To update the ad hoc Committee of the Cerebrovascular Diseases Study Group of The Spanish Neurological Society guidelines on prevention of ischaemic stroke (IS) and transient ischaemic attack (TIA).
MethodsWe reviewed available evidence on risk factors and means of modifying them to prevent IS and TIA. Levels of evidence and recommendation grades are based on the classification of the Centre for Evidence-Based Medicine.
ResultsThis first section summarises the recommendations for action on the following factors: blood pressure, diabetes, lipids, tobacco and alcohol consumption, diet and physical activity, cardio-embolic diseases, asymptomatic carotid stenosis, hormone replacement therapy (HRT) and contraceptives, hyperhomocysteinemia, prothrombotic states and sleep apnea syndrome.
ConclusionsChanges in lifestyle and pharmacological treatment for hypertension, diabetes mellitus and dyslipidemia, according to criteria of primary and secondary prevention, are recommended for preventing IS.
Actualizar las guías terapéuticas del Comité ad hoc del Grupo de Estudio de Enfermedades Cerebrovasculares de la SEN en el tratamiento preventivo de ictus isquémico (II) y ataque isquémico transitorio (AIT).
MétodosRevisión de evidencias disponibles sobre los factores de riesgo y la oportunidad de su modificación para prevenir el ictus isquémico y AIT. Los niveles de evidencia y grados de recomendación se han basado en la clasificación del Centro de Medicina Basada en la Evidencia.
ResultadosEn esta primera parte se resumen las recomendaciones sobre la actuación sobre los siguientes factores: presión arterial, DM, lípidos plasmáticos, consumo de tabaco y alcohol, dieta y actividad física, cardiopatías embolígenas, estenosis carotídea asintomática, terapia hormonal sustitutiva y anticonceptivos, hiperhomocisteinemía, estados protrombóticos y síndrome de apnea del sueño.
ConclusionesLa modificación de los estilos de vida y el tratamiento farmacológico de la hipertensión arterial, diabetes méllitus y dislipemia según criterios de prevención primaria y secundaria se recomiendan en la prevención de ictus isquémico.
In Spain, cerebrovascular diseases are the leading cause of death in women, the third most frequent in men,1 and the most common cause of disability. The measures we describe here are designed to prevent both first-ever ischaemic strokes (IS) and future episodes in patients who have already suffered a stroke. We will also list measures intended to reduce overall vascular risk in these patients2; given the length of this chapter, we decided to publish it in 2 parts. Firstly, we will review the risk factors for IS and any modifications to those factors which may be appropriate. Secondly, we will specify different preventive treatments according to the stroke subtype. Recommendations for each section are shown in tables for better readability. Levels of evidence and recommendation grades are based on the classification system proposed by the Centre for Evidence Based Medicine at the University of Oxford3 (Table 1). Following current criteria, we included transient ischaemic attack (TIA) in the definition of IS, which also improves readability. Therefore, all recommendations included in these clinical practice guidelines are directed at preventing focal cerebral ischaemia in general, without distinguishing between cerebral infarction and TIA.
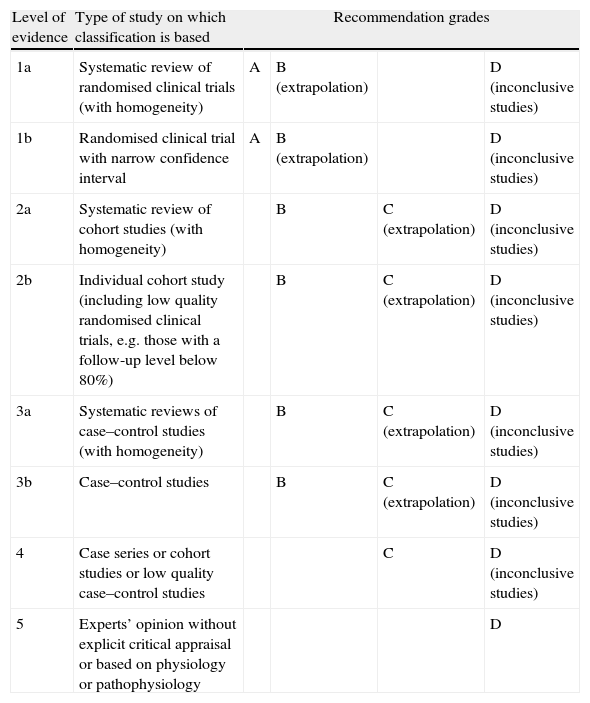
Levels of evidence and recommendation grades. Based on the classification drawn up by the Centre for Evidence Based Medicine (CEBM).1
| Level of evidence | Type of study on which classification is based | Recommendation grades | |||
| 1a | Systematic review of randomised clinical trials (with homogeneity) | A | B (extrapolation) | D (inconclusive studies) | |
| 1b | Randomised clinical trial with narrow confidence interval | A | B (extrapolation) | D (inconclusive studies) | |
