Reports on surgical outcomes in patients with drug-resistant temporal lobe epilepsy without histological abnormalities are scarce.
MethodsRetrospective review of data from patients with drug-resistant temporal lobe epilepsy and no histopathological alterations who underwent anterior mesial temporal lobectomy. We analysed the following variables: age, sex, age at seizure onset, age at surgery, time elapsed between diagnosis and the date of the surgery, follow-up time, and classification according to the Engel rating scale.
ResultsFrom a database of 256 temporal lobectomies, 21 were identified as meeting the inclusion criteria. The average age upon diagnosis of epilepsy was 8.3 years and average age at time of surgery was 28.6 years. The mean time elapsed between diagnosis and surgery was 20.2 years. After a mean follow-up of 6.5 years, 90.5% of the patients showed favourable outcomes (classes i and ii) and 42.9% were seizure-free (class IA). Comparative analysis of the variables revealed that average age at seizure onset was the only statistically significant difference between groups, with age at onset being lower in patients with favourable outcomes.
ConclusionAlthough long-term surgical outcomes were favourable for a large majority of patients, the percentage of seizure-free patients is lower than in patients with lesional epilepsy and comparable to that previously reported in the literature.
Los reportes de los resultados quirúrgicos en los pacientes con epilepsia fármaco-resistente del lóbulo temporal sin anormalidades histológicas son escasos.
MétodosFueron revisados retrospectivamente los datos de los pacientes con epilepsia del lóbulo temporal tratados con lobectomía temporal anterior con amígdalo-hipocampectomía que no presentaban alteraciones en los estudios histopatológicos. Fueron analizadas las variables: edad, género, edad al inicio de las crisis epilépticas, edad al momento de la cirugía, tiempo de evolución de la epilepsia desde el diagnóstico hasta la fecha de la cirugía, periodo de seguimiento y clasificación según la escala de Engel.
ResultadosDe una base de datos de 256 lobectomías temporales, fueron identificados 21 pacientes que cumplieron los criterios de inclusión. El promedio de edad a la cual se realizó el diagnóstico de epilepsia fue 8,3 años, al momento de la cirugía fue 28,6 años y de tiempo transcurrido desde el diagnóstico hasta la cirugía fue 20,2 años. Tras un seguimiento promedio de 6,5 años, el 90,5% obtuvo resultados favorables (clases i y ii) y el 42,9% estaba libre de crisis (clase iA). Tras el análisis comparativo de las variables estudiadas, solo existieron diferencias estadísticamente significativas en la edad de inicio de las crisis, siendo inferior en los pacientes que obtuvieron resultados favorables.
ConclusiónAunque en la gran mayoría de los pacientes los resultados quirúrgicos a largo plazo fueron favorables, la proporción de pacientes libres de crisis es inferior a la de los pacientes con epilepsia lesional y comparables con lo reportado previamente en la literatura.
Temporal lobe epilepsy (TLE) is a subtype of focal epilepsy that very frequently requires surgery; seizure control is achieved in most patients receiving this treatment.1–3
Results from TLE surgery are closely related to the presence of epileptogenic abnormalities which are detected in preoperative MRI studies. A recent meta-analysis found that 75% of the patients with mesial lesions of the temporal lobe were seizure-free throughout long-term postoperative follow-up, while only 51% of the patients with normal MR images had similar benefits with surgery.4
Several studies have shown that 20% to 30% of the patients with TLE present an epileptogenic lesion that cannot be identified or delimited in the preoperative images, even when high-resolution MRI is used.4–6 These cases are classified by some authors as non-lesional epilepsy (NLE).4 However, they may be accompanied by potentially epileptogenic histopathological alterations, which would make this label inappropriate. The microscopic study of the surgical specimen is still considered the gold standard test for determining the presence of a lesion underlying the seizures.
Some studies have linked the absence of structural alterations in the extirpated tissue to persistence of seizures after surgical treatment.7–9 The aim of the current study is to describe long-term surgical results in a series of patients with TLE and whose histopathological studies present no abnormalities.
