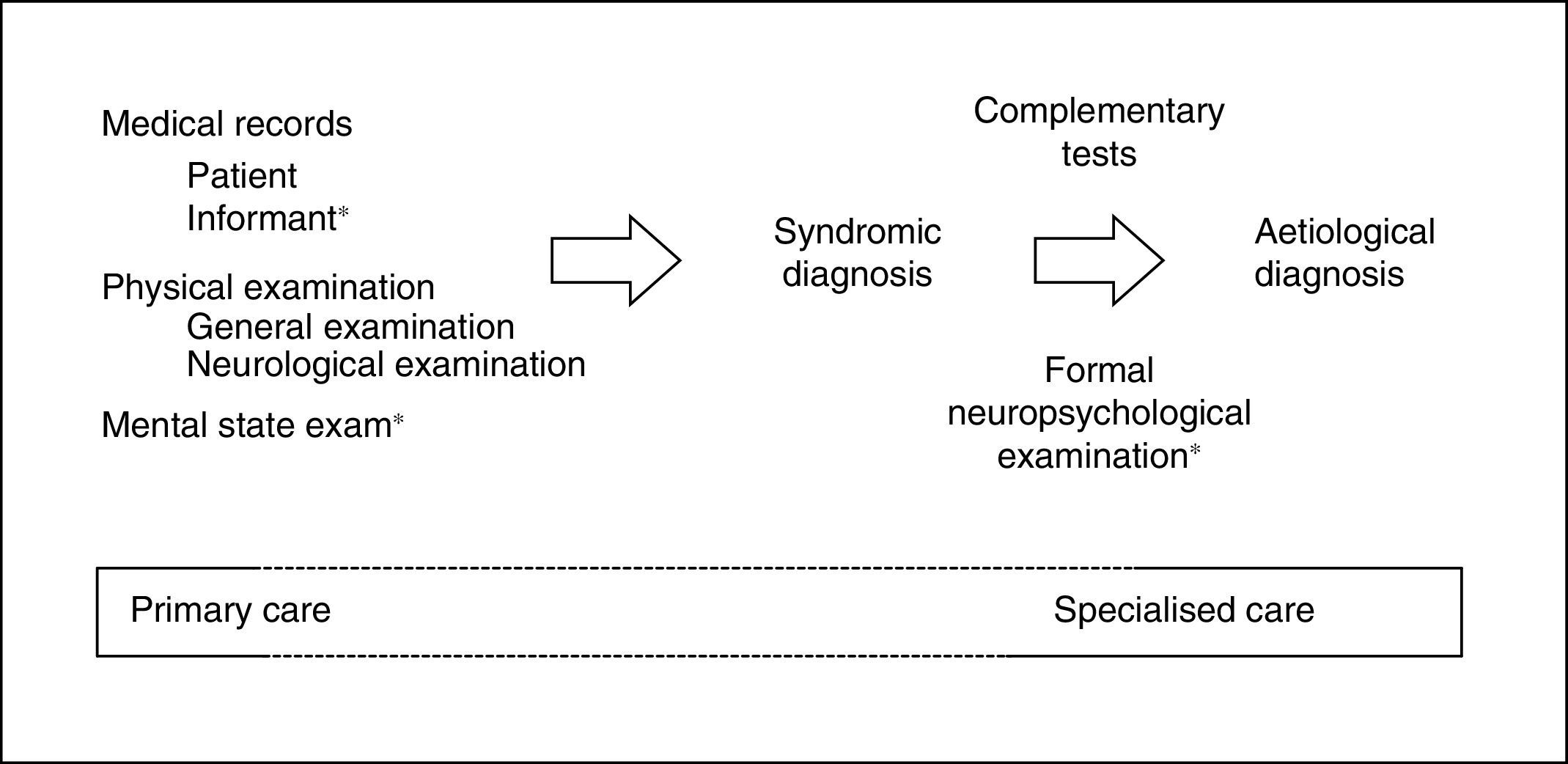
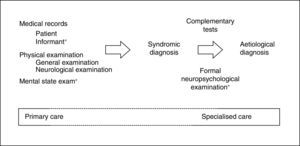
When MCI is suspected, it is fundamental to first rule out confusional syndrome or acute or subacute (less than 3 months of progression) focal neurological symptoms. These symptoms are markedly different from CI (of chronic nature), need emergency care (usually in-hospital),9 and require assessment with specific tools.16 By definition, CI must be detected and diagnosed using tests that assess the patient's cognitive capacities within the traditional yet irreplaceable framework of the clinical method.17 The information provided by a close acquaintance (regarding cognitive capabilities and performance of daily activities) and the results on the mental state examination (MSE)18 are especially important for the clinical method (Fig. 1).
Excellent review studies on the diagnostic value of brief cognitive tests (BCT) for detecting dementia have been conducted.19–21 However, their usefulness in detecting CI has been much less studied.21 The aim of this study is to review the usefulness of BCT and offer recommendations on their rational use in healthcare settings as the first approach to patients with suspected CI or cognitive complaints. We hope to aid in the achievement of more efficient and accurate diagnoses and early therapeutic intervention which could prevent or delay development of dementia, and reduce its high personal, family, and social costs.