| 2a | Systematic review of cohort studies (with homogeneity) | B | C (extrapolation) | D (inconclusive studies) | |
| 2b | Individual cohort study (including low quality randomised clinical trials, e.g. those with a follow-up level below 80%) | B | C (extrapolation) | D (inconclusive studies) | |
| 3a | Systematic reviews of case–control studies (with homogeneity) | B | C (extrapolation) | D (inconclusive studies) | |
| 3b | Case–control studies | B | C (extrapolation) | D (inconclusive studies) | |
| 4 | Case series or cohort studies or low quality case–control studies | C | D (inconclusive studies) | ||
| 5 | Experts’ opinion without explicit critical appraisal or based on physiology or pathophysiology | D | |||
Epidemiological studies and clinical trials have illustrated the relationship between blood pressure and stroke.4–8 Lifestyle changes (decreasing tobacco and alcohol consumption, losing weight, engaging in moderate physical activity, decreasing salt consumption, and increasing fruit and vegetable intake) are useful for reducing blood pressure levels.9 It has been shown that reduction in risk of stroke is proportional to a patient's decrease in blood pressure.10,11 Studies of different antihypertensive drugs present similar results with regard to reduction in vascular episodes. The most recent Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure indicates which levels of blood pressure are considered high and which treatments are the most appropriate in each situation.7
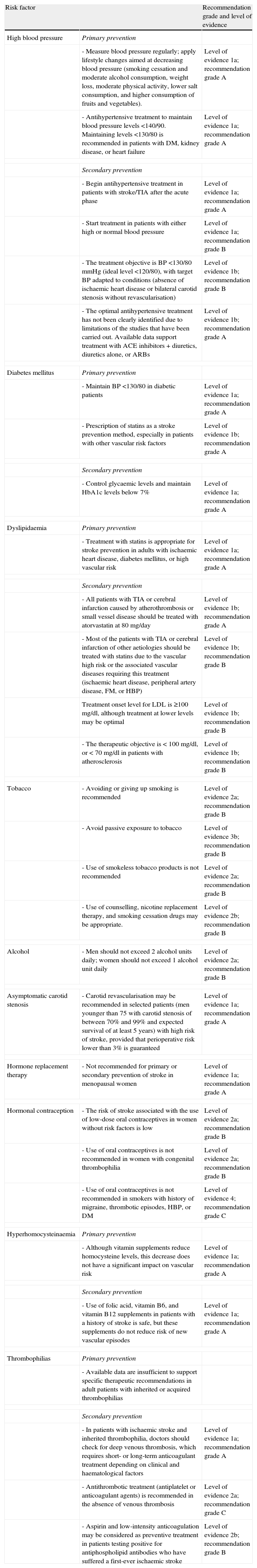
Modification of risk factors for stroke. Levels of evidence and recommendation grades.
| Risk factor | Recommendation grade and level of evidence | |
| High blood pressure | Primary prevention | |
| - Measure blood pressure regularly; apply lifestyle changes aimed at decreasing blood pressure (smoking cessation and moderate alcohol consumption, weight loss, moderate physical activity, lower salt consumption, and higher consumption of fruits and vegetables). | Level of evidence 1a; recommendation grade A | |
| - Antihypertensive treatment to maintain blood pressure levels <140/90. Maintaining levels <130/80 is recommended in patients with DM, kidney disease, or heart failure | Level of evidence 1a; recommendation grade A | |
| Secondary prevention | ||
| - Begin antihypertensive treatment in patients with stroke/TIA after the acute phase | Level of evidence 1a; recommendation grade A | |
| - Start treatment in patients with either high or normal blood pressure | Level of evidence 1a; recommendation grade B | |
| - The treatment objective is BP <130/80mmHg (ideal level <120/80), with target BP adapted to conditions (absence of ischaemic heart disease or bilateral carotid stenosis without revascularisation) | Level of evidence 1b; recommendation grade B | |
| - The optimal antihypertensive treatment has not been clearly identified due to limitations of the studies that have been carried out. Available data support treatment with ACE inhibitors+diuretics, diuretics alone, or ARBs | Level of evidence 1b; recommendation grade A | |
| Diabetes mellitus | Primary prevention | |
| - Maintain BP <130/80 in diabetic patients | Level of evidence 1a; recommendation grade A | |
| - Prescription of statins as a stroke prevention method, especially in patients with other vascular risk factors | Level of evidence 1b; recommendation grade A | |
| Secondary prevention | ||
| - Control glycaemic levels and maintain HbA1c levels below 7% | Level of evidence 1a; recommendation grade A | |
| Dyslipidaemia | Primary prevention | |
| - Treatment with statins is appropriate for stroke prevention in adults with ischaemic heart disease, diabetes mellitus, or high vascular risk | Level of evidence 1a; recommendation grade A | |
