Headaches (including migraines) and epilepsy have a high level of comorbidity and may be confused during diagnosis. Although physicians have known for centuries that these two conditions are somehow linked, their relationship remains poorly understood. Herein we describe the known associations between them, their underlying physiopathologic and genetic mechanisms, and the treatments recommended for them.
MethodWe have reviewed the most relevant publication of headache/migraine and epilepsy by using the PubMed data base.
DescriptionAn individual can suffer both from headaches (either migraine and/or other type of headache) and epilepsy, either by chance or because of a common underlying pathology. In these cases, the headache usually occurs at a different moment than the seizure (“interictal headache”). However, headaches sometimes occur simultaneously with, or very close in time to, the seizure: one that occurs at the same time as an epileptic seizure is known as an “ictal epileptic headache” or as “hemicrania epileptica”; one that precedes a seizure is known as a “pre-ictal headache”; and one that follows a seizure is known as a “post-ictal headache”. There is a particular type of pre-ictal headache, known as “migralepsy”, which occurs during or just after a migraine aura.
ConclusionsThe terminology and concepts employed to describe possible associations between headaches (mainly migraines) and epilepsy have evolved over time with increasing clinical and physiopathogenic knowledge. Some researchers have suggested eliminating the term migralepsy and using the terms ictal epileptic headache and hemicrania epileptica exclusively and uniformly in all classification systems.
Cefalea (especialmente la migraña) y epilepsia son entidades con elevada comorbilidad que pueden confundirse desde el punto de vista clínico. Existe una relación bidireccional entre ambas, conocida desde hace siglos, pero aún no bien comprendida. Describimos las distintas modalidades de asociación existentes entre ellas, los mecanismos fisiopatológicos y genéticos subyacentes y los tratamientos recomendados.
MétodoHemos revisado las publicaciones más relevantes sobre la asociación entre migraña/cefalea y epilepsia utilizando la base de datos de PubMed.
DescripciónEn un mismo individuo, la epilepsia puede coexistir con algún tipo de cefalea (sobre todo migraña) por azar o a través de una etiología subyacente común. En ambos casos, los ataques de una y otra se presentan en diferentes momentos temporales (“cefalea interictal”). Cuando la cefalea es parte de la propia crisis, estamos ante una hemicránea epiléptica o ante una cefalea epiléptica ictal. La cefalea que aparece tras la crisis, define una cefalea post-ictal. La cefalea que la precede, se denomina cefalea preictal. Un tipo especial de esta última es la migralepsia, término que hace referencia a las crisis que aparecen durante o poco después del aura migrañosa.
ConclusionesLa terminología y los conceptos que definen las posibles asociaciones entre cefalea/migraña y epilepsia han ido evolucionando a lo largo del tiempo, en virtud del mayor conocimiento clínico y fisiopatogénico. Se ha propuesto suprimir el término de migralepsia, y utilizar de forma restringida y uniforme los términos cefalea epiléptica ictal y hemicránea epiléptica en todos los sistemas de clasificación.
For centuries, people have been aware of a link between epilepsy and migraines (or headaches in general), but the relationship between the two is not yet fully understood. In 1898, an editorial in the Journal of the American Medical Association underlined the need to find “a plausible explanation of the long recognised affinities of migraine and epilepsy”.1 More than 100 years later, these affinities are still clinically and scientifically relevant. Migraine and epilepsy are both episodic disorders that are characterised by paroxysmal bursts of transient cerebral dysfunction. These conditions have a high rate of comorbidity and they are sometimes mistaken for each other in clinical practice.2–4
The term ‘comorbidity’ does not imply progression or causality, but rather the co-presence of diseases that cannot be explained by chance alone. The association between epilepsy and migraine (or other types of headache) is two-way: either may precede or follow the other, and they may also appear simultaneously.5 Furthermore, these disorders share pathophysiological mechanisms and genetic and/or environmental risk factors.
For purposes of this study, we completed a medical literature search on PubMed using the keyword ‘migralepsy’ or a combination of the terms ‘epilepsy’ or ‘epileptic seizure’ with ‘headache’, ‘migraine’, or ‘aura’. No time limits were imposed other than those inherent to the database. We then selected the most relevant original studies and reviews from the search results to write this review.
Epidemiology and relationship between epilepsy and headache (especially migraine)The prevalence of epilepsy in the migraine population ranges from 1% to 17%, with a median of 5.9%.6 This figure is significantly higher than the prevalence of epilepsy in the general population (0.5%–1%).7 Migraine prevalence in the epileptic population is also high, ranging between 8.4% and 23%.3 One retrospective Icelandic study carried out in epileptic children aged 5 to 15 years showed a fourfold risk of experiencing migraine, especially migraine with aura.8
Ottman and Lipton studied migraine–epilepsy comorbidity using a structured telephone interview. The cumulative incidence rate of migraine in subjects with epilepsy was 24%. Among their relatives with epilepsy, 23% also had a history of migraine compared to 12% of their non-epileptic relatives. Epilepsy increased the relative risk of migraine by a factor of 2.4 (Cox proportional hazard) in both subjects and their relatives compared to family members without epilepsy.9
Leniger et al.10 pointed out that headache as a symptom during epileptic seizures is often overlooked. That team studied 341 consecutive patients with epilepsy over a 15-month period. Within that group, 34% (n=115) experienced headache associated with seizures; headaches were preictal in 3%, peri-ictal in 27%, and postictal in 70%. Headaches were classified as migraines in 55.7% of those 115 patients, and as tension-type headaches in 36.5%. There were no significant associations with sex, type of seizure, or epileptic syndrome. In contrast, there was an association between presence of a migraine-type headache associated with seizures and history of migraine.