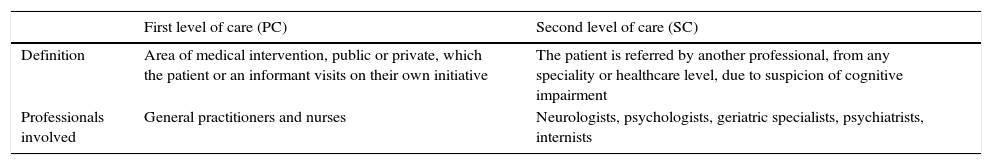
DevelopmentMethodsThis review article assesses the main characteristics of BCT and offers recommendations for use in clinical practice. In general, any cognitive test which takes less than 20minutes to be administered is a BCT. In issuing recommendations for use, we considered the 2 main levels in which healthcare systems are organised: first level or primary care (PC) and second level or specialised care (SC). The first level of care normally constitutes patients’ first encounter with the healthcare system. This level provides outpatient care at a healthcare centre or at the patients’ home and also includes prevention and early detection strategies. The second level provides care to patients transferred from the first and second levels through consultations, admitted patient units, home-based care units, and emergency services. Healthcare services also interact with other social and healthcare institutions that provide additional care services or cover specific needs (medium-stay hospitals, day care centres, institutions for the elderly, etc.). Table 1 shows the definition of levels of care, adapted to the goals of this study.
Definition of the levels of care used in this article.
| First level of care (PC) | Second level of care (SC) | |
|---|---|---|
| Definition | Area of medical intervention, public or private, which the patient or an informant visits on their own initiative | The patient is referred by another professional, from any speciality or healthcare level, due to suspicion of cognitive impairment |
| Professionals involved | General practitioners and nurses | Neurologists, psychologists, geriatric specialists, psychiatrists, internists |
The most accessible and immediate level of care is PC, and professionals at this level have a longitudinal perspective of the patient which is essential for detecting CI, especially when patients have a high education or intellectual level, or there is no informant available. Professionals at the SC level do not usually have previous knowledge of the patient. However, they do have more experience and resources for less common tasks. PC is, therefore, the correct level for detection and early management of CI, while SC is the right level for confirming diagnoses, determining the aetiology, and starting specific treatment.
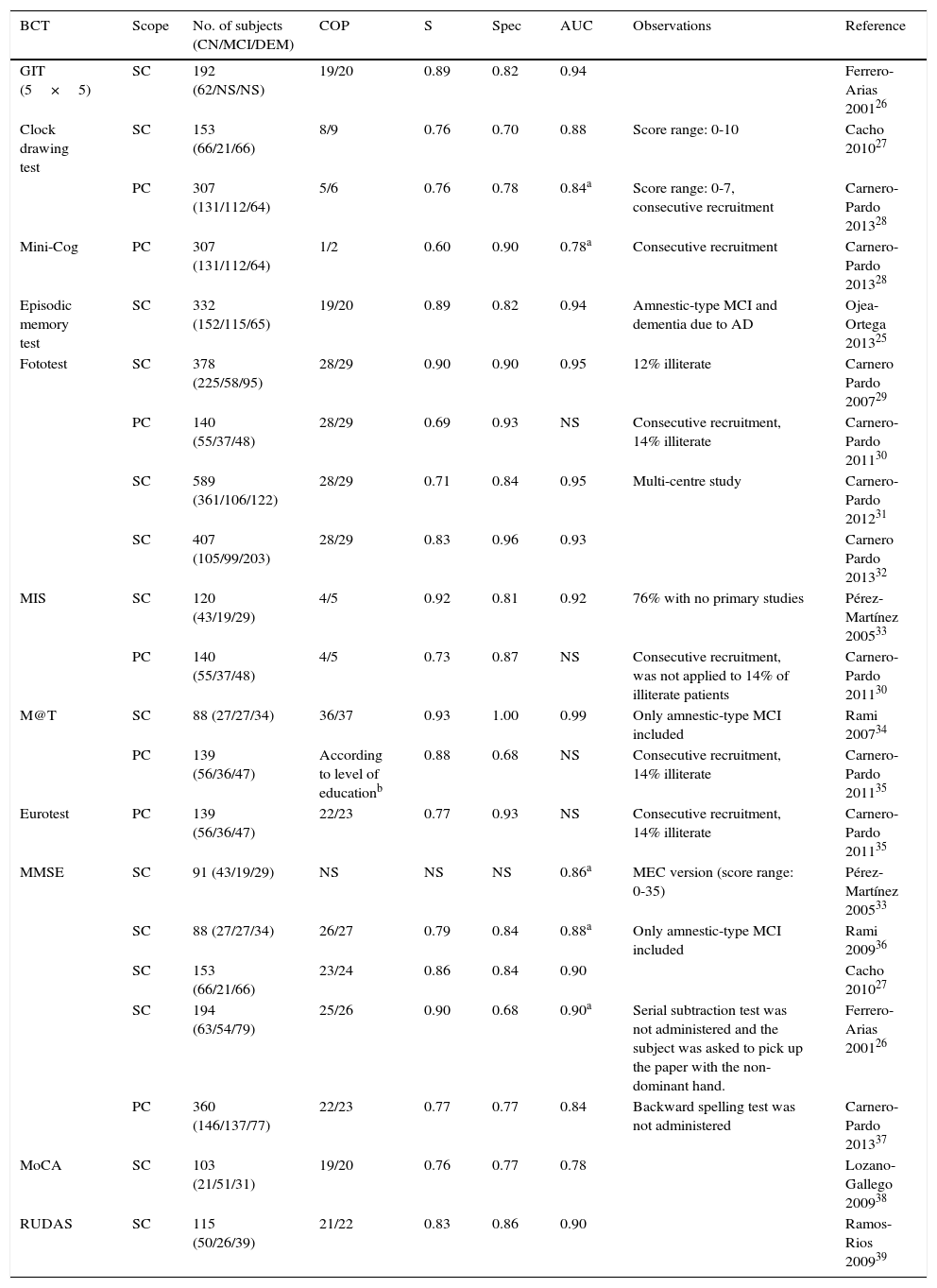
This article does not address systematic detection of CI in the population, since this process5 and its methodology are unclear.22 We have not assessed either telephone or internet-based cognitive tests, or self-administered tests or those administered by a caregiver. These may be useful options for patients living in isolated areas, having impaired mobility or other health problems, or with little motivation to go to a clinic, but evidence is still limited as to their efficacy.23–25 We briefly describe BCTs which have been validated for detecting CI in Spain, and offer guidelines for their use. Tests are presented according to their administration time and only relevant data are mentioned. More information on the characteristics, method of administration, and diagnostic yield of each test can be found in Table 2, as well as in the validation studies mentioned in the table,25–39 and in a recently published monograph.40 To make comparisons between different BCTs easier, we have described only diagnostic accuracy parameters (sensitivity, specificity, and the area under the ROC curve [AUC]) corresponding to the cut-off point that maximises the sum of the sensitivity and specificity values.