| Secondary prevention | ||
| - All patients with TIA or cerebral infarction caused by atherothrombosis or small vessel disease should be treated with atorvastatin at 80mg/day | Level of evidence 1b; recommendation grade A | |
| - Most of the patients with TIA or cerebral infarction of other aetiologies should be treated with statins due to the vascular high risk or the associated vascular diseases requiring this treatment (ischaemic heart disease, peripheral artery disease, FM, or HBP) | Level of evidence 1b; recommendation grade B | |
| Treatment onset level for LDL is ≥100mg/dl, although treatment at lower levels may be optimal | Level of evidence 1b; recommendation grade B | |
| - The therapeutic objective is < 100mg/dl, or < 70mg/dl in patients with atherosclerosis | Level of evidence 1b; recommendation grade B | |
| Tobacco | - Avoiding or giving up smoking is recommended | Level of evidence 2a; recommendation grade B |
| - Avoid passive exposure to tobacco | Level of evidence 3b; recommendation grade B | |
| - Use of smokeless tobacco products is not recommended | Level of evidence 2a; recommendation grade B | |
| - Use of counselling, nicotine replacement therapy, and smoking cessation drugs may be appropriate. | Level of evidence 2b; recommendation grade B | |
| Alcohol | - Men should not exceed 2 alcohol units daily; women should not exceed 1 alcohol unit daily | Level of evidence 2a; recommendation grade B |
| Asymptomatic carotid stenosis | - Carotid revascularisation may be recommended in selected patients (men younger than 75 with carotid stenosis of between 70% and 99% and expected survival of at least 5 years) with high risk of stroke, provided that perioperative risk lower than 3% is guaranteed | Level of evidence 1a; recommendation grade A |
| Hormone replacement therapy | - Not recommended for primary or secondary prevention of stroke in menopausal women | Level of evidence 1a; recommendation grade A |
| Hormonal contraception | - The risk of stroke associated with the use of low-dose oral contraceptives in women without risk factors is low | Level of evidence 2a; recommendation grade B |
| - Use of oral contraceptives is not recommended in women with congenital thrombophilia | Level of evidence 2a; recommendation grade B | |
| - Use of oral contraceptives is not recommended in smokers with history of migraine, thrombotic episodes, HBP, or DM | Level of evidence 4; recommendation grade C | |
| Hyperhomocysteinaemia | Primary prevention | |
| - Although vitamin supplements reduce homocysteine levels, this decrease does not have a significant impact on vascular risk | Level of evidence 1a; recommendation grade A | |
| Secondary prevention | ||
| - Use of folic acid, vitamin B6, and vitamin B12 supplements in patients with a history of stroke is safe, but these supplements do not reduce risk of new vascular episodes | Level of evidence 1a; recommendation grade A | |
| Thrombophilias | Primary prevention | |
| - Available data are insufficient to support specific therapeutic recommendations in adult patients with inherited or acquired thrombophilias | ||
| Secondary prevention | ||
| - In patients with ischaemic stroke and inherited thrombophilia, doctors should check for deep venous thrombosis, which requires short- or long-term anticoagulant treatment depending on clinical and haematological factors | Level of evidence 1a; recommendation grade A | |
| - Antithrombotic treatment (antiplatelet or anticoagulant agents) is recommended in the absence of venous thrombosis | Level of evidence 2a; recommendation grade C | |
| - Aspirin and low-intensity anticoagulation may be considered as preventive treatment in patients testing positive for antiphospholipid antibodies who have suffered a first-ever ischaemic stroke | Level of evidence 2b; recommendation grade B | |
BP, blood pressure.
