Epigenetics is the study of heritable modifications in gene expression that do not change the DNA nucleotide sequence. Some of the most thoroughly studied epigenetic mechanisms at present are DNA methylation, post-transcriptional modifications of histones, and the effect of non-coding RNA molecules. Gene expression is regulated by means of these mechanisms and disruption of these molecular pathways may elicit development of diseases.
DevelopmentWe describe the main epigenetic regulatory mechanisms and review the most recent literature about epigenetic mechanisms and how those mechanisms are involved in different epileptic syndromes.
ConclusionIdentifying the epigenetic mechanisms involved in epilepsy is a promising line of research that will deliver more in-depth knowledge of epilepsy pathophysiology and treatments.
La epigenética es el estudio de los cambios heredables en el ADN sin afectar a las secuencia de nucleótidos. Entre los mecanismos de regulación epigenética, los más estudiados y conocidos hasta la fecha son la metilación del ADN, la modificación de las histonas y los ARN no codificantes. Mediante estos mecanismos se regula la expresividad génica y la alteración de los mismos puede llevar al desarrollo de patologías.
DesarrolloDescribimos los principales mecanismos de regulación epigenética y realizamos una revisión de la bibliografía reciente sobre los mecanismos de regulación epigenética y su implicación en distintos síndromes epilépticos.
ConclusiónLa identificación de los mecanismos epigenéticos implicados en la epilepsia constituye una prometedora vía de investigación para profundizar en el conocimiento de la fisiopatología y terapéutica de esta enfermedad.
The term ‘epigenetics’ is attributed to Conrad Waddington, who in 1942 defined it as ‘the branch of biology that studies the causal interactions between genes and their products, which bring the phenotype into being’.1 Today, this area of study is expanding rapidly and delivering results that will hold medical relevance.
In simple terms, we can define epigenetics as the study of inheritance of heritable changes in gene expression that occur with no modifications to the DNA sequence.2 Epigenetic processes may therefore be understood as gene expression regulating mechanisms that determine not only expression or silencing but also where, when, and how intensely genes are expressed.
Evidence shows that these epigenetic processes may be modified by physical, chemical, nutritional, and even psychosocial factors. As such, our environment and life habits are able to modify genetic expression through epigenetic mechanisms.3,4
Epigenetics may help us answer questions that have yet to be fully answered: for example, how is it that 2 individuals with the same genetic load –monozygotic twins, for example– may demonstrate differences in appearance, behaviour, and illness?5 And how can the environment affect the genome?
Epigenetic regulatory mechanisms in epilepsyBasic epigenetic mechanismsSeveral epigenetic mechanisms are now known. Although novel mechanisms are being discovered, along with new effects produced by known mechanisms, three main types have received the most study: DNA methylation, histone modification, and the action of non-coding RNA.
DNA methylationDNA methylation is the most thoroughly studied epigenetic mechanism. It consists of the addition of a methyl group to the carbon 5 of those cytosine molecules that are followed by guanine (CpG dinucleotides). These molecules are not distributed in the genome in a uniform manner; rather, they appear in clusters called CpG islands, which are primarily located in gene promoters. CpG islands are not usually methylated, but methylation of these clusters is required by some physiological processes, such as genomic imprinting or X-inactivation in females. In contrast, the other CpG dinucleotides that are not included in CpG islands are methylated and mainly located in repetitive sequences or near the centromere. Methylation is carried out by DNA methyltransferases (DNMT) that catalyse the transfer of a methyl group from S-adenosyl-l-methionine to carbon 5 of cytosine. Five types are known in mammals: DNMT1, DNMT2, DNMT3A, DNMT3B, and DNMT3L.6 Maintaining correct metabolism of vitamin B12, folic acid, and homocysteine is of vital importance to this process.