BCT validation studies for the detection of CI conducted in Spain.
| BCT | Scope | No. of subjects (CN/MCI/DEM) | COP | S | Spec | AUC | Observations | Reference |
|---|---|---|---|---|---|---|---|---|
| GIT (5×5) | SC | 192 (62/NS/NS) | 19/20 | 0.89 | 0.82 | 0.94 | Ferrero-Arias 200126 | |
| Clock drawing test | SC | 153 (66/21/66) | 8/9 | 0.76 | 0.70 | 0.88 | Score range: 0-10 | Cacho 201027 |
| PC | 307 (131/112/64) | 5/6 | 0.76 | 0.78 | 0.84a | Score range: 0-7, consecutive recruitment | Carnero-Pardo 201328 | |
| Mini-Cog | PC | 307 (131/112/64) | 1/2 | 0.60 | 0.90 | 0.78a | Consecutive recruitment | Carnero-Pardo 201328 |
| Episodic memory test | SC | 332 (152/115/65) | 19/20 | 0.89 | 0.82 | 0.94 | Amnestic-type MCI and dementia due to AD | Ojea-Ortega 201325 |
| Fototest | SC | 378 (225/58/95) | 28/29 | 0.90 | 0.90 | 0.95 | 12% illiterate | Carnero Pardo 200729 |
| PC | 140 (55/37/48) | 28/29 | 0.69 | 0.93 | NS | Consecutive recruitment, 14% illiterate | Carnero-Pardo 201130 | |
| SC | 589 (361/106/122) | 28/29 | 0.71 | 0.84 | 0.95 | Multi-centre study | Carnero-Pardo 201231 | |
| SC | 407 (105/99/203) | 28/29 | 0.83 | 0.96 | 0.93 | Carnero Pardo 201332 | ||
| MIS | SC | 120 (43/19/29) | 4/5 | 0.92 | 0.81 | 0.92 | 76% with no primary studies | Pérez-Martínez 200533 |
| PC | 140 (55/37/48) | 4/5 | 0.73 | 0.87 | NS | Consecutive recruitment, was not applied to 14% of illiterate patients | Carnero-Pardo 201130 | |
| M@T | SC | 88 (27/27/34) | 36/37 | 0.93 | 1.00 | 0.99 | Only amnestic-type MCI included | Rami 200734 |
| PC | 139 (56/36/47) | According to level of educationb | 0.88 | 0.68 | NS | Consecutive recruitment, 14% illiterate | Carnero-Pardo 201135 | |
| Eurotest | PC | 139 (56/36/47) | 22/23 | 0.77 | 0.93 | NS | Consecutive recruitment, 14% illiterate | Carnero-Pardo 201135 |
| MMSE | SC | 91 (43/19/29) | NS | NS | NS | 0.86a | MEC version (score range: 0-35) | Pérez-Martínez 200533 |
| SC | 88 (27/27/34) | 26/27 | 0.79 | 0.84 | 0.88a | Only amnestic-type MCI included | Rami 200936 | |
| SC | 153 (66/21/66) | 23/24 | 0.86 | 0.84 | 0.90 | Cacho 201027 | ||
| SC | 194 (63/54/79) | 25/26 | 0.90 | 0.68 | 0.90a | Serial subtraction test was not administered and the subject was asked to pick up the paper with the non-dominant hand. | Ferrero-Arias 200126 | |
| PC | 360 (146/137/77) | 22/23 | 0.77 | 0.77 | 0.84 | Backward spelling test was not administered | Carnero-Pardo 201337 | |
| MoCA | SC | 103 (21/51/31) | 19/20 | 0.76 | 0.77 | 0.78 | Lozano-Gallego 200938 | |
| RUDAS | SC | 115 (50/26/39) | 21/22 | 0.83 | 0.86 | 0.90 | Ramos-Rios 200939 |
ACE-III, Addenbrooke's Cognitive Examination (3rd version); MCI, mild cognitive impairment; SC, specialised care; PC, primary care; AUC, area under the ROC curve; CN, cognitively normal; CI, cognitive impairment; DEM, dementia; S, specificity; AD, Alzheimer disease; MEC, Mini-Examen Cognoscitivo; MIS, Memory Impairment Screen; MMSE, Mini-mental State Examination; MoCA, Montreal Cognitive Assessment; NS, non-specified; COP, cut-off point; RUDAS, Rowland Universal Dementia Assessment; S, sensitivity; T@M, memory alteration test; BCT, brief cognitive tests; GIT, general information test; FT, Fototest.