Studies of different antihypertensive agents used in secondary IS prevention have yielded inconsistent results.12 Beta blockers have not been shown to deliver significant benefits.13–15 Results from diuretics have been contradictory16; a single study of indapamide at 2.5mg/day pointed to a positive effect.17 Using ACE inhibitors, such as ramipril at 10mg/day18 or combination therapy with perindopril (4mg/day) and indapamide (2.5mg/day), decreases the risk of stroke recurrence, even in patients without a history of HBP.19 Regarding results from ARBs, trials have shown that eprosartan performed better than nitrendipine20; telmisartan performed no better than a placebo21 and similarly to ramipril22; and combination therapy with telmisartan and ramipril was associated with a higher frequency of side effects and no significant benefits.22 Major vascular events decreased by an additional 15% with intensive antihypertensive treatments, regardless of the drug employed.23 Some studies show differing levels of IS risk reduction for different classes of antihypertensive drugs. This could be due to the fact that some antihypertensive drugs, such as ACE inhibitors or ARBs, may have an added pleiotropic effect as well as an antihypertensive effect.12,24,25
Diabetes mellitusPatients with DM are more susceptible to developing arteriosclerosis and display a higher prevalence of HBP, obesity, and dyslipidaemia. The risk entailed by DM is considered equal to that entailed by coronary disease, which is why secondary prevention measures must be applied in these cases.26 The risk of IS is 2 to 6 times higher in diabetic patients,27 and 9.1% of recurrences are directly attributable to DM.28 Adjusting glycaemic control and normalising HbA1c are more effective for reducing the complications of microangiopathy, whereas the approach to stroke prevention should be more global and include strict blood pressure control29 and administration of statins.30 Results from studies of close glycaemic control are controversial, with the ACCORD study31 showing a possible negative effect on mortality rates. The ADVANCE study32 and Veterans Affairs Diabetes Trial33 showed that glycaemic control did not reduce risk of IS.33 Long-term follow-up in the DCCT and UKPDS trials suggests that maintaining HbA1c levels under or near 7% at the onset of DM is associated with a long-term reduction in macrovascular disease.34 In a subanalysis of the PROactive study,35 treatment with pioglitazone in patients with prior stroke was associated with a 47% decrease in risk of recurrence.36 Based on these results, it has been recommended for preventing secondary stroke in patients with DM.37 Nevertheless, the safety of glitazone treatment has recently been called into question.38 It is therefore advisable to wait for the results of the Insulin Resistance Intervention after Stroke (IRIS) study.39
Plasma lipids and lipid-lowering drugsHigh levels of cholesterol increase risk of IS. The Asia-Pacific Cohort Studies Collaboration found a 25% higher risk of IS for each mmol/L (38.7mg/dL) increase in total cholesterol levels.40 The US Women's Pooling Project detected a similar risk increase in women aged 30 to 54.41 Several primary prevention studies have shown that statins are effective for reducing cholesterol levels and risk of stroke (by 27%–32%).42,43 Treatment with rosuvastatin decreases the risk of IS,44 even in patients with high levels of high-sensitivity C-reactive protein and no hyperlipidaemia. Earlier guidelines already recommended statin use for secondary prevention of stroke or TIA in most patients based on NCEP-ATP III criteria.45 According to the SPARCL study, 80mg/day of atorvastatin significantly decreases the risk of recurrence in patients with non-cardioembolic stroke or TIA, and those with other vascular complications such as ischaemic heart disease or revascularisation procedures.46 We detected an increase in haemorrhagic stroke frequency in the atorvastatin group, especially in cases with a history of haemorrhagic stroke at time of inclusion, men, elderly subjects, and patients with stage 2 HBP, without significant interactions in baseline and recent LDL cholesterol levels.47 Another exploratory study showed that decreases ≥50% with respect to baseline figures achieve the greatest reductions in risk of stroke and major coronary episodes without risk of cerebral haemorrhage.48 A meta-analysis of randomised clinical trials suggested that fibrates may play a role in patients with DM.49
TobaccoTobacco consumption is associated with increased risk of all stroke subtypes, especially atherothrombotic stroke50–55 and in young patients.56 Additionally, it has a synergistic effect through its link to other vascular risk factors, such as HBP, DM, use of oral contraceptives, or physical inactivity. Smoking cessation reduces the risk of stroke, coronary disease, peripheral vascular disease and death from vascular causes proportionally to the length of the period during which the subject stopped using tobacco.57–60 Passive smoking also entails a risk of cerebrovascular disease.61–63 Smokeless tobacco products are associated with increased risk of fatal stroke as well.64
AlcoholRobust evidence shows that excessive consumption of alcohol is a risk factor for all types of stroke.65–70 However, whether or not light-to-moderate alcohol consumption is related to ischaemic or haemorrhagic stroke is a matter of debate.71,72 Risk of stroke with light (<12g/day) or moderate (between 12 and 24g/day) alcohol consumption is less than risk of stroke with total abstinence from alcohol. High levels of alcohol consumption (>60g/day) increases total risk of stroke and risk of both ischaemic and haemorrhagic stroke.69 We found a J-shaped relationship between the risk of coronary morbidity and mortality73 and IS,68–70 as well as a linear association for the risk of haemorrhagic stroke.65–67 Moderate red wine consumption is associated with lower vascular risk.73 There are not enough studies to establish a relationship between alcohol consumption and recurrent stroke.