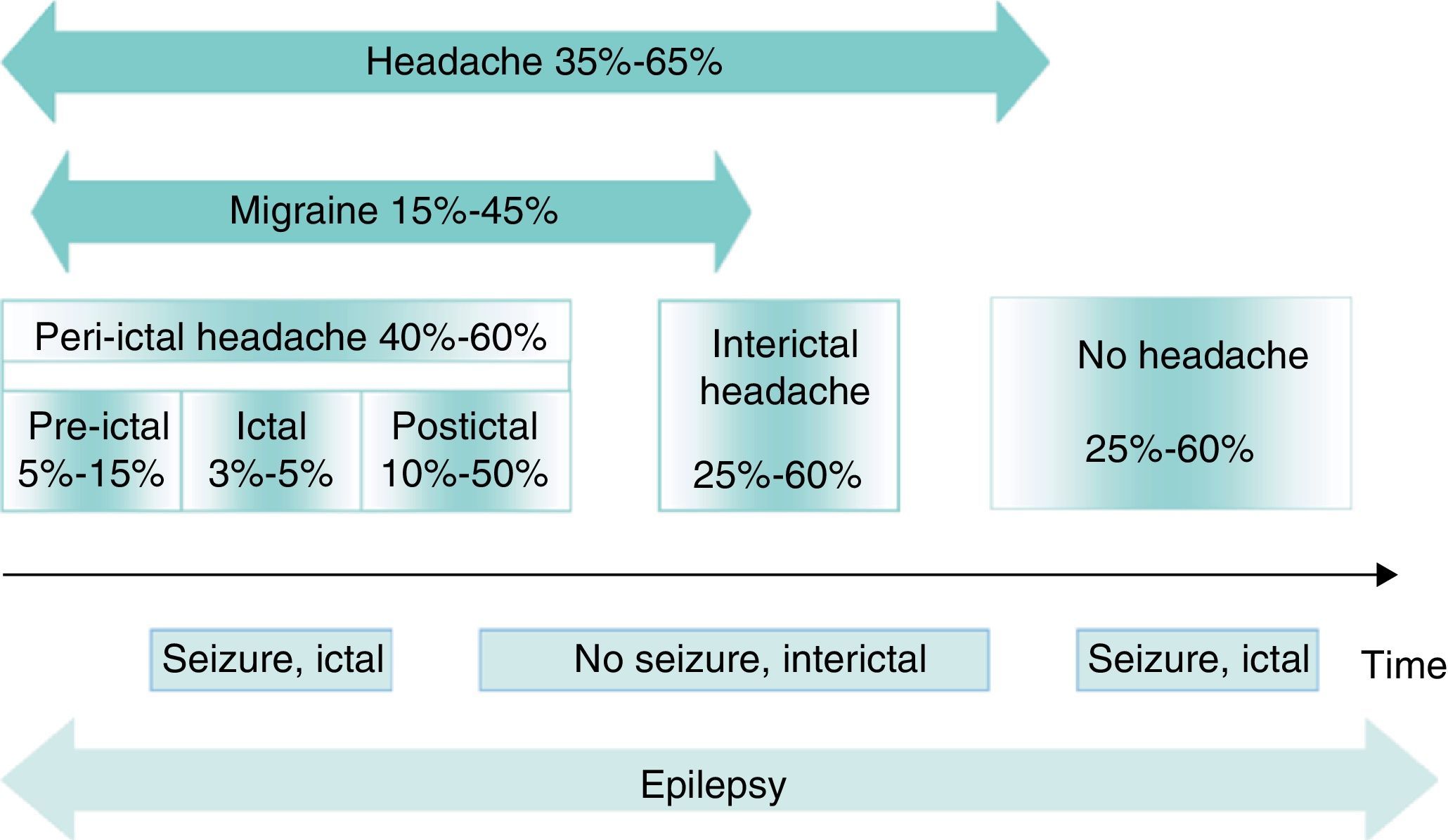
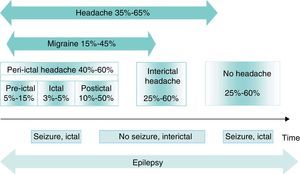
Different types of association can be found between headache and epilepsy, giving rise to different temporal relationships between the conditions (Fig. 1)11,12:
- 1.
Casual co-presence in the same individual.
- 2.
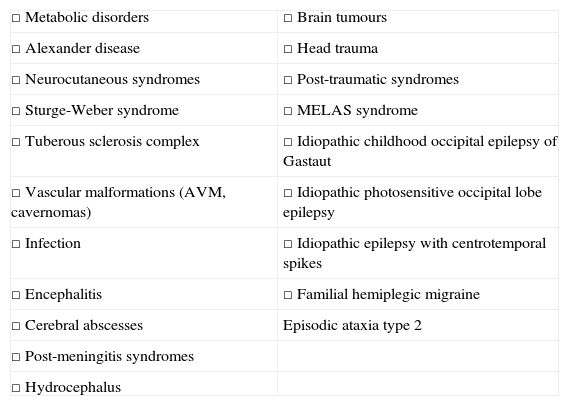
Common underlying aetiology explaining both disorders (Table 1).
Table 1.Diseases that may present with headache and epilepsy.
□ Metabolic disorders □ Brain tumours □ Alexander disease □ Head trauma □ Neurocutaneous syndromes □ Post-traumatic syndromes □ Sturge-Weber syndrome □ MELAS syndrome □ Tuberous sclerosis complex □ Idiopathic childhood occipital epilepsy of Gastaut □ Vascular malformations (AVM, cavernomas) □ Idiopathic photosensitive occipital lobe epilepsy □ Infection □ Idiopathic epilepsy with centrotemporal spikes □ Encephalitis □ Familial hemiplegic migraine □ Cerebral abscesses Episodic ataxia type 2 □ Post-meningitis syndromes □ Hydrocephalus In both cases, we refer to interictal headache, since the 2 types of attacks present at different moments in time.
