Hypophosphataemia is defined as a phosphorus level below 2.5mg/dL.1 Its incidence in hospitalised patients is high, especially in septic, malnourished, or alcoholic patients, in whom it is associated with higher morbidity and mortality rates. Clinical manifestations present with phosphorus levels below 1mg/dL, although these manifestations are highly diverse and unspecific, which may delay diagnosis.2
Our patient is a 69-year-old man who had kidney failure following kidney transplant from a deceased donor, and transient episodes of loss of consciousness due to severe hypophosphataemia.
Clinical caseOur patient had a personal history of smoking, arterial hypertension, dyslipidaemia, ischaemic heart disease with percutaneous transluminal coronary angioplasty (PTCA) and stenting of the anterior descending artery, diagonal branch, and right coronary artery with subsequent restenosis and new PTCA-stent, stenosis, moderate aortic insufficiency, mild mitral regurgitation, mild-moderate tricuspid insufficiency, ischaemic dilated cardiomyopathy, atrial flutter, nephrectomy due to papillary kidney cancer, and chronic kidney disease stage 5-D secondary to ischaemic kidney disease treated with haemodialysis from February 2009 and kidney transplant from a deceased donor in March 2013. He also presented surgically treated colonic diverticulitis due to colonic perforation after colonoscopy, duodenal ulcus, avascular necrosis of the head and patella of the femur, brucellosis, and boutonneuse fever. He was receiving tacrolimus, mycophenolic acid, furosemide, prednisone, acenocoumarol, ramipril, lansoprazole, acetylsalicylic acid, eplerenone, allopurinol, metoprolol, solifenacin, and nitroglycerin.
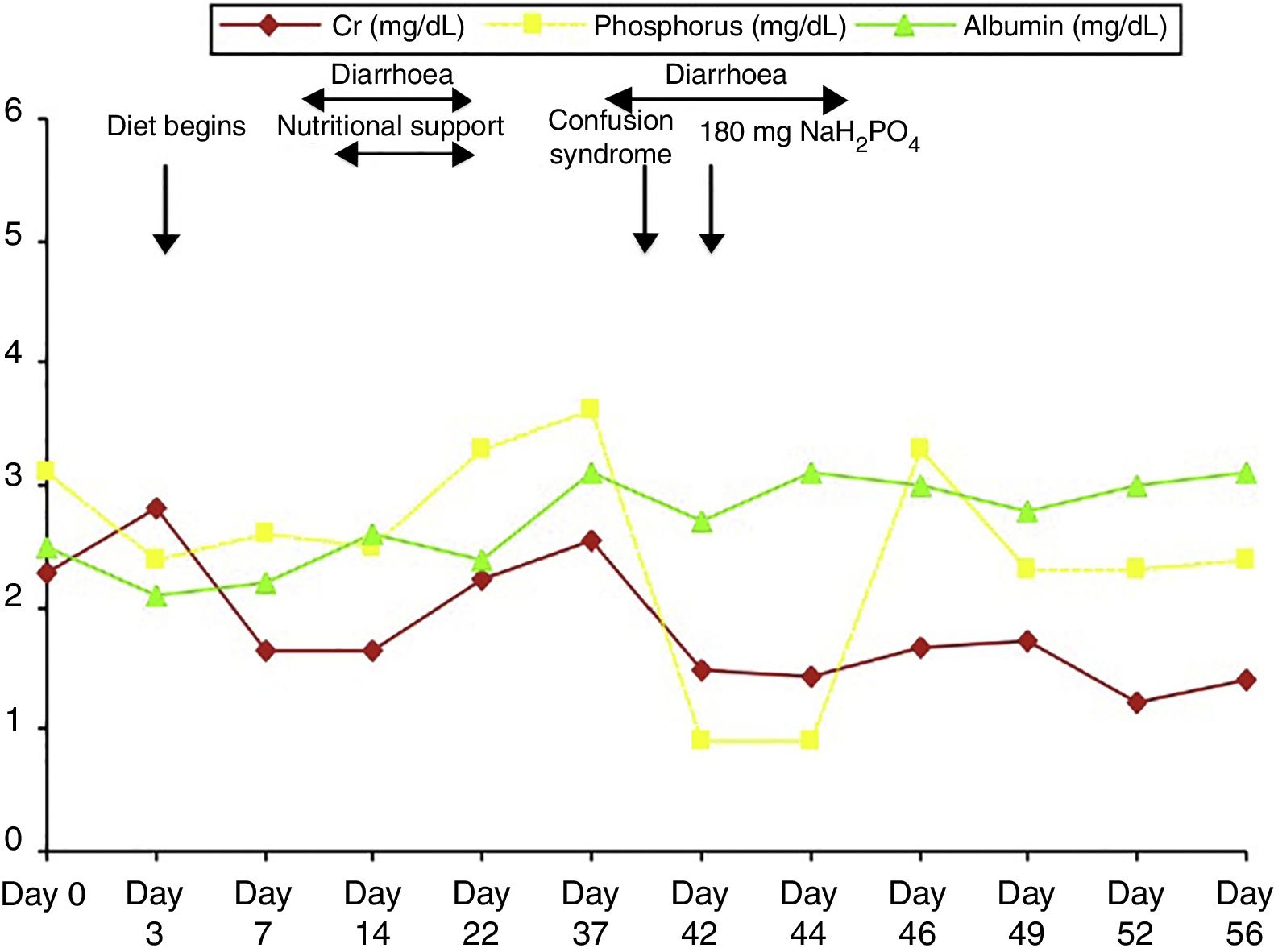
The patient was admitted to the intensive care unit due to septic shock secondary to acute cholecystitis after undergoing open cholecystectomy. He was on a nil-by-mouth diet for several days. Clinical outcomes were positive and inotropes and steroids were progressively discontinued (they were prescribed at high doses due to suspicion of relative adrenal insufficiency: hydrocortisone at 100mg/8hours). However, the patient presented pronounced anorexia; recurrent episodes of