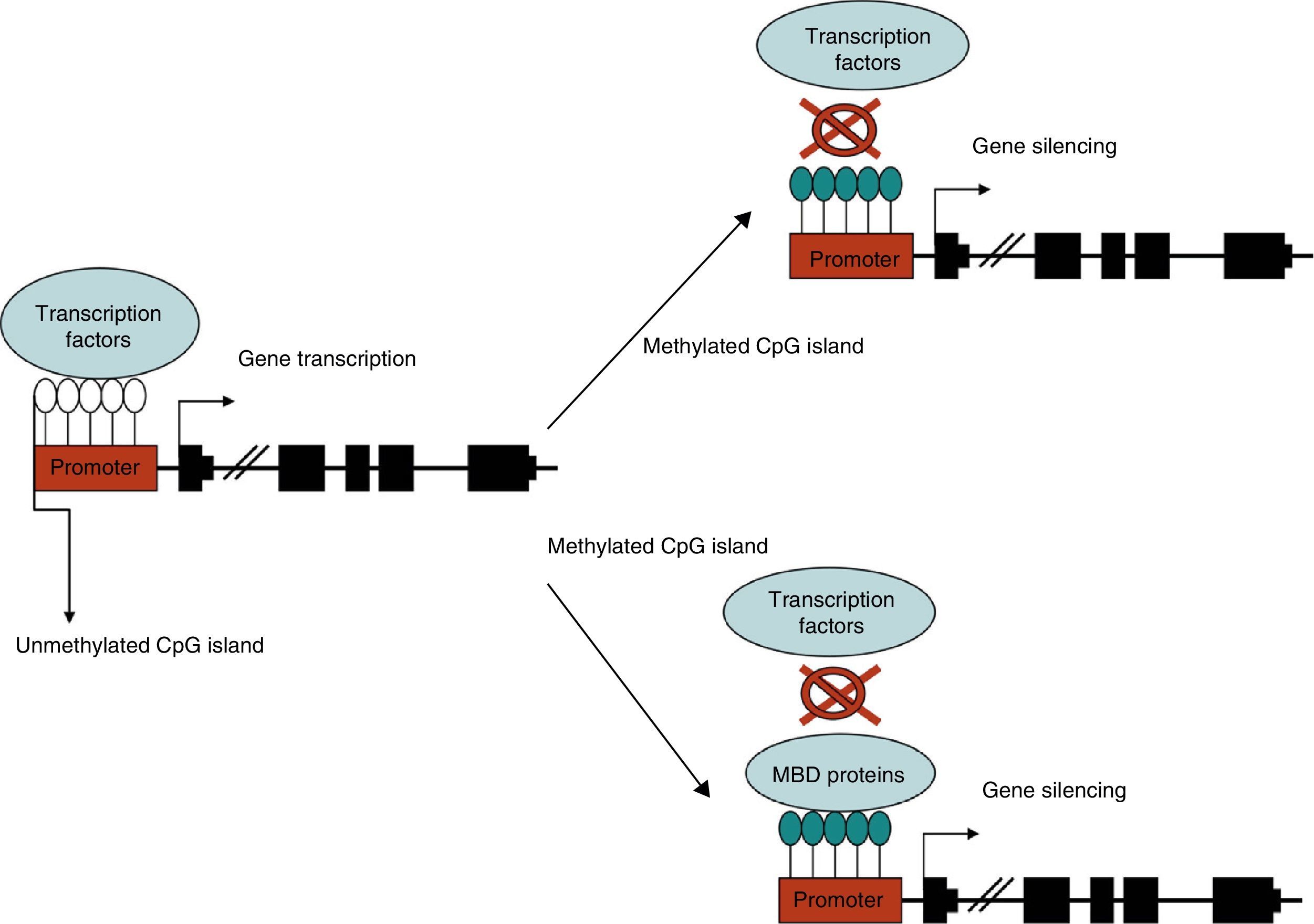
Methylation of the gene promoter is generally associated with transcription inhibition, otherwise known as gene silencing. Methylation inhibits transcription through 2 basic mechanisms. The first impedes binding of transcription regulatory factors whose recognition sites contain CpG. The second mechanism involves protein complexes that specifically bind to methylated CpG sites and indirectly prevent transcription factor binding by limiting the access of regulatory elements.6 These protein complexes are known as methyl-CpG binding domains (MBDs). There are 5 families in mammals: MeCP2, MBD1, MBD2, MBD3, and MBD4. In this way, DNA methylation indirectly regulates the structure of chromatin and DNA accessibility for transcription factors (Fig. 1).7
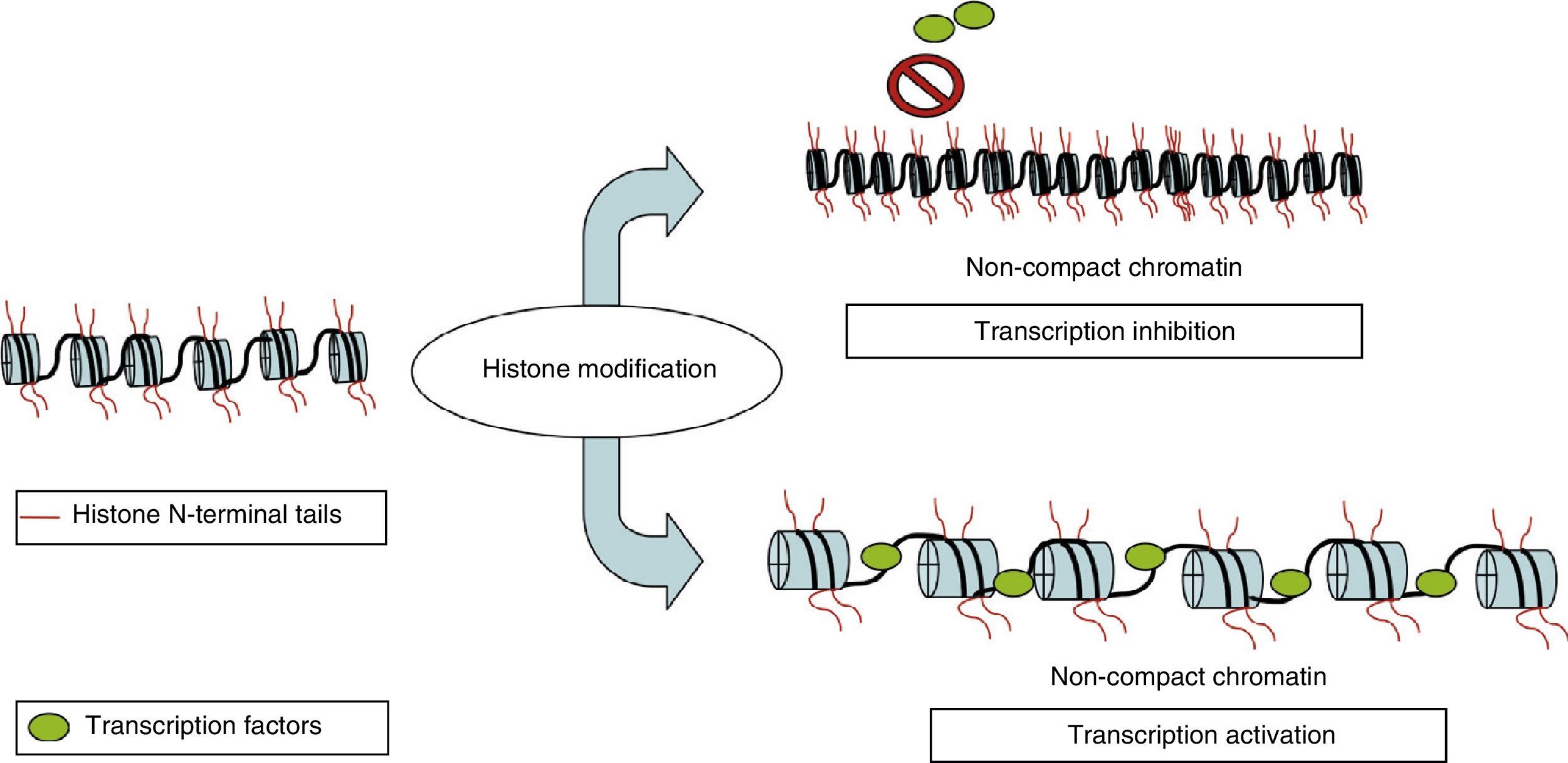
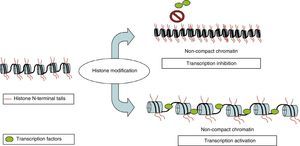
Histone modificationNuclear DNA is associated with a multiprotein complex that forms chromatin. The nucleosome is the basic structural unit of chromatin. It consists of 146 base pairs of DNA wound around 8 proteins called histones (a H3/H4 tetramer, 2 H2A and H2B dimers, and a H1 molecule). In addition to providing structural