- 3.
Headache is part of the seizure itself (or, less frequently, the sole manifestation): hemicrania epileptica and ictal epileptic headache. Headaches and seizures present simultaneously.
- 4.
Headache appears after the seizure (seizure-headache/migraine sequence): post-ictal headache. In these cases, epileptic manifestations may resemble migraine aura, as occurs in benign childhood epilepsy. Seizure and headache appear one after the other.
- 5.
Epileptic seizure begins during or after a migraine aura (migraine/headache-seizure sequence): migralepsy. The migraine and seizure episodes appear consecutively or simultaneously.
Timelines for headache and migraines in epilepsy. The numbers represent headache prevalence in epilepsy. Modified from Bianchin et al.,11 2010.
These last 3 types of headache presenting near the time of onset of a seizure will be described in the next section.
Headaches related to epileptic seizuresBetween 40% and 60% of all patients with epilepsy present peri-ictal headache at some time during the course of the disease. These headaches may be preictal, ictal, or postictal. However, types are not mutually exclusive; the same patient may experience more than one type of peri-ictal headache, or even concurrent interictal headaches. Peri-ictal headaches may have diagnostic significance; for example, in temporal lobe epilepsy, the location of the headache is correlated with the side of the epileptogenic focus and therefore displays lateralising value.13
Preictal headachePreictal headaches precede an epileptic seizure without forming part of the seizure itself; they occur more than 5minutes before seizure onset.14 Although there are few systematic studies of these headaches, they have been described in 5% to 15% of all patients with epilepsy.15,16 Yankovsky et al. found 11 cases of preictal headache when studying 100 patients with drug-resistant partial epilepsy. In 7 cases, headache appeared in the 30minutes prior to the seizure (early preictal headache); in the 4 remaining cases, it appeared between 30minutes and 24hours earlier, with irregular presence throughout that time (prodromal headache). Except for one case of frontal lobe epilepsy, all patients in this group had temporal lobe epilepsy. Headache was in a frontotemporal location in all cases and ipsilateral to the epileptogenic focus in most cases with temporal lobe epilepsy.16 Headache preceded epileptic seizures in 75% of this patient group. In 36%, headache showed migraine-like characteristics but was less intense than a typical migraine headache. When epilepsy surgery stopped the seizures, headaches disappeared as well.
A different type of preictal headache refers to seizures triggered by migraine (migralepsy); in these cases, seizures appear during or soon after the migraine aura. While its prevalence is unknown, it has been associated with basilar migraine and menstrual migraines in particular.2 The term ‘migralepsy’ was first used by Lennox and Lennox17 to describe “ophthalmic migraine with perhaps nausea and vomiting followed by symptoms characteristic of epilepsy”.
Migralepsy is a controversial entity. The ICHD-II (second edition of the International Classification of Headache Disorders) includes it among the complications of migraine (epigraph 1.5.1) and establishes its diagnostic characteristics (Table 2).18 Nevertheless, the term does not appear in the ILAE classification (International League Against Epilepsy), nor does it appear among the recent recommendations of the ILAE Commission on Classification and Terminology.19
Diagnostic criteria for migralepsy (ICHD-II).18
| 1.5.5 Migraine-triggered seizure (migralepsy): episode triggered by a migraine aura. Must meet the following criteria: |
| A. Migraine fulfilling criteria for ‘migraine with aura’ |
| B. A seizure fulfilling diagnostic criteria for one type of epileptic attack occurs during or within 1hour after a migraine aura |
In fact, doctors today consider a clinical sequence like that described in migralepsy (migraine aura-epileptic seizure or migraine-epileptic seizure) to be quite unlikely.20,21 The existence of this nosological entity is now questioned for various reasons.
- 1.
In one study of more than 1500 individuals with epilepsy, none experienced seizures after a migraine aura or concomitantly with aura.22
- 2.