The animal category fluency trial which lasts just a minute is the briefest dementia detection test studied.41 It addresses linguistic and executive skills exclusively, so this type of test is not recommended as the first option to detect CI. Verbal fluency tests (VFT) offer extremely useful qualitative and quantitative data within the context of a more comprehensive evaluation. The animal naming test and the test that asks the patient to produce as many words beginning with ‘p’ in a minute as possible are the most recommended, since they are complementary and have been well studied in Spain.42,43 There are some variants, such as the Isaac Set Test44 and the general information test or the five by five test,26 which make administration longer and more difficult without improving diagnostic usefulness. Therefore, they are not recommended as alternatives.
The clock drawing test (CDT) assesses visuospatial ability (VA) and executive functions (EF).45 Despite the appeal of its simplicity and short administration time, the CDT is not enough in and of itself to detect CI,27 not even in the context of PC28 (Table 2). Nevertheless, the CDT is very sensitive to damage to the right parietal lobe and is definitely helpful as a supplement to other brief tests, especially when no abnormal performance in memory tests has been detected. In fact, CDT has been included successfully in several more comprehensive tests, as we will describe later. Its main disadvantage is that minimum drawing skills are necessary to complete the test, which makes the CDT inappropriate as a tool for illiterate patients or those with limited graphomotor skills. There are several proposals for scoring; the simplest proposals are the most appropriate for detecting CI while the most complex may be more useful for monitoring studies and case analysis.46,47
Mini-Cog includes the most frequently affected areas at CI onset. It includes a 3 word learning test similar to the Folstein Mini-mental State Examination (episodic memory [EM] and CDT [VA, EF]). Its score range is narrow (0-5: one point for every recalled word, and 2 additional qualitative assessment points if the clock has all numbers, in the correct order, and the clock hands point to the requested time) and has little sensitivity for detecting CI28 (Table 2) and its monitoring study. Mini-Cog is most useful in supporting a diagnosis of dementia, especially in relatively clear cases according to medical records.
The episodic memory test has been recently created and validated in a sample of patients with amnestic-type MCI and Alzheimer disease (AD)25 (Table 2). This is a user-friendly test which does not require paper or pencil (presumably it is little influenced by education level). It exclusively assesses EM through questions regarding the recent past. Its main disadvantage is that a reliable informant must be present to corroborate the veracity of the answers.
Fototest comprises a visual-verbal naming task with 6 photographs of objects (language), a verbal fluency task (language, EF), and an EM task with free and cued recall of the previously named images. Together with the Folstein Mini-Mental State Examination (MMSE), Fototest is the most extensively studied BCT for the detection of CI in Spain. This is a widely accepted tool that in both PC and SC has shown a satisfactory usefulness in detecting CI, with a stable cut-off point (28/29).29,31,32,35 Fototest has also shown a higher cost-effectiveness ratio than MMSE in PC, with the additional advantage of having equivalent parallel versions which may be of help in reducing the practice effect in the case of repeated administrations (PC, follow-up)48 (Table 2).
The Memory Impairment Screen (MIS) assesses verbal learning (EM) through reading and subsequent free and cued recall of 4 words. There are several validation studies conducted in Spain that have shown acceptable results for detecting CI30,33 (Table 2) and good results for dementia,30,33,49 especially if due to AD.50 The MIS also presents a good correlation with hippocampal formation and entorhinal cortex volumes,51 which supports its potential for early detection of AD.