Patients and methodsA cross-sectional study was performed to retrospectively review data from patients with drug-resistant TLE and no neuroimaging or histopathological abnormalities, treated by the multidisciplinary epilepsy surgery group pertaining to the Colombian Foundation and Centre for Epilepsy and Neurological Diseases (Cartagena de Indias, Colombia).
Preoperative assessmentPatients were assessed during a single hospital stay according to the institutional protocol for epilepsy surgery.10
Patients with clinical and semiological characteristics and abnormal results in the ictal surface electroencephalogram who showed focal motor seizures with typical automatisms (with or without generalisation) were initially assessed using video-EEG monitoring and neuroimaging tests (MRI/CT without contrast). Patients were also evaluated individually by each member of the epilepsy surgery group according to the institutional protocol for epilepsy surgery.
Patients who did not present abnormal results in imaging or electrophysiological tests that would demonstrate the laterality of the epileptogenic focus were studied by invasive video-EEG after temporary implantation of subdural subtemporal electrodes. After seizures were recorded, the laterality of the temporal epileptogenic region could be determined.
Before each procedure, all patients were assessed in joint meetings of the multidisciplinary epilepsy surgery group, which included epileptology, epilepsy neurosurgery, clinical neurosurgery, neuropsychology, physiotherapy, and speech therapy experts. During meetings, experts determined whether patients met the criteria for the following: drug-resistant epilepsy, impact on quality of life, and indication for surgery (anterior temporal lobectomy with amygdalohippocampectomy). Each patient and his or her family members were informed of the surgical plan, after which doctors obtained informed consent for surgery.
All patients underwent the classic procedure of anterior temporal lobectomy with amygdalohippocampectomy. None of the extracted specimens showed microscopic morphological changes.
Patients with non-specific histological anomalies (such as gliosis) and those with a postoperative follow-up period of less than 12 months were excluded from the study.
During data collection, we recorded the following variables: age, sex, age at epileptic seizure onset, age at surgery, progression timeline for epilepsy (from diagnosis to surgery), and follow-up period.
Results for surgery were classified according to the Engel scale; using a dichotomous assessment, results for seizure control were classified as favourable (Engel I or II) or unfavourable (Engel III or IV).11 Patients identified as Engel class I were further subdivided according to the recommendations of the Neurosurgery Commission of the International League Against Epilepsy Since this group included both seizure-free subjects and patients with seizures that were not disabling, seizure freedom was defined as the total absence of seizures after surgery (Class IA).11
Statistical analysisData were recorded in an SPSS (Statistical Package for the Social Sciences) database, version 17.0 (SPSS, Inc., Chicago, IL, USA). Quantitative variables were expressed as measures of central tendency (mean±standard deviation and range), while qualitative variables were presented as percentages.
For comparisons between groups, Pearson's chi-squared test was used for qualitative variables, whereas Fisher's exact test was used to assess the differences between quantitative variables (values of P<.05 were considered statistically significant).
Ethical considerationsBecause of the study's observational design, there are no ethical limitations concerning treatment indications.12 In any case, patients’ family members signed an informed consent form for data collection. This study was approved by the institutional ethics committee (memorandum of agreement number 005, Centro Latinoamericano de Investigación en Epilepsia) and it also applied Colombian scientific and technical standards for human research (Resolution Number 8430 of 1993, Colombian Ministry of Social Protection) and good clinical practice guidelines (International Conference on Harmonisation/Good Clinical Practice).
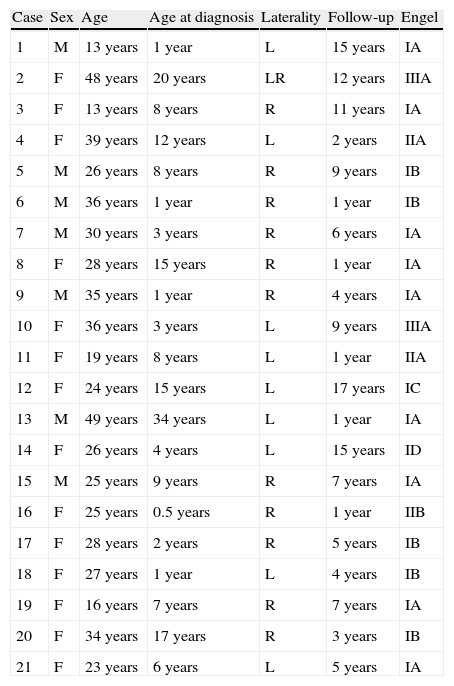
ResultsOf a database including 256 patients who were treated surgically for TLE, 21 did not present histological changes; 14 (66.7%) were women and 7 (33.7%) were men. All were right-handed. The mean age at which epilepsy was diagnosed was 8.3±8.3 years (range, 0–34) and the mean time between diagnosis and surgery was 20.2±8.9 years (range, 5–35). Patients’ ages at time of surgery ranged from 13 to 49 years, with a mean of 28.6±9.8 years. Most of the resections affected the right temporal lobe (57.1%). There were no cases of perioperative mortality.