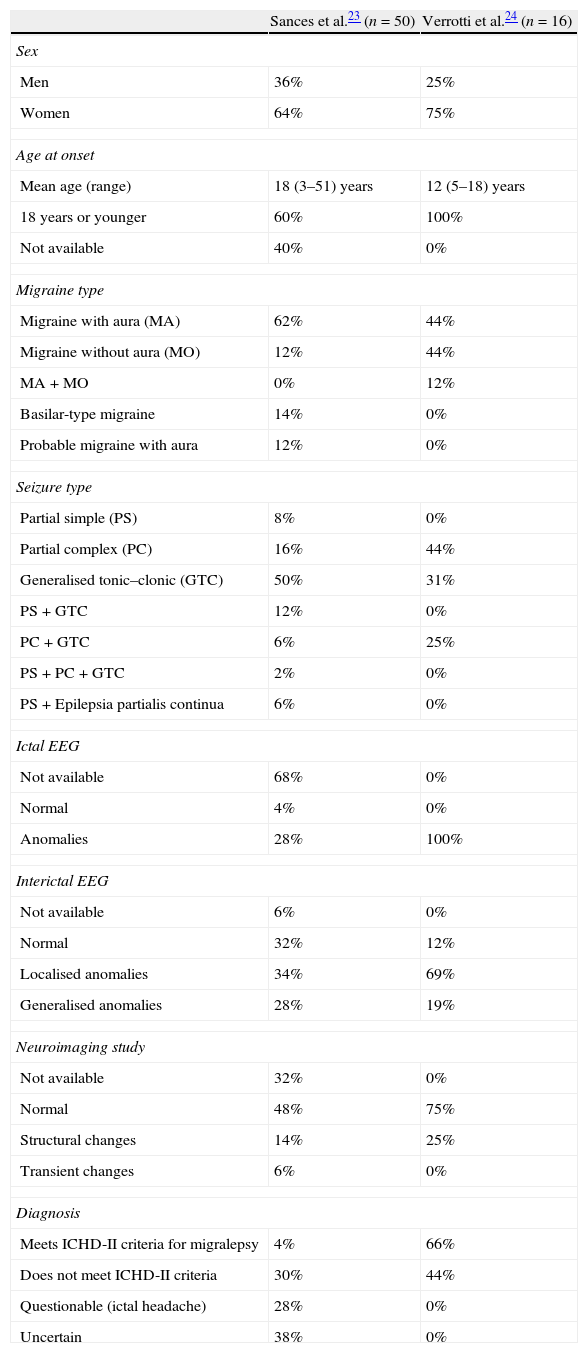
A review of the approximately 50 cases of migralepsy published in the literature before 2010 (Table 3) shows the diagnosis to be quite questionable in most cases, and none demonstrates a clear migraine–epilepsy sequence.23 Only two patients of these 50 (4%) strictly comply with ICHD-II diagnostic criteria. Instead, some of the cases described are associated with migraine without aura; in other cases, the epileptic seizure manifested more than an hour after migraine with aura.23
Table 3.Data from cases of migralepsy reported in the literature.23,24
Sances et al.23 (n=50) Verrotti et al.24 (n=16) Sex Men 36% 25% Women 64% 75% Age at onset Mean age (range) 18 (3–51) years 12 (5–18) years 18 years or younger 60% 100% Not available 40% 0% Migraine type Migraine with aura (MA) 62% 44% Migraine without aura (MO) 12% 44% MA+MO 0% 12% Basilar-type migraine 14% 0% Probable migraine with aura 12% 0% Seizure type Partial simple (PS) 8% 0% Partial complex (PC) 16% 44% Generalised tonic–clonic (GTC) 50% 31% PS+GTC 12% 0% PC+GTC 6% 25% PS+PC+GTC 2% 0% PS+Epilepsia partialis continua 6% 0% Ictal EEG Not available 68% 0% Normal 4% 0% Anomalies 28% 100% Interictal EEG Not available 6% 0% Normal 32% 12% Localised anomalies 34% 69% Generalised anomalies 28% 19% Neuroimaging study Not available 32% 0% Normal 48% 75% Structural changes 14% 25% Transient changes 6% 0% Diagnosis Meets ICHD-II criteria for migralepsy 4% 66% Does not meet ICHD-II criteria 30% 44% Questionable (ictal headache) 28% 0% Uncertain 38% 0% - 3.
Although an ictal EEG during the migraine phase is only available for 32% of these 50 described cases, they do display an elevated prevalence of pure epileptic disorders.23 MRI scans showed transient cerebral anomalies in 6% of the patients with migralepsy. This was probably caused by changes to the blood–brain barrier eliciting oedema, which explains why the changes are reversible. In addition, several of the patients described as having this diagnosis responded rapidly to intravenous diazepam administration; response was both clinical and detectable by EEG.23
- 4.
In 2011, clinical and EEG characteristics were published for a series of patients diagnosed with migralepsy. These 16 patients were selected retrospectively from among 4600 epileptic children who had experienced a seizure less than an hour after having had a migraine with or without aura. It included only those subjects whose data included an EEG recording taken during the migraine phase (Table 3).24 While 69% of these children had complex partial seizures, the other 31% presented primary generalised epilepsy. Visual symptoms such as amaurosis or visual, elemental, or complex hallucinations affected 56% of the patients. Forty-four per cent had migraine without aura, another 44% migraine with aura, and 12% experienced both types. Neuroimaging studies (MRI) yielded normal results in 75%, whereas the remaining 25% exhibited anomalies such as neuronal migration disorder, hydrocephalus, leukoencephalopathy, and periventricular gliosis. The 16 patients included in the study showed focal or generalised EEG anomalies during their migraine episodes, including spike-waves, spikes only, or theta waves at different topographies. Most patients (88%) also showed anomalies in interictal EEG.24 No correlations have been found between the type and/or cortical localisation of ictal EEG anomalies and synchronic migraine onset, or episodes of migraine with or without aura.
It has therefore been suggested that the migraine–epilepsy sequence defined as ‘migralepsy’ might possibly be an epileptic seizure that begins with an ictal headache, i.e. headache is part of the seizure itself. The headache may or may not be followed by a sensory, motor, autonomic, or generalised partial seizure.24
Ictal headacheHeadache as a manifestation of epileptic seizure has been documented in less than 5% of all patients with epilepsy, and it does not meet diagnostic criteria for migraine.10 One reason for this small percentage may be that if headache coincides with the seizure, it may go unnoticed, especially in patients with altered cognition or consciousness. When headache does appear, it is generally only for a few minutes.5
Ictal headache manifests with multiple, varied symptoms, such as a bilateral sensation of pressure across the frontal region, dull headache, piercing retro-orbital pain, or an electric sensation with various intensities and localisations.25 It is often accompanied by other symptoms26 but in rare cases it may be the sole symptom of a seizure or focal status epilepticus.27 This phenomenon is even less likely to present in all seizures experienced by the same patient. According to some authors, this manifestation is similar to pain in other parts of the body, and they suggest that it may be of parietal origin (ictal headache).28 Nevertheless, clinical experience finds this symptom to be exceptional in parietal epilepsy.