Diet and physical activityIt has been shown that dietary habits are related to cerebrovascular risk.74–76 Low intakes of salt77 and fat78 along with regular consumption of fish,79 pulses, fibre, fruits, and vegetables80–82 are associated with lower vascular mortality rates and a significant decrease in risk of cerebrovascular diseases. Sedentary lifestyle is very prevalent in the general population.83 It is associated with cerebrovascular diseases84 and related to other factors such as HBP, hypercholesterolaemia, obesity, and DM.85,86 Physical activity increases HDL cholesterol, reduces LDL cholesterol and triglycerides, decreases blood pressure, fosters insulin homeostasis, helps in losing and maintaining weight, promotes good mental health, and is helpful in quitting smoking.86–90 Individuals who are physically active have a lower risk of ischaemic heart disease and stroke.84,87,91 This relationship is independent of age and sex, but it should be noted that data from patients older than 80 are limited.92 The benefits of exercise can be observed whether the activity is undertaken during work or leisure time.84 The general recommendation is at least 30minutes of moderate-intensity exercise, most days of the week.93
Asymptomatic carotid stenosisPrevalence of asymptomatic carotid artery stenosis (≥50%) increases with age. The prevalence is 0.5% in individuals younger than 50 years and higher than 10% in men older than 80 years.94 After a 15-year follow-up period, 16.6% of patients may suffer an episode of stroke.95 There are 2 alternative strategies: medical treatment or revascularisation using an endarterectomy or endovascular treatment with angioplasty and stenting. Initial studies of endarterectomy96,97 indicated that this treatment was beneficial, since it reduced risk of stroke after 5 years of follow-up. Nevertheless, new developments in medical treatment have raised questions about the benefit of surgical techniques, which deliver a decrease in absolute risk of stroke of only 1% per year, according to several meta-analyses.98–100 According to the CREST study,101 there is a 2.5% risk of suffering periprocedural stroke during an angioplasty and a 1.4% risk during endarterectomy. Therefore, indiscriminate use of revascularisation treatment in asymptomatic patients does not seem justified provided that they receive appropriate medical treatment. Revascularisation is only recommended in selected cases according to factors such as age, comorbidity, life expectancy, and perioperative risk.102 Based on the above, revascularisation can be considered for 70% to 99% carotid stenosis in men younger than 75, provided that perioperative risk is <3% and expected post-operative survival is at least 5 years.37,103 Of all patients with asymptomatic carotid stenosis, those with the highest level of risk have a history of contralateral TIAs,104 silent cerebral infarcts ipsilateral to stenosis,105 stenosis progression,106 or microemboli detected using transcranial Doppler.107 The SPACE 2 trial will compare results from current medical treatment with anti-platelet and antihypertensive drugs and statins with results from endovascular therapy or open surgical revascularisation.
Heart diseaseHeart disease is the second leading cause of IS. In addition, patients with cardioembolic stroke present a higher risk of death and of new vascular events over the long term than patients with IS of arterial origin (non-cardioembolic).108 Findings regarding risk of IS for different types of emboligenic heart disease are listed below. The second part of this guide will list specific recommendations for preventing IS.
Atrial fibrillationAtrial fibrillation (AF) is the most frequent type of arrhythmia109 with a prevalence of 6% in patients older than 65. In patients older than 85, prevalence rises to 12%.110 AF is one of the main risk factors for stroke. It is estimated that 1 out of 6 strokes occur in patients with AF,111 which is the cause of nearly half of all cardioembolic ISs.112 AF is associated with an increase in IS risk by a factor of 3 to 4 after adjusting for other risk factors.113 In cases with associated rheumatic heart disease, risk of stroke is 17 times higher than in healthy controls.114 Several factors significantly increase risk of embolism: age >75 years, congestive heart failure (CHF), HBP, DM, history of thromboembolism, left ventricular dysfunction with left ventricular ejection fraction (LVEF) <35%, atrial size, presence of mitral annular calcification (MAC), presence of spontaneous echo contrast, and detection of a left atrial thrombus.39 Based on the above, researchers have designed IS risk stratification systems for patients with AF.115 The CHADS2 score116,117 is the most well-known and validated risk classification method. It factors in CHF (1 point), HBP (1 point), DM (1 point), age >75 years (1 point), and history of cardiovascular disease or systemic embolism (2 points). The recently validated CHA2-DS2-VASc score118 divides the study population into 2 age groups: 65–74 (1 point) and >75 (2 points). It also accounts for the following factors: vascular disease (prior AMI, peripheral artery disease, and complex aortic plaque) (1 point); and female sex (1 point). All other scores were the same as for CHADS2.