support, histones regulate accessibility for transcription factors, thereby determining gene expression. This occurs through epigenetic modifications that take place in each of the N-terminus tails that extend beyond the nucleosome, mainly in the N-terminals of H3 and H4. These modifications include methylation, acetylation, phosphorylation, ubiquitination, and ADP-ribosylation.8 The best-studied modification is the acetylation of lysine residues. The state of acetylation of H3 and H4 histones increases gene expression by promoting the open configuration of chromatin; the hypoacetylation state is typical of transcriptionally inactive areas of the genome (Fig. 2).9 This process is carried out by histone acetyltransferase and reversed by histone deacetylases (HDAC). Another modification that takes place is methylation, a process that occurs due to the activity of methyltransferase and by histone demethyltransferase. The effect of this modification depends on the type of modified residue and the degree of methylation.7 All of these modifications constitute an array of information, the histone code, which mediates the state of chromatin, thereby determining gene expression.
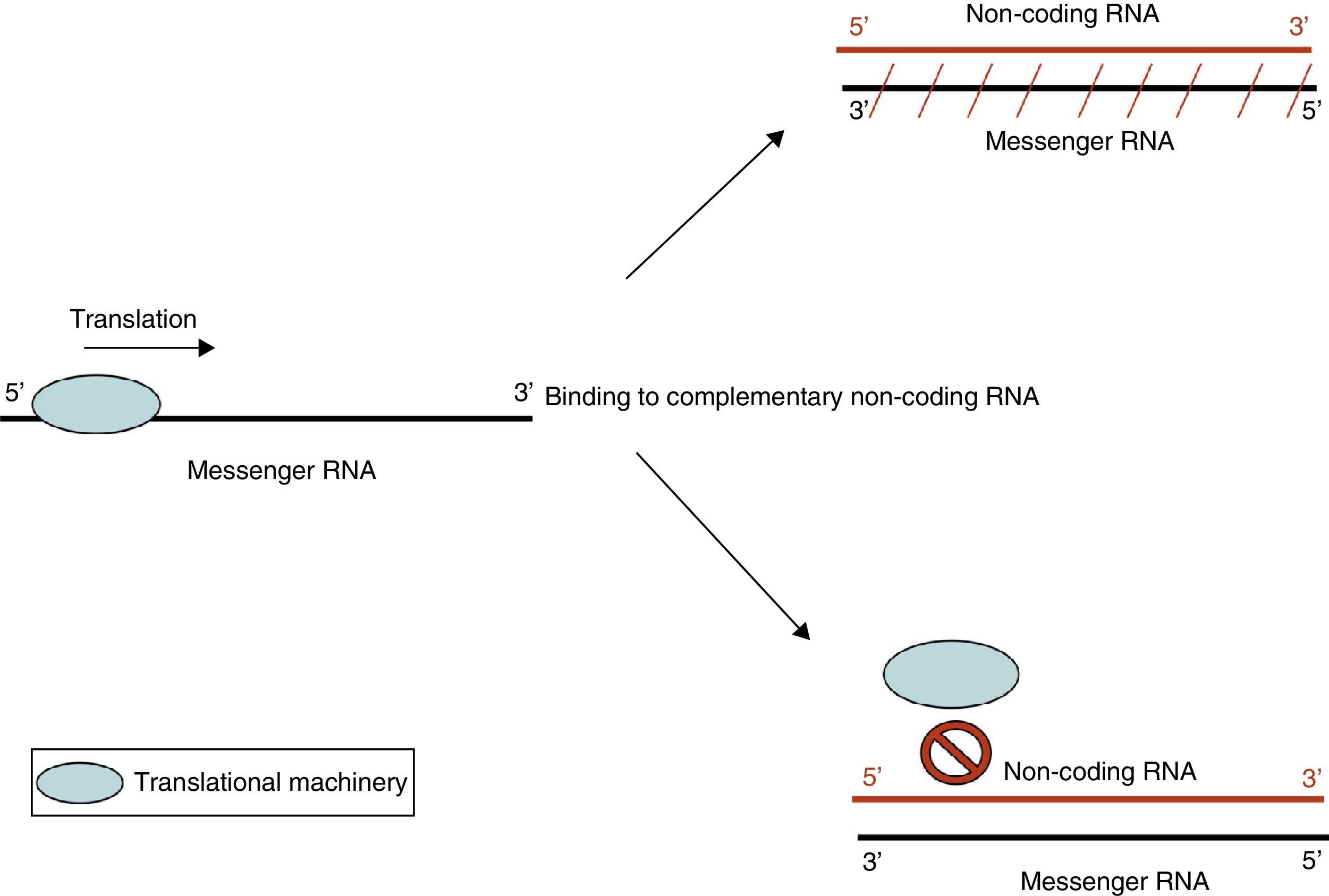
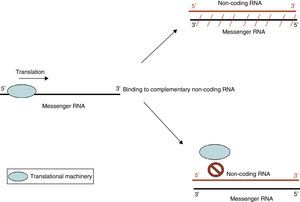
Action by non-coding RNANon-coding RNA refers to small endogenous RNA molecules that do not code for proteins. Micro-RNA (miRNA) is a type of non-coding RNA (ncRNA) that participates in the post-transcriptional regulation of gene expression.10 miRNA acts by 2 main mechanisms: degradation of target messenger RNA (mRNA) or by preventing translation (Fig. 3). Activation of one mechanism or the other depends on target mRNA. It is believed that each miRNA species may have more than one target, and in turn, each mRNA species may be regulated by more than one miRNA. In addition to miRNA, other types of ncRNA have been described. Their exact function is not yet known, but they too may be involved in epigenetic regulatory mechanisms.
This academic classification of the main mechanisms of epigenetic regulation that have been identified to date does not reflect the true complexity of epigenetic regulation. As may be expected, these events are not isolated, but instead interact with and affect each other. In summary, epigenetic regulatory mechanisms act in a coordinated way and play a key role in an organism's normal development and function.
Relevance of studying epigenetics in different diseasesEpigenetic regulation is of the utmost importance to an individual's proper development and function. Modifications in regulatory mechanisms allow the body to adapt to fluctuating situations; as a result, changes in these regulatory responses may lead to the development of different diseases.
The first data to show a link between alterations in epigenetic regulatory mechanisms and disease came from the field of oncology, specifically from a study of colorectal cancer. Here, researchers observed less methylation in patients with colorectal cancer than in healthy controls, such that hypomethylation resulted in abnormal gene activation. Similarly, they observed hypermethylation in several different tumour suppressor genes.11 This initial experience gave rise to a series of studies in other areas of medicine, such as neurodevelopmental anomalies and neurodegenerative diseases.12
Another important aspect of advances in epigenetics is the possibility of new treatments that will let doctors intervene directly in disease-associated epigenetic changes.13 Several of these treatments are currently being evaluated in clinical trials.