The memory alteration test (M@T) includes items related with EM (free and cued recall, time orientation) and general information items (long-term/semantic memory [SM]) to a lesser extent. These characteristics, together with the wide score range (0-50), make it especially appropriate for detecting and monitoring the first clinical stages of typical AD34,36 (Table 2). The M@T distinguishes between subjective memory complaints and MCI, and has been validated using biochemical and neuroimaging markers.52 Although its usefulness in PC has been shown,35 limiting this test to SC seems more realistic and appropriate.
Eurotest is an original proposal for brief cognitive evaluation that uses currency coins and consists of 3 parts: knowledge of current coins (SM), operations with coins (calculation), and recall of the coins used (EM). Eurotest, which enables to some degree the functional evaluation of the ability to handle money, has obtained acceptable results in the detection of CI35 (Table 2) and good results for dementia.53,54 It is an ecologically valid test applicable to illiterate patients which has additionally been used with different currency systems, including Chinese.
The MMSE, with its countless adaptations and translations,55–59 is the most widely used BCT in the world, both in PC and SC settings. This extensive use, which enables the comparison and follow-up of subjects from different origins, has 2 main disadvantages, which are mainly due to the existence of multiple versions and the lack of clear instructions for scoring60: a questionable standard administration procedure and unreliability. Other limitations of the MMSE are the limited representation of EF, which diminishes its sensitivity in detecting MCI and frontotemporal dementia, and restrictions to its free use due to its recent copyright licence.61 The MMSE has been traditionally employed for the detection of dementia55,59,62,63; most of the information regarding its usefulness in CI detection comes from validation studies of other tools which included the MMSE as a comparison test. In other studies, the usefulness of the MMSE was, in general, lower than that of the tests to which it was compared27,30,33,36 (Table 2). In the only specific evaluation study of the MMSE for CI, the best performance was obtained with a lower than usual (22/23) cut-off point (COP) and in addition, adjusting for age and education did not improve its discriminant ability, unlike in common practice.28 In any case, MMSE helps to obtain qualitative profiles or features that give confidence to the clinician in the diagnosis and follow up, especially in the case of patients with typical AD. Some examples are the selective alteration of items related to EM (deferred recall, day of the month) in the case of prodromal AD, transformation of pentagons into quadrilaterals in AD with mild dementia, and impaired orientation to month and problems with naming a pencil in the case of moderate AD.
The Montreal Cognitive Assessment (MoCA) has an eclectic and balanced design for the detection of MCI regardless of aetiology. It includes items regarding attention, free recall (list of 5 words), EF (Trail Making Test, similarities), calculation, VA (copy a cube, CDT), orientation, and language. The first validation studies, conducted in Canada, obtained promising results.64 However, a Spanish study showed a lower effectiveness in the detection of MCI (69% of the subjects were correctly classified vs 90% in the original study)38 (Table 2). Education level exerts a strong influence on the MoCA, as was shown in validation studies conducted in other countries.
The Rowland Universal Dementia Assessment (RUDAS) was developed in Australia for its administration to multicultural and illiterate populations. It assesses identification of body parts, EM, visuoconstructive praxis (drawing a cube), and reasoning. A preliminary study conducted in Spain obtained good results in the detection of CI39 (Table 2).
There are 2 BCTs which have been validated in non-clinical community settings and which therefore do not meet inclusion criteria in this review article. However, we mention them here since they can be useful in PC.
The Abbreviated Mental Test (AMT) by Hodkinson assesses orientation, attention, and memory. The test, which includes 10 items, is easy to administer (except the item requiring the identification of 2 people), and education level is less influential. A COP of 7/8 showed a good performance in the detection of MCI65 and dementia.66 The AMT is rarely used in Spain, possibly due to its limited distribution and availability.
The Leganés cognitive test (LCT) was designed to assess the cognitive capacities of an elderly population with a low level of education.67 It yielded excellent results for the detection of dementia (COP 22/23) and good for CI (COP 26/27).68 Since most of its items are related to EM, the LCT can be useful for detecting and monitoring typical AD when advanced age, low education level, or other circumstances advise against administering more complex tests.