diarrhoea due to antibiotic treatment, exacerbating kidney failure and secondary metabolic acidosis, which was treated with bicarbonate; and progressive malnutrition (Fig. 1). He was transferred to the general surgery department on day 21 after admission due to slow healing of the surgical wound, and continued treatment with antibiotics and underwent surgical debridement and daily curettage. Progressive malnutrition was treated with nutritional support consisting of a lipid, amino-acid, and glucose emulsion containing 24mmol of phosphate (744mg). Treatment also included insulin, which had to be discontinued due to increased diarrhoea. On day 38, the patient experienced a transient episode of low level of consciousness with disorientation and fluctuating disconnection, and did not respond to verbal or painful stimuli. Clinical symptoms recurred at 24hours; given suspicion of benzodiazepine poisoning, 1/2 vial of flumazenil was administered and alprazolam was discontinued; this achieved no clinical improvement. On day 45, we were alerted to a new clinical event similar to the episode reported above. The physical examination revealed blood pressure of 110/80mmHg, heart rate of 90bpm, and oxygen saturation of 98%; the patient was dazed (Glasgow Coma Scale score of 9/15), disoriented in time and place, unable to follow simple instructions, and displayed echolalia. The results from an examination of the cranial nerves and reflexes were normal; the patient could move all 4 limbs, despite weakness and tremor in all 4. Assessment of sensory deficit was hindered by the patient's lack of cooperation. Laboratory blood testing revealed urea at 51mg/dL, Cr 1.43mg/dL, Ca 10.3mg/dL, P 0.9mg/dL, Na 136mmol/L, K 3.6mmol/L, Cl 99mmol/L, with normal results for CK and CK-MB; venous blood gas analysis showed pH of 7.46, PCO2 of 43mmHg, and actual bicarbonate of 30.6mmol/L. A brain CT scan showed no sign of ischaemia. Given suspicion of neurological impairment secondary to severe hypophosphataemia, we started treatment with 180mg of monosodium phosphate every 8hours, with progressive improvement of symptoms and disappearance of clinical manifestations (Fig. 1); the patient was discharged on day 56 after admission.