In summary, epigenetics is an expanding field that is expected to provide a deeper knowledge of numerous diseases and how to treat them.
Epigenetics and diseases that present with epilepsyEpilepsy is one of the most prevalent chronic neurological diseases and up to 30% of all patients do not respond well to pharmacological treatment.14 Epilepsy is clinically and pathophysiologically heterogeneous; despite advances in recent years consisting of identifying various genes responsible for epileptic syndromes, the cause of most types of epilepsy remains unclear. The study of epigenetic mechanisms in the field of epilepsy provides evidence of how changes in epigenetic regulation affect the susceptibility to and the development and maintenance of epilepsy.15–18 We will now describe some of this evidence, classified here into 3 groups for clarity.
Mutations in ‘epigenetic’ genes that cause epilepsyCertain neurological diseases and neurodevelopmental defects are caused by mutations in genes that code for the proteins involved in epigenetic regulation.7,12 These neurological disorders are characterised by intellectual disability that may be accompanied by other neurological manifestations, including epileptic seizures, autism-spectrum disorders, and psychomotor retardation.12
One of the best-known neurological disorders in this group is Rett syndrome, caused by mutations in the MECP2 gene, which codes for the MECP2 subfamily of MBP. This protein binds to specific methylated DNA sequences, resulting in suppression of gene expression. The mutation responsible for this syndrome was discovered in 1999 in the MECP2 gene, which is located on the Xq28 chromosome.19 The mutation therefore displays a sex-linked inheritance pattern and more than 99% of all cases are de novo mutations.
The course of this disease, which affects girls, consists of normal development for 6 to 18 months, followed by gradual loss of acquired abilities, deceleration of growth, language loss, and presentation of stereotyped movements (hand-wringing is especially characteristic). The disease is progressive and evolves to a stage with autistic features and cognitive decline. Other common features include epileptic seizures, hyperventilation, and apnoea.7 Epilepsy affects between 50% and 80% of these patients, and it is associated with greater clinical severity.20 The types of epileptic crises that may arise are varied (generalised tonic-clonic seizures, simple or complex partial seizures, atonic seizures, tonic seizures, photosensitive seizures). The same patient may present multiple types.21 There are no antiepileptic drugs specifically recommended in these cases; carbamazepine as monotherapy or in combination with clobazam is the most effective treatment.22
Changes in methylation patterns. Defects in genomic imprintingAs stated above, CpG dinucleotides located in promoter regions are normally non-methylated, although they are methylated in the physiological state in cases of X-chromosome inactivation in women, and in cases of genomic imprinting.7
Genomic imprinting is a highly important mechanism arising from the fact that specific chromosomal regions express an allele from only one of the progenitors, while the other allele is silenced by an epigenetic mechanism. Imprinting affects particular regions and not the entire genome. Imprinted genes tend to form clusters controlled by imprint control elements, which consist of regions with abundant CpG dinucleotides that are regulated by methylation of one of the alleles.23 Alterations in this process result in inappropriate gene expression that can disrupt normal development and give rise to some diseases.12
Angelman syndrome (AS) is one example of a neurodegenerative disease presenting with epilepsy that is caused by an imprinting abnormality. In 1987, Magenis et al. identified a deletion in chromosome 15q 11-13 in 2 patients with AS.24 Subsequent studies evidenced that AS may be caused by a variety of genetic mechanisms, including deletions, paternal uniparental disomy, or imprinting defects, all of which affect the expression of the maternal allele of the UBE3A gene. AS has a wide range of clinical symptoms including intellectual delay, potentially severe language impairment, ataxia, stereotyped behaviours (hand-flapping while walking), epileptic seizures, facial dysmorphia, and behaviour disorders including an unusually happy disposition with unmotivated or easily provoked laughter.25 The first signs of the syndrome in the form of mild delayed psychomotor development appear between the ages of 6 and 12 months. However, since these children do not display major losses of ability, they are usually diagnosed between the ages of 3 and 7 years, when symptoms are more apparent.26 Epileptic seizures affect up to 80% of these cases.25 Typical onset occurs between 1 and 5 years of age and seizures often present as febrile convulsions. After that time, seizures are variable; the most common types are atypical absence seizures, myoclonic seizures, generalised tonic-clonic seizures, and atonic seizures. Controlling seizures are often difficult in adolescence. The most effective drug is valproic acid, which may be employed in monotherapy or in association with clonazepam or other benzodiazepines.27
Epigenetic changes in epilepsyMethylation alterationsEvidence from recent studies in animal models and tissues from epileptic patients has revealed altered methylation patterns in these patients compared to those in healthy controls.