Other commonly used BCTs in Spain which have been specifically evaluated for use with dementia although not for CI are the following:
The Pfeiffer questionnaire (Short Portable Mental Status Questionnaire [SPMSQ]) is similar to the AMT, although more focused on SM. Mistakes instead of correct answers are scored. The SPMSQ obtained good results in the detection of dementia in PC69 and SC (COP 2/3),70 and excellent results in geriatric settings (COP 4/5).71
The 7 Minute Screen (7MS), which requires somewhat more than 7minutes to administer, presents some characteristics (16 figure learning task, complex score system) that make it difficult to use as a first approach to CI in the healthcare setting. However, being halfway between MSE and formal neuropsychological examination (Fig. 1), it can be a useful tool in the assessment of cognitive areas usually impaired in AD. The 7MS achieves an excellent performance in the detection of dementia (COP 20/21),70,72 but an Argentinian study has questioned its usefulness in MCI.73
The Addenbrooke's Cognitive Examination (ACE) was developed from the MMSE, adding frontal/executive and visuospatial elements. Its third version (ACE-III) is currently available and has shown good results with regards to dementia detection,74 and excellent results with subjects with more than 9 years of schooling (COP 73/74). The ACE can also be useful in distinguishing the main aetiological types of dementia.75 An abbreviated version has been recently validated (M-ACE) which may have more discriminant ability than the original version.76
Limits of the brief cognitive tests: new action and research linesWithout questioning its usefulness and convenience, the diagnostic capability of BCT is far from being completely satisfactory, especially regarding the detection of CI. One solution would be adding more items and areas in order to test more cognitive skills with more detail, but then the test would become less efficient and require more time and specialised personnel. In that case the screening tool and the gold standard would be nearly the same. Another possible solution might consist of using more complex tasks involving diverse cognitive skills, including processing speed (divided attention tasks, figural fluency, etc.).77 However, a more complex and difficult task is likely to lead to greater influence by non-pathological factors (ageing, stress, etc.), thereby reducing specificity. Using questionnaires on cognitive complaints or metacognition is another option, but this has been demonstrated as less than useful due to the influence of lesion topography, psychiatric comorbidity, and psychological and social factors.78
Informant questionnaires are generally simple, easy to administer tools that offer a perspective (longitudinal and that of the informant) supplementary to the one offered by cognitive tests.79 The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) or informant test is the most widely studied questionnaire in Spain. Both the complete version (26 items) and the abbreviated version (17 items) have shown a good performance in the detection of dementia,80–82 achieving an AUC of 0.91 in one of the studies.82 Furthermore, this questionnaire is not influenced by age, sex, mental health, or prior intellectual level.62 Performance of the IQCODE for the detection of CI was lower (AUC 0.75) in another study, while its incorporation added predictive value to the MMSE (AUC 0.86 vs 0.82 with MMSE only). The Functional Activities Questionnaire (FAQ) comprises 11 items and has also obtained good results in the detection of dementia (AUC 0.91), although results are poorer for detecting CI (AUC 0.77). The FAQ, like the IQCODE, improved the performance of the MMSE for detecting dementia (AUC 0.95 vs 0.91 with MMSE only).83 The Ascertain Dementia-8 (AD 8) is a very brief proposal, designed for PC. It includes 8 yes or no questions and presents a good performance for diagnosing CI (AUC 0.90). Furthermore, its use associated with a BCT (Fototest) adds predictive value (AUC of Fototest 0.93 vs AUC of Fototest plus AD 8 0.96).32
Physical examination (motor alterations, frontal signs, etc.) or biological sample analysis (blood, saliva, etc.) could provide other complementary markers for the detection of CI. But these lines of research, though interesting for aetiological diagnosis, have not yet provided evidence of practical usefulness.84,85
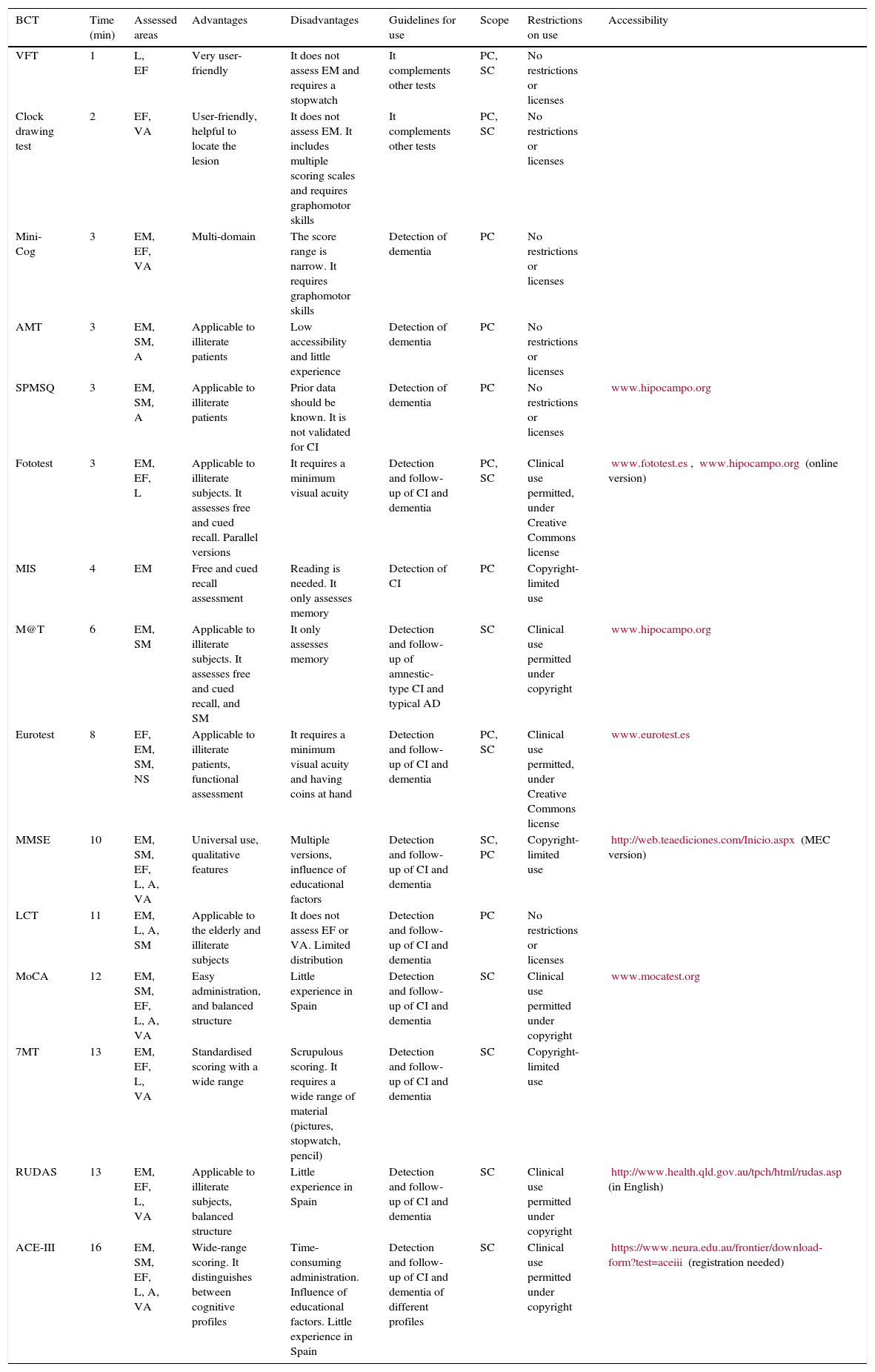