Mitral stenosisBetween 10% and 20% of patients with mitral stenosis present systemic embolism. In cases of rheumatic stenosis (the most frequent cause) and a history of prior embolism, the level of risk reaches 30% to 65%. Moreover, up to 40% of patients with mitral stenosis develop AF.119 They therefore have a higher risk of stroke and a poorer prognosis than those who maintain sinus rhythm.120 Regarding patients with mitral stenosis and no AF or prior embolism, treatment is controversial since there are no data supporting the prescription of oral anticoagulant therapy. It is true that these patients may experience episodes of paroxysmal AF which are difficult to detect. At present, experts agree that oral anticoagulant drugs should be prescribed for patients with severe mitral stenosis and dilation of the left atrium ≥55mm or spontaneous contrast in the echocardiographic study.119 One observational study comparing medical treatment, percutaneous balloon valvuloplasty, and valve replacement suggests that balloon commissurotomy is the most effective method for reducing the incidence of IS.121 MAC is an infrequent non-rheumatic cause of mitral stenosis, and it is associated with distal complex aortic atheroma.122,123 The relationship between MAC and the risk of stroke is unclear; some cohort studies observe an increased risk of ischaemic heart disease and vascular death but no increase in IS,124 while other studies do observe a significant increase of IS risk after adjusting for other risk factors.125
Mitral valve prolapseMitral valve prolapse (MVP), the most frequent valve anomaly in adults, is present in 2.5% of population. It is particularly prevalent in individuals with connective tissue diseases. It is considered innocuous since different population-based observational studies have not detected a higher risk of stroke in subjects with MVP.126
Ischaemic heart diseaseAMI is associated with a 2% absolute risk of stroke during the first 30 days after the infarct. This is due to the presence of mural thrombi in the left ventricle (LV), most of which occur during the first 2 weeks following the previous AMI (12% incidence). The majority of these cases are characterised by extensive infarcts that reduce left ventricular function or provoke dyskinesia/apical akinesia, although AMIs at any location may cause thrombi to form. The incidence of early embolism is >22%, especially in cases of moving or protruding thrombi in the LV. It is estimated that 10% of these patients will suffer from IS if they are not treated. Risk increases in cases of left ventricular dilation, ventricular dyskinesia, ejection fraction <30%, or CHF.127–129
CardiomyopathyIn dilated cardiomyopathy and other conditions with decreased LVEF, such as CHF, there is an increased risk of emboli due to stasis of blood in the LV. This risk is relatively low (1%–3% yearly) in cases of depressed LVEF and echocardiographic signs of intramural thrombi.128 Stable CHF (NYHA Class II and Class III) is associated with an absolute risk of stroke of 1% per year. The relative risk increases by 18% when there is a 5% decrease in the LVEF. With more severe conditions (NYHA Class IV), risk of stroke is 4% per year.128 Approximately 10% of patients with IS have a LVEF of ≤30%.130
Anomalies of the interatrial septumPatent foramen ovale (PFO) is very prevalent in the general population (15%–25%), while atrial septal aneurysm (ASA) is less frequent (2.3%). The risk of stroke in patients with PFO is similar to that in the general population,131,132 but there seems to be a well-documented correlation between persistent PFO and cryptogenic stroke, especially in the younger population. This anomaly was detected in 35% to 50% of patients with cryptogenic IS vs only 4% to 10% of control subjects.133 According to the PFO-ASA study, risk of stroke recurrence in PFO patients treated with acetylsalicylic acid is low after 4 years of follow-up (2.3% vs 4.2% in patients without PFO), with a higher risk when PFO is associated with ASA.134 Subsequent studies did not detect any differences in risk of recurrence in patients with massive right-to-left shunt compared to patients without this anomaly.135 According to a recent meta-analysis, the risk of stroke recurrence in patients with PFO does not differ from that in patients with cryptogenic stroke and no PFO.136
Other risk factors for strokeHormone replacement therapy (HRT) and oral contraceptivesInitial observational studies with hormone replacement therapy137 along with subsequent published articles related to the Women's Health Initiative (WHI) showed that use of HRT favoured the onset of vascular episodes.138,139 Several meta-analyses have confirmed that HRT is associated with a 30% increase in risk of stroke.140,141 This risk is not age-related, and progesterone replacement in addition to oestrogen doubles the risk of venous thromboembolism.142 However, a systematic review showed that risk of stroke from use of low doses of oestrogens administered transdermally may be no higher than that associated with the oral route of administration. The review was unable to present any conclusions due to the presence of confounding factors.143 Other alternative therapies also entail cerebrovascular risks. For example, tibolone is associated with risk of stroke in postmenopausal women144,145 and raloxifene may increase risk of fatal stroke in at-risk women.146 HRT in postmenopausal women with prior stroke does not seem to present benefits and the treatment is associated with increased risk of stroke and stroke-related death.141,147–149