For example, the autopsy study by Kobow et al.28 of hippocampus tissue from both controls and patients with temporal lobe epilepsy found a greater degree of methylation of the reelin promoter among the patients with epilepsy. Reelin is an extracellular matrix protein that performs key functions in neuronal migration and synaptic plasticity. It is also involved in proper maintenance of the laminar structure of hippocampal granule cells. It has been shown that loss of this structure in the hippocampal dentate nucleus (granule dispersion) is present in up to 50% of the patients affected by temporal lobe sclerosis. Earlier studies29 had already demonstrated the importance of reelin in granule dispersion in temporal lobe epilepsy, but Kobow et al.28 were the first to show that this was followed by altered epigenetic regulation.
Zhu et al.,30 who were also investigating temporal lobe epilepsy, examined DNMT1 and DNMT3A expression in patients with temporal lobe epilepsy compared to healthy controls. As described in the introductory sections, DNMTs are the enzymes that carry out methylation; DNMT1 is responsible for maintaining methylation patterns, whereas DNMT3 promotes de novo methylation during development. These researchers concluded that both DNMT types are more abundant in patients with temporal lobe epilepsy, a tendency which indicates that they contribute to the pathogenesis of this type of epilepsy.
A study recently published by Kobow's group31 analysed overall DNA methylation in the hippocampus of rats with chronic epilepsy and in controls; the group with chronic epilepsy showed more overall methylation. The group then evaluated the effect of administering a ketogenic diet and observed a reduction in seizure frequency and a change in the DNA methylation pattern. It should be noted that this is the first study to provide a genome-wide analysis of DNA methylation.
In another study of the rodent hippocampus (field C3), Miller-Delaney et al.32 found different methylation patterns in animals with status epilepticus and in those with epileptic tolerance. The latter group consisted of mice subjected to preconditioning using chemical or electrical stimuli prior to induction of status epilepticus. As a result, they showed tolerance to status epilepticus. Furthermore, these researchers found that some of the differences in the methylation profile are apparent in genes that had not previously been described in epilepsy.
Histone modificationStudies in animal models of epilepsy are beginning to yield evidence of changes in chromatin that are mediated by histone modifications and occur after epileptic seizure.
An early example of these studies was performed by Huang et al.33 This team analysed rat hippocampal tissue 3hours after having induced status epilepticus with pilocarpine and found histone H4 hypoacetylation (marker for gene repression) in the promoter of the glutamate 2 receptor (GluR2, a subunit of the AMPA receptor), as well as hyperacetylation (marker for gene transcription) in the brain-derived neurotrophic factor promoter. These findings clearly show that status epilepticus rapidly triggers modulations in histone acetylation. The same study found that prior administration of an HDAC inhibitor prevented hyperacetylation of the GluR2 promoter.