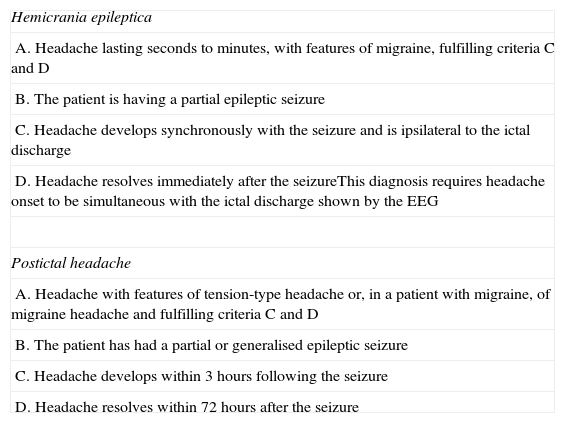
The only type of ictal headache described in the ICHD-II18 is hemicrania epileptica, included in section 7 (headache attributed to non-vascular intracranial disorder) under heading 7.6 (headache attributed to epileptic seizure). The condition is extremely rare, and when it does appear, it is unlikely to meet all proposed diagnostic criteria (Table 4).18 Hemicrania epileptica is not listed among the nosological or terminological recommendations of the ILAE Commission on Classification and Terminology.19 In an introductory article, Isler et al.29 studied 91 patients with drug-resistant epilepsy; of the total, 18 presented hemicranial headache with migraine-like characteristics lasting seconds to minutes at the time of onset of epileptic activity, which was partial seizure in all patients. In rare cases, ictal headache lasted for hours.
Diagnostic criteria for hemicrania epileptica and postictal headache (ICHD-II).18
| Hemicrania epileptica |
| A. Headache lasting seconds to minutes, with features of migraine, fulfilling criteria C and D |
| B. The patient is having a partial epileptic seizure |
| C. Headache develops synchronously with the seizure and is ipsilateral to the ictal discharge |
| D. Headache resolves immediately after the seizureThis diagnosis requires headache onset to be simultaneous with the ictal discharge shown by the EEG |
| Postictal headache |
| A. Headache with features of tension-type headache or, in a patient with migraine, of migraine headache and fulfilling criteria C and D |
| B. The patient has had a partial or generalised epileptic seizure |
| C. Headache develops within 3hours following the seizure |
| D. Headache resolves within 72hours after the seizure |
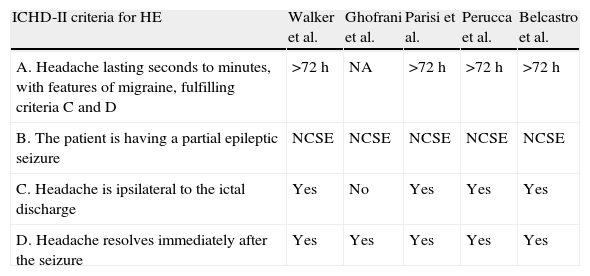
Between 1988 and the present, researchers reported 5 cases of status epilepticus migrainosus.27,30–33 None met the current ICHD-II criteria for hemicrania epileptica (Table 5).18,34 In all cases, headache seemed to be the sole manifestation of non-convulsive status epilepticus, whether partial30–33 or generalised.27 The nature of this state can only be revealed by an EEG recording.32–36 There are no reports of any specific patterns or localisations of the epileptic discharges. Intriguingly, diffusion MRI has shown changes in the region of critical activity, which confirms the technique's usefulness for determining the epileptic nature of these events.32,33 On the other hand, studies have also shown that complete resolution of the headache and epileptic anomalies is achieved by using antiepileptic drugs, not anti-migraine drugs, in most of these patients.32,33
Review of 5 cases of ictal epileptic headache described in the literature, compared to current ICHD-II criteria for hemicrania epileptica (HE).
Modified from Belcastro et al.,34 2011.
| ICHD-II criteria for HE | Walker et al. | Ghofrani et al. | Parisi et al. | Perucca et al. | Belcastro et al. |
| A. Headache lasting seconds to minutes, with features of migraine, fulfilling criteria C and D | >72h | NA | >72h | >72h | >72h |
| B. The patient is having a partial epileptic seizure | NCSE | NCSE | NCSE | NCSE | NCSE |
| C. Headache is ipsilateral to the ictal discharge | Yes | No | Yes | Yes | Yes |
| D. Headache resolves immediately after the seizure | Yes | Yes | Yes | Yes | Yes |
HE: hemicrania epileptica; NCSE: non-convulsive status epilepticus; NA: not available.
Diagnostic difficulty is inherent to the description of an epileptic seizure presenting with ictal pain. The situations listed below contribute to making the diagnostic scenario even more complicated.
- 1.
If ictal headache is associated with other ictal epileptic manifestations, especially visual or sensory manifestations, without generalisation of the seizures, it is easy to conclude that the headache is migraine with aura.
- 2.
If ictal headache is the sole epileptic manifestation, the mere step of considering this rare possibility and detecting any epileptiform anomalies in an ictal EEG will be helpful in the diagnostic process. However, we must be mindful of the difficulty involved in taking an ictal recording.
This headache type is also included in the ICHD-II under heading 7.6 (Table 4).18 It presents in 50% of all patients with epilepsy37 and constitutes the type of headache most frequently linked to epileptic seizures. A list of circumstances promote or act as risk factors for the condition: young adulthood and prior history of headache, especially migraine (in this case, postictal headache is typically migrainous); experiencing epileptic seizures at a young age; longer histories of epilepsy; drug-resistant epilepsy; generalised tonic–clonic seizures; and occipital epilepsy, whether idiopathic or symptomatic.38 A study by Ito et al. found that 41% of all patients with temporal lobe epilepsy had postictal headache. This was also true in 40% of the cases of frontal epilepsy and in 59% of the cases of occipital epilepsy.39
Postictal headaches tend to appear 3 to 15minutes after an epileptic seizure18,22 and may be accompanied by vomiting and photophobia. Their duration varies, although it is usually less than 12hours; headache intensity is moderate and typically requires analgesics.14,40 Postictal headache presenting after an occipital seizure will require differential diagnosis to rule out typical migraine with aura or basilar migraine.