ConclusionsA first approach to the patient with complaint or suspicion of CI should at minimum include the examination of the more frequently impaired areas (EM and EF). In PC we recommend using some BCT as that could offer objective support to the clinical suspicion and facilitate an efficient referral to SC to obtain an accurate diagnosis. Fototest and MIS have been well studied and are the most recommended options for PC. Other tests (CDT, VFT) can be added, especially in the case of negative results and persistent complaints or suspicions (stepped-care approach). Eurotest and MMSE are also valid alternatives in PC, depending on the circumstances (Eurotest for illiterate patients and MMSE if more time is available) (Table 3). The patient's performance in his or her day-to-day social environment should be clearly defined both in PC and SC (money handling, medication, etc.). If impairment is observed, suspicion of dementia and not of MCI should be considered.
Brief cognitive tests: general characteristics and guidelines for use.
| BCT | Time (min) | Assessed areas | Advantages | Disadvantages | Guidelines for use | Scope | Restrictions on use | Accessibility |
|---|---|---|---|---|---|---|---|---|
| VFT | 1 | L, EF | Very user-friendly | It does not assess EM and requires a stopwatch | It complements other tests | PC, SC | No restrictions or licenses | |
| Clock drawing test | 2 | EF, VA | User-friendly, helpful to locate the lesion | It does not assess EM. It includes multiple scoring scales and requires graphomotor skills | It complements other tests | PC, SC | No restrictions or licenses | |
| Mini-Cog | 3 | EM, EF, VA | Multi-domain | The score range is narrow. It requires graphomotor skills | Detection of dementia | PC | No restrictions or licenses | |
| AMT | 3 | EM, SM, A | Applicable to illiterate patients | Low accessibility and little experience | Detection of dementia | PC | No restrictions or licenses | |
| SPMSQ | 3 | EM, SM, A | Applicable to illiterate patients | Prior data should be known. It is not validated for CI | Detection of dementia | PC | No restrictions or licenses | www.hipocampo.org |
| Fototest | 3 | EM, EF, L | Applicable to illiterate subjects. It assesses free and cued recall. Parallel versions | It requires a minimum visual acuity | Detection and follow-up of CI and dementia | PC, SC | Clinical use permitted, under Creative Commons license | www.fototest.es, www.hipocampo.org (online version) |
| MIS | 4 | EM | Free and cued recall assessment | Reading is needed. It only assesses memory | Detection of CI | PC | Copyright-limited use | |
| M@T | 6 | EM, SM | Applicable to illiterate subjects. It assesses free and cued recall, and SM | It only assesses memory | Detection and follow-up of amnestic-type CI and typical AD | SC | Clinical use permitted under copyright | www.hipocampo.org |
| Eurotest | 8 | EF, EM, SM, NS | Applicable to illiterate patients, functional assessment | It requires a minimum visual acuity and having coins at hand | Detection and follow-up of CI and dementia | PC, SC | Clinical use permitted, under Creative Commons license | www.eurotest.es |
| MMSE | 10 | EM, SM, EF, L, A, VA | Universal use, qualitative features | Multiple versions, influence of educational factors | Detection and follow-up of CI and dementia | SC, PC | Copyright-limited use | http://web.teaediciones.com/Inicio.aspx (MEC version) |
| LCT | 11 | EM, L, A, SM | Applicable to the elderly and illiterate subjects | It does not assess EF or VA. Limited distribution | Detection and follow-up of CI and dementia | PC | No restrictions or licenses | |
| MoCA | 12 | EM, SM, EF, L, A, VA | Easy administration, and balanced structure | Little experience in Spain | Detection and follow-up of CI and dementia | SC | Clinical use permitted under copyright | www.mocatest.org |
| 7MT | 13 | EM, EF, L, VA | Standardised scoring with a wide range | Scrupulous scoring. It requires a wide range of material (pictures, stopwatch, pencil) | Detection and follow-up of CI and dementia | SC | Copyright-limited use | |
| RUDAS | 13 | EM, EF, L, VA | Applicable to illiterate subjects, balanced structure | Little experience in Spain | Detection and follow-up of CI and dementia | SC | Clinical use permitted under copyright | http://www.health.qld.gov.au/tpch/html/rudas.asp (in English) |
| ACE-III | 16 | EM, SM, EF, L, A, VA | Wide-range scoring. It distinguishes between cognitive profiles | Time-consuming administration. Influence of educational factors. Little experience in Spain | Detection and follow-up of CI and dementia of different profiles | SC | Clinical use permitted under copyright | https://www.neura.edu.au/frontier/download-form?test=aceiii (registration needed) |