In a more recent study,34 the same author reported greater HDAC2 expression in tissue from patients with temporal lobe epilepsy, as well as from animal subjects with status epilepticus, than in controls. HDAC2, a type of HDAC expressed by the central nervous system, is active in neurodevelopment. It is also essential for cognitive function since it intervenes in the repression of genes associated with synaptic plasticity and creating memory.35 Results from the study show that HDAC2 is significantly involved in the pathogenesis of temporal lobe epilepsy, and in the cognitive impairment that may sometimes also be associated with this type of epilepsy.
In another animal model of epilepsy using electrically induced seizures, Tsankova et al.36 found changes in the acetylation of histones H3 and H4 at the CREB promoter region in the rat hippocampus. CREB is an important transcriptional factor; among other activities, it modulates the expression of the GABAa receptor in the hippocampus and plays an important role in the epileptogenic process.
The above studies illustrate the effect of epigenetic mechanisms, in the form of histone modifications, on the regulation of genes implicated in control of epileptiform activity.
Micro-RNA and epilepsySeveral studies of the expression profile of miRNA in epilepsy have been published recently, and they offer promising information about the potential role of miRNA as a biomarker.
One example, published by Liu et al.,37 describes the miRNA expression profile in rats with kainate-induced status epilepticus based on analyses of brain tissue and blood samples. The authors found similar expression profiles for one miRNA subtype in blood and hippocampal tissue and therefore support the possibility that these molecules might serve as blood biomarkers.
On the other hand, studies of miRNA are also providing additional knowledge about the epileptogenic process. For example, 3 recent studies carried out in animal models38–40 have all shown increased expression of miRNA-132 in the hippocampus of rats with induced status epilepticus. It is understood that miRNA-132 has anti-inflammatory functions, and inflammation has been shown to play a role in epileptogenesis. Therefore, increased miRNA-132 may contribute to the development of epilepsy.
ConclusionEpigenetics and its relationship with human diseases constitute an emerging line of study that may contribute greatly to our knowledge of pathophysiology and treatment of common illnesses.
Studies of epigenetic modifications in the field of epilepsy, both in animal models and using human tissue specimens, are providing encouraging results that build on existing knowledge of epileptogenesis (Table 1). Furthermore, detection of epigenetic modifications observed in both brain and blood provides a means of using these modifications as epigenetic markers that may refine the diagnosis, prognosis, and treatment of different epileptic syndromes.
Study of epigenetic modifications in the field of epilepsy
| Epilepsy model | Tissue studied | Epigenetic mechanism | References |
|---|---|---|---|
| Temporal lobe epilepsy in humans | Hippocampus | Hypermethylation of reelin promoter | Kobow et al.28 |
| Temporal lobe epilepsy in humans | Temporal cortex | Alterations of DNMT 1 and 3A expression | Zhu et al.30 |
| Rats. Pilocarpine-induced seizures | Hippocampus | Different overall methylation profile | Kobow et al.31 |
| Rats. Kainate-induced status epilepticus | Hippocampus | Different overall methylation profile | Miller-Delaney et al.32 |
| Mice. Pilocarpine-induced seizures | Hippocampus | H4 hypoacetylation of GluR2H4 hyperacetylation of BDNF | Huang et al.33 |
| Rats. Pilocarpine-induced seizures Temporal lobe epilepsy in humans | Temporal cortex | Altered HDAC2 expression | Huang et al.34 |
| Rats. Electrically induced seizures | Hippocampus | H4 hypoacetylation of CREBH3 hyperacetylation of CREB | Tsankova et al.36 |
| Rats. Kainate-induced seizure | Hippocampus, peripheral blood | miRNA | Liu et al.37 |
| Rats. Status epilepticus | Hippocampus | Increased miRNA 132 expression | Hu et al.38; Jimenez-Mateos et al.39; Song et al.40 |
These advances contribute greatly to research aimed at finding new ways of managing a neurological disorder which continues to take a heavy toll on healthcare resources, patients and families, and society at large.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pulido Fontes L, Quesada Jimenez P, Mendioroz Iriarte M. Epigenética y epilepsia. Neurología. 2015;30:111–8.