In general, postictal headache is underestimated and undertreated. This occurs because the epileptic seizure is a much more eloquent symptom than pain. However, this condition does decrease quality of life considerably for those patients who present it.
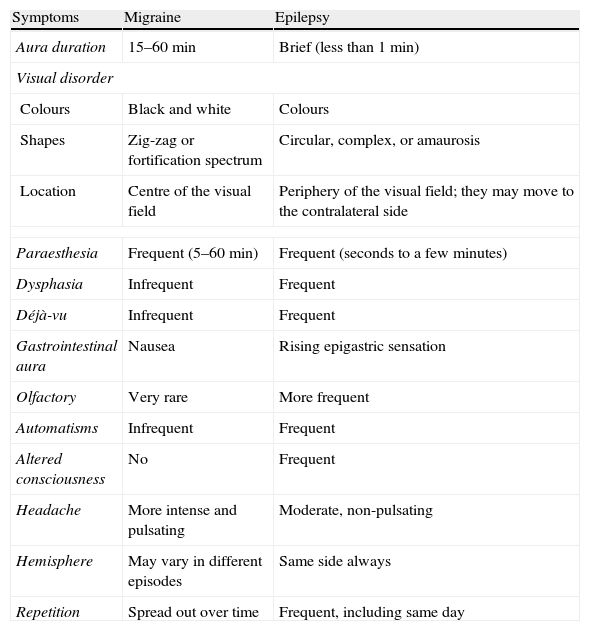
Differential diagnosis for epileptic seizures and migraine: clinical and electroencephalographic characteristicsClinical aspectsVisual symptoms of occipital seizures can be mistaken for visual auras in migraine, even though most cases display typical traits that differentiate them (Table 6). Visual auras in migraine appear as colourless blinking lights, or often as zig-zagging lines that begin in the centre of the visual field and progress to the edge of the hemifield in 4 to 30minutes. They are frequently followed by scotoma.20 The total duration of aura is usually 60minutes or less. In contrast, elemental visual hallucinations in occipital lobe epilepsy are mainly colourful and circular; they develop in a few seconds and are of short duration (2–3min). They frequently appear on the edge of a temporal hemifield and they expand and multiply during the seizure, often moving horizontally towards the contralateral side.22 Nevertheless, it is not always easy to distinguish migraine from epilepsy based on the above criteria. Alice in Wonderland syndrome may present as macropsia, micropsia, metamorphopsia, or size distortion phenomena. It has been described in both temporal-occipital epilepsy and migraine.41
Useful clinical data in the differential diagnosis of aura in migraines and in epilepsy.
| Symptoms | Migraine | Epilepsy |
| Aura duration | 15–60min | Brief (less than 1min) |
| Visual disorder | ||
| Colours | Black and white | Colours |
| Shapes | Zig-zag or fortification spectrum | Circular, complex, or amaurosis |
| Location | Centre of the visual field | Periphery of the visual field; they may move to the contralateral side |
| Paraesthesia | Frequent (5–60min) | Frequent (seconds to a few minutes) |
| Dysphasia | Infrequent | Frequent |
| Déjà-vu | Infrequent | Frequent |
| Gastrointestinal aura | Nausea | Rising epigastric sensation |
| Olfactory | Very rare | More frequent |
| Automatisms | Infrequent | Frequent |
| Altered consciousness | No | Frequent |
| Headache | More intense and pulsating | Moderate, non-pulsating |
| Hemisphere | May vary in different episodes | Same side always |
| Repetition | Spread out over time | Frequent, including same day |
While EEG is not useful for routine evaluation of headache, 24-hour video-EEG studies may play a useful role in specific cases. Marks and Ehrenberg3 employed this technique on 2 patients with migralepsy and observed EEG changes that were not typical of epilepsy during the migraine aura (burst of spikes resembling EEG findings in an epileptic seizure). In most cases, this activity did not display the timeline typical of ictal epileptiform activity with its progressive increases and decreases in frequency and amplitude.42,43 EEG during migraine aura may also show ‘alternating’ patterns separated by completely normal activity despite the persistence of clinical symptoms.44
In published cases of ictal epileptic headache,27,30–33 EEG during headache did not show a specific pattern. Some cases displayed high-voltage rhythmic activity at 11 to 12Hz with alternating spikes in the right temporal-occipital region,32,33 while others showed high-voltage theta wave activity alternating with acute waves in the occipital region,30,31 or discharges of spikes and continuous bilateral slow spike-waves.27 Intermittent light stimulation has evoked photoparoxysmal responses30 together with low-intensity pulsating headache.31 On some occasions, no anomalies may be found on a surface EEG during the ictal headache. This finding is common in certain types of epilepsy, such as those originating in deep-structure focal epilepsy, for example, in the orbitomedial frontal region,2,36,45–47 or in epileptic syndromes such as Panayiotopoulos syndrome that have autonomic manifestations.20 This means that absence of clear spike-wave epileptic activity does not rule out a diagnosis of epilepsy. In these cases, using deep electrodes greatly improves the diagnostic sensitivity of the technique.2