A, attention; ACE-III, Addenbrooke's Cognitive Examination (3rd version); SC, specialised care; AMT, Abbreviated Mental Test; PC, primary care; VA, visuospatial area; NS, numerical skills; CI, cognitive impairment; FTLD, frontotemporal lobar degeneration; AD, Alzheimer disease; EF, frontal/executive functions; L, language; EM, episodic memory; MEC, Mini-Cog; min, minutes; MIS, Memory Impairment Screen; MMSE, Mini-mental State Examination; MoCA, Montreal Cognitive Assessment; SM, remote/semantic memory; LCT, Leganés cognitive test; RUDAS, Rowland Universal Dementia Assessment; SPMSQ, Short Portable Mental Status Questionnaire; M@T, Memory alteration test; VFT, verbal fluency test (animals, words beginning with ‘p’); 7MT, 7 Minute Screen.
A systematic review of the main cognitive areas (attention, language, EM, EF, VA) must be conducted in SC, so a formal neuropsychological examination would be the ideal test (Fig. 1). For this reason, the most recommended tools for this level of care are, theoretically, those permitting evaluation of the highest number of cognitive domains, such as MoCA, MMSE, RUDAS, or ACE-III. These tests should be selected according to patient characteristics (MoCA if the patient is close to normality, MMSE if advanced CI is suspected, RUDAS if the patient is illiterate, ACE-III if in doubt between AD and frontotemporal lobar degeneration), the clinician's experience, and the available time (ACE-III administration requires more than 15minutes). Maybe due to the lack of specific studies (ACE-III), modest or negative results available (MMSE, MoCA),30,38 or the presence of other disadvantages (MMSE is not free),60,61 it is still to be determined whether the use of these tools is more efficient than the use of other brief and simple cognitive tests. This determination should consider that tests can be administered separately (MIS,33 M@T,36 and Fototest30), combined (VFT, CDT, and MMSE)27,28 or in a stepped-care approach (Fototest and Eurotest),86 particularly in situations with little time available.
Possible actions to avoid false negatives are varied and efficacious, and implementable in developed countries with enough healthcare and social resources. These include promoting a healthy lifestyle, controlling vascular risk factors, advising, researching, etc. These measures are not risky or costly, and all of them can be addressed or monitored by PC. Using cut-off points other than those recommended is acceptable in order to increase sensitivity and facilitate early detection, especially in cases of persistent complaint or suspicion or in subjects with a prior high intellectual level. Cut-off points serve as a guide and support, but never as confirmation of the diagnosis of CI, which is obtained by clinically assessing all the information available (Fig. 1).
To diagnose dementia, informant questionnaires perform the same or even better than BCT. This is most likely because a large number of older people in Spain have a low level of schooling, resulting in lower specificity of cognitive tests. However, an MSE should be performed whenever possible, since the target is CI (MCI or dementia) and the informant's perceptions might not be valid, or might be distorted by primarily non-cognitive aspects (physical health, sensory deficit, etc.), or even self-interest. Informant questionnaires provide added value to BCT, and this synergy should be further investigated. Rigorous, non-stop and imaginative research here in Spain has led to the designing of varied and complementary BCT, which are useful to increase clinicians’ confidence and accuracy in their first approach to the patient with complaint or suspicion of CI. The recommended BCT as well as their cut-off points and instructions, must be accessible in the electronic medical histories in PC and SC when possible in order to facilitate use and make it more cost-effective. BCT potential should be further explored, especially regarding aetiological diagnosis (for example, Eurotest may help detect CI caused by anything other than typical AD)87 and its usefulness in progression monitoring. Tests and questionnaires should reinforce but never replace medical opinion, fluid communication with patients and their acquaintances, coordination between the different healthcare levels, and dialogue between professionals in healthcare settings.
Conflicts of interestC. Carnero-Pardo is the creator of Fototest and Eurotest. The remaining authors have no conflicts of interest to declare.
Please cite this article as: Olazarán J, Hoyos-Alonso MC, del Ser T, Garrido Barral A, Conde-Sala JL, Bermejo-Pareja F, et al. Aplicación práctica de los test cognitivos breves. Neurología. 2016;31:183–194.