The relationship between oral contraceptives and risk of stroke is still a matter of debate. Recommendations are based on observational studies, since no randomised trials are available. Another systematic review found no association between oral contraceptives and stroke in cohort studies, while it did detect a significant increase in risk in case–control studies. This increase in risk was more evident for IS than for haemorrhagic stroke. Higher risk levels were observed in hospital-based populations than in community-based populations.150 A different systematic review showed increased risk of AMI or stroke associated with the use of first and second-generation oral contraceptives. The increase in risk was more evident for stroke.151 A systematic review showed that progesterone-only contraceptive use is not associated with an increased risk of stroke.152 Women older than 35 years who smoke and have HBP, DM, history of migraine, or history of thrombotic complications may be at increased risk.150,153–157 Certain congenital thrombophilias, such as Factor V Leiden, prothrombin G20210A, methylenetetrahydrofolate reductase (MTHFR) mutation, or hyperhomocysteinaemia, are associated with an increased risk of cerebral vein thrombosis.158,159
HyperhomocysteinaemiaA meta-analysis and various systematic reviews have confirmed the association between homocysteine levels and stroke.160,161 The high risk thymidine–thymidine (TT) genotype is associated with a 21% increase in risk of ischaemic heart disease and an increase in risk of stroke that is not statistically significant.162 Several studies have analysed the efficacy of folic acid or vitamin B supplements. Although vitamin supplements reduced homocysteine levels, this decrease did not have any significant effect on vascular risk.163–165 A single systematic review described an 18% decrease in risk of stroke in a group taking folic acid supplements with or without B complex vitamins compared to a control group. Researchers have observed this effect mainly in trials lasting more than 36 months in countries in which cereals are supplemented with folic acid.166 According to a meta-analysis, patients with history of vascular disease show similar risks of vascular disease, coronary disease, stroke, or death whether they receive folic acid supplements or a placebo.167 The VISP and VITATOPS studies have shown that folic acid, vitamin B6 and vitamin B12 supplements are safe in patients with prior stroke, but they do not reduce risk of new vascular episodes.163,168 Nevertheless, some authors point to a probable benefit in patients with markedly higher or lower homocysteine levels.169 A systematic review has confirmed that there is not enough evidence to determine whether or not treatments affecting homocysteine levels can prevent stroke recurrence.170
Prothrombotic statesInherited thrombophiliasObservational studies have not demonstrated a clear association between inherited thrombophilias and IS in adults.171–175 One review even concluded that routine testing for factor V Leiden, prothrombin G20210A, protein C, protein S, and antithrombin III in IS was unproductive.176 Nevertheless, doctors can detect a hypercoagulable state in more than 40% of young patients with IS.177 A systematic review in a paediatric population (<18 years) concluded that all the thrombophilias which it evaluated (antithrombin III deficiency, protein C deficiency, protein S deficiency, factor V Leiden, prothrombin G20210A, thermolabile MTHFR polymorphism, and antiphospholipid antibodies) were associated with increased risk of suffering new IS or cerebral venous thrombosis. Patients with multiple defects had higher levels of risk.178 Two meta-analyses have researched the potential relationship between stroke and prothrombotic disorders. The first meta-analysis found significant associations between stroke and factor V Leiden, MTHFR C677T variant, and factor II G20210A mutation.179 The risk of suffering stroke in the general population with these polymorphisms is low. The second meta-analysis studied the association between prothrombotic factors and arterial thrombotic episodes. Researchers could not verify a significant association between stroke and factor V Leiden; they did find slight associations between stroke and factor II G20210A mutation and MTHFR C677T mutation. These findings were more evident in young subjects (<55 years).180 The relationship between cerebral venous disease and thrombophilias has been clearly demonstrated. For example, a review showed a significant relationship with cerebral venous thrombosis in patients with factor V Leiden, factor II G20210A mutation and MTHFR C677T mutation.158 The risk of suffering cerebral thrombosis is high in patients who take oral contraceptives and have factor V Leiden mutation, prothrombin G20210A mutation, or hyperhomocysteinaemia.159 Doctors still debate whether or not hypercoagulation studies should be carried out in patients with venous thrombosis. Additional studies are needed, and doctors should follow clinical criteria in these situations.181
Acquired thrombophiliasA retrospective study in patients with antiphospholipid antibodies revealed a 4.4% probability of developing IS.182 The presence of anticardiolipin antibodies was associated with an increase by a factor of 1.5 to 2.2 of risk of developing stroke in men,183 and a cohort study showed a similar relationship in women.184 Nevertheless, there have been no clinical trials in patients with antiphospholipid antibody syndrome and no prior thrombosis.185–187
Sleep apnoeaSleep apnoea affects up to 24% of the adult male population.188 It has traditionally been associated with a risk of severe vascular episodes such as ischaemic heat disease, stroke,189 CHF,190 and sudden death.191 In population studies, sleep apnoea showed a slight association with CHF and ischaemic heart disease after adjusting for other risk factors.192 It showed a more marked association with risk of stroke.193–195
Conflicts of interestThe authors have no conflicts of interest to declare.