The mean postoperative follow-up time was 6.5±5.1 (range, 1–17) years, after which outcomes were favourable for 90.5% of the patients according to the pre-established criteria (Classes I and II) and 42.9% were seizure free (Class IA) (Table 1).
Clinical characteristics and classification of postoperative outcomes according to the Engel scale.
| Case | Sex | Age | Age at diagnosis | Laterality | Follow-up | Engel |
| 1 | M | 13 years | 1 year | L | 15 years | IA |
| 2 | F | 48 years | 20 years | LR | 12 years | IIIA |
| 3 | F | 13 years | 8 years | R | 11 years | IA |
| 4 | F | 39 years | 12 years | L | 2 years | IIA |
| 5 | M | 26 years | 8 years | R | 9 years | IB |
| 6 | M | 36 years | 1 year | R | 1 year | IB |
| 7 | M | 30 years | 3 years | R | 6 years | IA |
| 8 | F | 28 years | 15 years | R | 1 year | IA |
| 9 | M | 35 years | 1 year | R | 4 years | IA |
| 10 | F | 36 years | 3 years | L | 9 years | IIIA |
| 11 | F | 19 years | 8 years | L | 1 year | IIA |
| 12 | F | 24 years | 15 years | L | 17 years | IC |
| 13 | M | 49 years | 34 years | L | 1 year | IA |
| 14 | F | 26 years | 4 years | L | 15 years | ID |
| 15 | M | 25 years | 9 years | R | 7 years | IA |
| 16 | F | 25 years | 0.5 years | R | 1 year | IIB |
| 17 | F | 28 years | 2 years | R | 5 years | IB |
| 18 | F | 27 years | 1 year | L | 4 years | IB |
| 19 | F | 16 years | 7 years | R | 7 years | IA |
| 20 | F | 34 years | 17 years | R | 3 years | IB |
| 21 | F | 23 years | 6 years | L | 5 years | IA |
R: right; F: female; L: left; M: male.
Among patients with favourable results, differences between women (85.7%) and men (100%) were not statistically significant; (χ2: 1.1; P=.29). Likewise, differences between seizure-free men (71.4%) and women (28.6%) were not statistically significant (χ2: 3.5; P=.06).
There were no statistically significant differences between right- and left-sided operations among patients with favourable outcomes (right: 91.7% vs left 88.9%; χ2: 0.046; P=0.83) and among seizure-free patients (right: 50% vs left 33.3%; χ2: 0.6; P=.45).
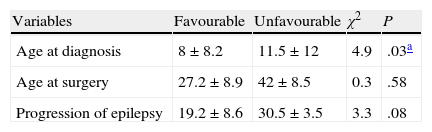
Table 2 shows comparisons between age at seizure onset, age at surgery, and epilepsy duration prior to surgery with regard to postoperative results.