Common pathogenic and genetic mechanisms in migraine and epilepsyThe causes that explain the high rate of comorbidity between epilepsy and headaches, especially migraines, are completely unknown. Although the pathophysiology of the migraine–epilepsy sequence has not yet been clarified, one hypothesis is that the threshold for cortical excitation in migraine patients is lower than in healthy subjects, and that this situation fosters onset of seizures. This cerebral hyperexcitability may be due to several factors, whether isolated or combined, including mitochondrial alterations, magnesium metabolism disorders, or ion channel anomalies.48
Cortical spreading depression (CSD) may constitute the link between migraine and epilepsy.35,49 This phenomenon consists of a wave of neuronal and glial depolarisation that begins in the occipital cortex and propagates at a velocity of 2 to 5mm/minute towards the anterior regions of the cortex. During this process, chemical mediators including glutamate are released into the extravascular and perivascular space.48 CSD is able to activate the trigeminal-vascular system (TVS), inducing the release of vasoactive peptides (CGRP and substance P) in the leptomeningeal area. This produces the vasodilation and sterile inflammation that together cause migraine pain.48,50 Glutamate and NMDA receptors are also active participants in epileptic seizures.5 On the other hand, the biochemical mechanism in CSD (transient loss of membrane ion gradients and increase in extracellular potassium, neurotransmitters, and intracellular calcium) produces a state of local hyperexcitability.51
Nevertheless, most cases of temporary association of migraine with epilepsy are in fact genuine occipital epileptic seizures. Researchers postulate that critical discharges in the occipital lobes trigger a genuine migraine by initiating the CSP phenomenon and activating the TVS, as well as other mechanisms mediated by brainstem nuclei.45
The most plausible pathophysiological explanation for presence of ictal migraine is that the epileptic focus activating TVS might be a purely autonomic focus, and discharge at that site manifests with the development of a migraine.35,45 Another possibility involves a subclinical focus that initiates CSP by activating TVS, but with discharges below the symptomatic threshold required to elicit sensory-motor manifestations since underlying depolarisation would not propagate to these other cortical areas. In such cases, migraine would be the only epileptic manifestation. Another hypothesis is that the threshold required to initiate CSP might be lower than that required to elicit an epileptic seizure.35,45 In fact, we know that central autonomic circuits have a lower threshold for epileptogenic activation than circuits producing cortical focal sensory-motor signs.4,35 This explains why, in a clinical context, we are more likely to observe epileptic patients with perictal or interictal migraines than migraine patients with epileptic seizures.
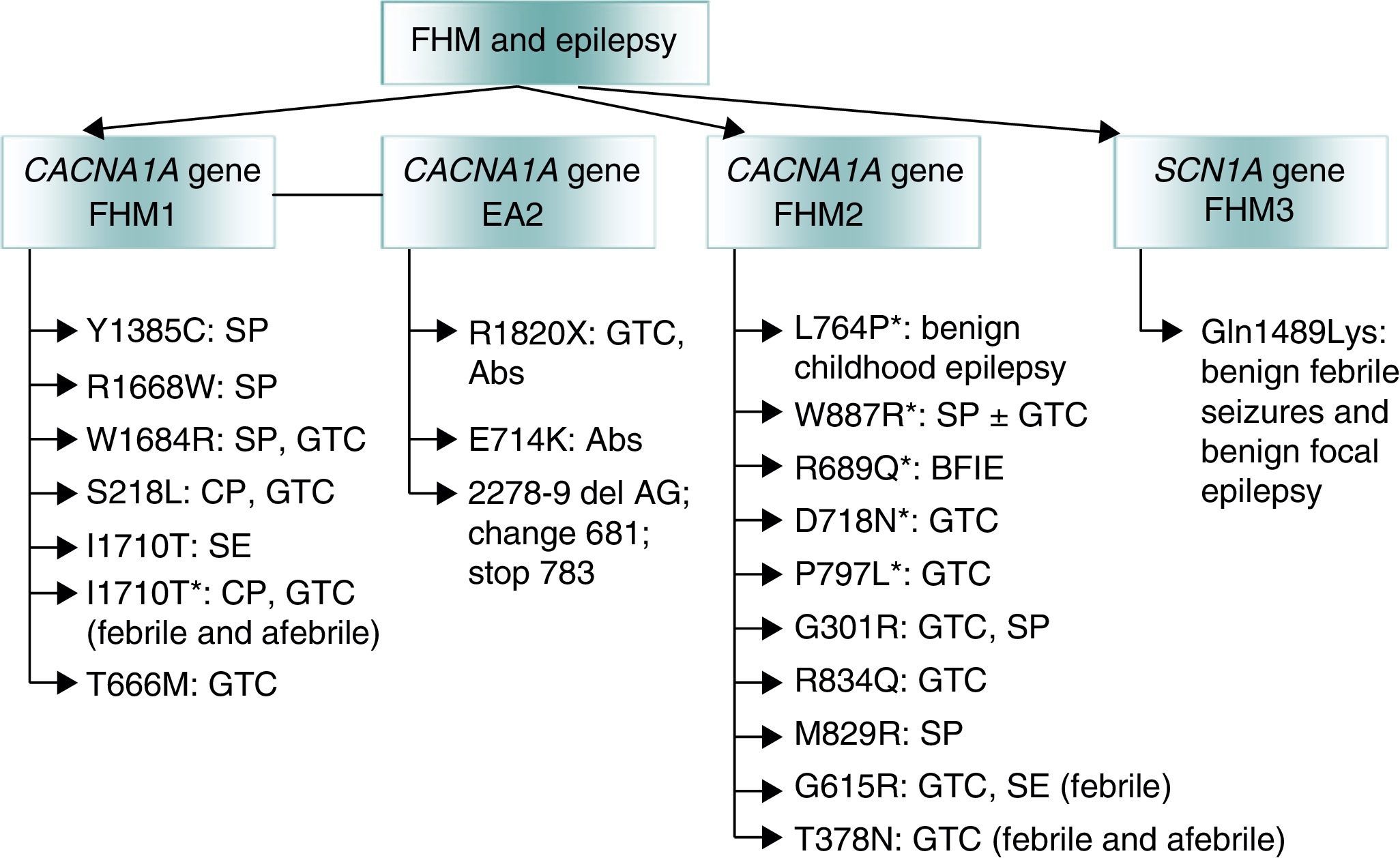
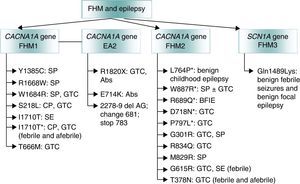
Lastly, mutations that give rise to channelopathies change neuronal or glial ionic homeostasis or affect GABA-ergic or glutamatergic systems or mitochondrial functions. These mutations may explain the association between migraine and epilepsy. Regarding familial hemiplegic migraine (FHM),52 an autosomal dominant form of migraine with aura, doctors have described co-presence of epilepsy in all 3 of its genetic subtypes (Fig. 2)53:
- (a)
The FHM1 gene CACNA1A (chromosome 19p) codes for the pore-forming Cav2.1 subunit of P/Q type calcium channels.54 These mutations may affect CSP given that P/Q calcium channels mediate glutamate release in cortical neurons.55
- (b)
The FHM2 gene ATP1A2 (chromosome 1q)56 encodes subunit α2 Na+/K+ ATPase, which is responsible for pumping K+ ions to the cell interior and Na+ ions out of the cell.57 An imbalance between Na+ and K+ ions triggers CSD.