DiscussionHypophosphataemia is defined by abnormally low serum or plasma phosphorus levels.1 A serum concentration between 1 and 2.5mg/dL is considered moderate hypophosphataemia, which typically does not lead to any clinical sign or symptom; however, severe hypophosphataemia, with a phosphorus level below 1mg/dL, does cause symptoms.2
Moderate hypophosphataemia is observed in 5% of hospitalised patients, with severe hypophosphataemia being less frequent (0.1% to 0.2%).1,2 Nevertheless, in certain risk groups such as alcoholic or septic patients, prevalence may reach 30% or even 80%, respectively. Its presence is associated with a higher rate of mortality in both hospitalised and dialysis patients.
Hypophosphataemia may be caused by 4 mechanisms2,3: 1) redistribution of phosphorus from the extracellular to the intracellular space, 2) a decrease in intestinal absorption, 3) an increase in urinary excretion, and 4) decreased phosphorus levels due to replacement therapy.
Clinical manifestations present with values below 1-1.5mg/dL and in cases of chronic phosphorus depletion. The pathogenic basis of these manifestations is a decrease in levels of intraerythrocytic 2,3-bisphosphoglycerate (2,3-BPG) and intracellular ATP. Hypercalciuria and osteomalacia are frequent; patients may also present a decrease in cardiac output, which may cause congestive heart failure and respiratory failure due to muscle involvement. Proximal myopathy, and even rhabdomyolysis, are frequent in the skeletal muscle. At the nervous system level, the presence of metabolic encephalopathy has been associated with seizures and coma in patients with phosphorus levels below 0.5mg/dL and haemolysis.1,2 Treatment is not necessary for mild hypophosphataemia. In moderate cases, in addition to treating the cause, oral supplements should be administered. Intravenous administration should be used in severe, life-threatening cases of hypophosphataemia with respiratory failure, seizures, or coma, or in cases in which oral administration is not possible.4 Depending on the remaining ion concentration, administration of monosodium or monopotassium phosphate may be started at a dose of 2.5-5mg/kg body weight, depending on severity.5 Prevention is the best treatment for hypophosphataemia. Patients with total parenteral nutrition should receive phosphorus at 1000mg/day.
Cardiovascular disease is the leading cause of death after kidney transplantation. The current risk of mortality due to cardiovascular disease is 50 times higher in these patients than in the general population, due to the accumulation of vascular risk factors. Almost half of deaths of cardiovascular cause following transplants are due to cerebrovascular disease secondary to stroke, with cerebrovascular atheromatosis being more frequent in kidney transplant patients. However, symptoms suggesting cerebrovascular accident may be secondary to severe electrolyte imbalances, which should be considered when performing differential diagnosis.
Our patient was a kidney transplant recipient with many cardiovascular risk factors and normal baseline phosphorus levels, who presented severe hypophosphataemia secondary to progressive malnutrition caused by sepsis, diarrhoea, prolonged hospitalisation with limited food intake, and prescribed nutritional support with limited phosphorus intake. Furthermore, the patient was prescribed treatment with corticosteroids, bicarbonate, insulin, and diuretics, all of which can cause hypophosphataemia. Absence of phosphorus level determination in routine (almost daily) laboratory testing delayed both diagnosis and treatment.
In summary, despite the fact that kidney transplant patients present high cardiovascular morbidity and mortality rates, very frequently secondary to cerebrovascular disease, such electrolyte imbalances as severe hypophosphataemia may mimic cerebrovascular accidents, which should be considered in differential diagnosis. We should include phosphorus level determination in serial laboratory analyses of patients who are critical or undergoing long-term hospitalisation, in order to establish an early diagnosis and implement the appropriate nutritional preventive measures.
FundingThe authors received no funding for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fraile P, Segurado O, Lizarazo A, Martínez AI, García-Cosmes P. Paciente trasplantado renal con disminución del nivel de conciencia de etiología poco frecuente: a propósito de un caso. Neurología. 2018;33:480–482.