Ad hoc committee of the SEN Study Group for Cerebrovascular Diseases constituted to draw up clinical practice guidelines for stroke.
Coordinator: Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid.
Exuperio Díez-Tejedor (Coordinator), Hospital Universitario La Paz, Madrid; Blanca Fuentes (Secretary), Hospital Universitario La Paz, Madrid; María Alonso de Leciñana, Hospital Universitario Ramón y Cajal, Madrid; José Álvarez-Sabin, Hospital Universitari Vall d’Hebron, Barcelona; Juan Arenillas, Hospital Universitario Clínico de Valladolid; Sergio Calleja, Hospital Universitario Central de Asturias, Oviedo; Ignacio Casado, Hospital San Pedro, Cáceres; Mar Castellanos, Hospital Josep Trueta, Gerona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Antonio Dávalos, Hospital Universitari Germans Trias i Pujol, Badalona Fernando Díaz-Otero, Hospital Universitario Gregorio Marañón, Madrid; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; José Antonio Egido, Hospital Clínico Universitario San Carlos, Madrid; Juan Carlos Fernández, Hospital Universitario Dr. Negrín, Las Palmas; Mar Freijo, Hospital Universitario de Basurto, Bilbao; Blanca Fuentes, Hospital Universitario La Paz, Madrid; Jaime Gállego, Hospital General de Navarra, Pamplona; Andrés García Pastor, Hospital Universitario Gregorio Marañón, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; Francisco Gilo, Hospital Universitario La Princesa, Madrid; Pablo Irimia, Clínica Universitaria de Navarra, Pamplona; Aida Lago, Hospital Universitario La Fe, Valencia; José Maestre, Hospital Universitario Virgen de las Nieves, Granada; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Joan Martí-Fábregas, Hospital de la Santa Creu i Sant Pau, Barcelona; Patricia Martínez-Sánchez, Hospital Universitario La Paz, Madrid; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Carlos Molina, Hospital Universitario Vall d’Hebron, Barcelona; Ana Morales, Hospital Universitario Virgen de la Arrixaca, Murcia; Florentino Nombela, Hospital Universitario La Princesa, Madrid; Francisco Purroy, Hospital Universitario Arnau de Vilanova, Lérida; Marc Ribó, Hospital Universitari Vall d’Hebron, Barcelona; Manuel Rodríguez-Yañez, Hospital Clínico Universitario, Santiago de Compostela; Jaime Roquer, Hospital del Mar, Barcelona; Francisco Rubio, Hospital Universitario de Bellvitge, Barcelona; Tomás Segura, Hospital Universitario de Albacete, Albacete; Joaquín Serena, Hospital Josep Trueta, Gerona; Patricia Simal, Hospital Clínico Universitario San Carlos, Madrid; Javier Tejada, Hospital Universitario de León, León; José Vivancos, Hospital Universitario La Princesa, Madrid.
José Álvarez-Sabín, Hospital Universitario ValldHebron, Barcelona; José Castillo, Hospital Clínico Universitario, Santiago de Compostela; Exuperio Díez-Tejedor, Hospital Universitario La Paz, Madrid; Antonio Gil-Núñez, Hospital Universitario Gregorio Marañón, Madrid; José Larracoechea, Hospital de Cruces, Bilbao; Eduardo Martínez-Vila, Clínica Universitaria de Navarra, Pamplona; Jaime Masjuan, Hospital Universitario Ramón y Cajal, Madrid; Jorge Matías-Guiu, Hospital Clínico Universitario San Carlos, Madrid; Francisco Rubio, Hospital de Bellvitge, Barcelona.
The affiliations of the authors and committee members are listed in the appendix.
Please cite this article as: Fuentes B, et al. Guía para el tratamiento preventivo del ictus isquémico y AIT (I). Actuación sobre los factores de riesgo y estilo de vida. Neurología. 2012;27:560–74.