- (c)
The FHM3 gene SCN1A (chromosome 2q24) is associated with epilepsy.58 Mutations at this location may cause genetic epilepsy with febrile seizures, Dravet syndrome, and other rare epileptic syndromes.59 More than 150 mutations of this gene have been described in families with epilepsy.60
Genetic mutations involved in familial hemiplegic migraine (FHM) and associated with epilepsy. Adapted from Haan J.53 *Epileptic seizures independent from episodes of hemiplegic migraine. EA2: episodic ataxia type 2; Abs: absence seizure: BFIE: benign familial infantile epilepsy; SE: status epilepticus; GTC: generalised tonic–clonic seizures; FHM: familial hemiplegic migraine; CP: complex partial seizures; SP: simple partial seizures.
Other genetic mutations suggesting a relationship between migraine and epilepsy have also been described. Examples include mutations in SLC1A3, a member of the solute carrier family that codes for transporter 1 of excitatory aminoacids,61 and POLG62 and C10orF2,63 genes that code for mitochondrial DNA polymerase and Twinkle helicase, respectively.
TreatmentWhen a patient has epilepsy and any type of headache, especially migraines, the best solution is to find a drug that effectively targets both conditions. Multiple double-blind placebo-controlled studies have demonstrated the efficacy of antiepileptic drugs such as sodium valproate, topiramate, and gabapentin; the first 2 options have been approved by the FDA as migraine prophylaxis. Effective doses for migraine are typically lower than those for epilepsy; 500mg/day valproate,64,65 1200mg/day gabapentin,66 or 100mg/day topiramate67,68 are often sufficient. Lamotrigine is especially effective for the aura of migraine patients, although it is less effective against pain.69 Other antiepileptic drugs, including pregabalin, levetiracetam, and zonisamide have been proven effective in some open trials.70–72 Antiepileptic drugs are probably effective for treating migraine due to their effect on cerebral excitability. Topiramate reduces CSP in rat brains73; a magnetoencephalographic study showed that neuronal excitability had decreased after 30 days of treatment with sodium valproate.74 Nevertheless, a study of epileptic patients with interictal migraines found no antiepileptic drugs to be superior to others for migraine prevention.75 On the other hand, headache is one of the most common side effects of antiepileptic drugs.
Some preventive treatments for migraine or other headaches, such as tricyclic antidepressants and neuroleptic drugs, should be avoided because they lower the epileptogenic threshold.76 In recent years, studies have also suggested that vagus nerve stimulation may have a preventive effect on migraines in epileptic patients. In 2 studies containing 4 and 10 patients with epilepsy and migraines, 75% of those in the first study and 80% of those in the second experienced decreased migraine frequency after stimulators had been implanted.77,78
Lastly, between 66% and 90% of patients with postcritical headache may benefit from analgesic treatment.15,16,79 Furthermore, triptanes have been used effectively in some patients with postcritical migraine-like headaches.80,81
ConclusionsFurther studies are needed to clarify the association between epilepsy and headache/migraine. At present, some propose discontinuing the use of ‘migralepsy’ since the term does not clearly reflect a defined nosological entity. Consensus should also be reached regarding the most appropriate terminology to be used. Meanwhile, Verrotti et al.24 propose the following change to both the IHS and the ILAE classifications:
- -
The term ‘ictal epileptic headache’ should be used to identify episodes in which headache is the only ictal manifestation of epilepsy. Ictal epileptic headache is a type of autonomic epilepsy.
- -
The term ‘hemicrania epileptica’ should remain in the classification schemes to identify those cases in which ictal epileptic headaches coexist and are associated with other ictal sensorimotor events occurring synchronically or sequentially.
Ideally, an ictal EEG should be performed during the migraine event for all patients presenting clinical characteristics of migraine and epilepsy. The aim is to detect the pathogenic mechanism underlying these episodes, although this is not always possible.2,47 However, we often find that these ideal situations are not achievable.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Caminero A, Manso-Calderón R. Vínculos existentes entre cefalea y epilepsia: terminología y conceptos actuales. Neurología. 2014;29:453‐463.