Comparison of temporal variables with regard to postoperative results.
| Variables | Favourable | Unfavourable | χ2 | P |
| Age at diagnosis | 8±8.2 | 11.5±12 | 4.9 | .03a |
| Age at surgery | 27.2±8.9 | 42±8.5 | 0.3 | .58 |
| Progression of epilepsy | 19.2±8.6 | 30.5±3.5 | 3.3 | .08 |
| Variables | Seizure-free | Persistent seizures | χ2 | P |
| Age at diagnosis | 9.3±10.2 | 7.6±6.9 | 0.22 | .64 |
| Age at surgery | 25.8±11.6 | 30.7±8.1 | 1.3 | .26 |
| Progression of epilepsy | 16.4±8.9 | 23.1±8 | 3.2 | .09 |
Many studies have shown that most lesional TLE patients have a very favourable prognosis after anterior temporal lobectomy with amygdalohippocampectomy (in cases of mesial sclerosis)13 or lesionectomies (in cases of other focal abnormalities such as malformations of vascular or cortical development or tumours).14–17 In some cases, despite semiological and electrophysiological evidence pointing to a temporal focus, MRI studies cannot identify any lesions. This has repeatedly been considered one of the main factors predicting a poor postoperative outcome due to technical difficulties involved in localising and resecting the entire area in which seizures originate.18,19 Studies have established that only 34% to 45% of the TLE patients with no lesions according to the MRI scan will attain seizure freedom. In contrast, this proportion reaches 75% for patients with pre-surgery imaging studies that show identifiable lesions.4
Currently, there is no consensus on this topic despite proposals for defining TLE, which are based on the MRI findings or histopathological studies. Definitions based on MRI findings have been widely criticised, given that microscopic alterations related to epilepsy have been found in 32% to 57% of the surgical specimens from patients with no MRI changes. Malformations of cortical development are the most frequent changes, especially focal cortical dysplasias (types Ia and Ib).7,20–24 This illustrates why a definition of TLE based on MRI findings is inappropriate; histopathological criteria should be used exclusively.
There are few studies on postoperative follow-up for patients with negative histopathology studies. In the series published by Bien et al., only 2.6% of the patients who underwent surgery for TLE presented no histological alterations, while in our study this group accounted for 8.2% of the total (21/256).7 We have observed that there are considerably fewer seizure-free patients with normal histology studies than patients with lesions, even if they have not been detected by the preoperative MRI.4,7,9,25 In a study by Kasasbeh et al. including 4 patients, only 2 remained seizure-free during 2 years of follow-up. Likewise, Bien et al. treated 20 patients, among which only 4 (20%) remained seizure-free. This percentage increased to 77.8% in those patients with normal MRI results and histopathological evidence of a lesion (P=.003).7
In the current study, 90.5% of the patients experienced a favourable outcome (Engel I and II), while 42.9% remained seizure-free during follow-up. This observation is similar to the findings of Téllez-Zenteno et al. in patients with a normal histological appearance. They found a seizure freedom rate of 36%, with a 95% confidence interval for 29% to 43%.4 We should stress that although most patients included in the study benefited visibly from surgery, the low seizure freedom rate reflects the possible presence of residual epileptogenic tissue beyond the resection margins described according to the classic antero-medial temporal lobectomy technique.7 Another cause of seizure recurrence may be the failure to identify multiple foci when they lie outside the resection area.7 Based on these postulates, we recommend a new assessment using intracranial recordings to look for remaining epileptogenic foci, which would raise the possibility of additional surgery. However, the literature still lacks such cases.
Preliminary evidence suggests that these difficulties may be resolved using positron emission tomography (PET) studies, which would allow doctors to better detect the affected side and delimit the specific area responsible for ictal discharges.20,26–28 Three studies carried out in different epilepsy surgery centres found that surgical results from patients with normal MR images and decreased metabolism of the positron-emitting agent on the PET scan were no different from those from patients with visible lesions on MRI. These studies highlight how important this method is for determining the true extension of the epileptogenic focus and delimiting the resection area more successfully.20,26,28 Some authors have even suggested that invasive EEG may be skipped in TLE patients whose PET and seizure semiology results are not contradictory.28 Given that this method was not available in Colombia, all patients from our study were assessed using subdural subtemporal electrodes.
At present, the pathological basis underlying an epileptogenic focus with a normal histological appearance remains undetermined. Furthermore, few theories attempt to explain the origin of ictal discharges and how they propagate.29 The only hypothesis to have been conclusively demonstrated involves functional alterations in the TSC2 gene, which are similar to those described in patients with other diseases that may present clinically with drug-resistant epilepsy, such as tuberous sclerosis complex, gangliogliomas, or cortical dysplasia. Some authors venture that these alterations may present as formes frustes of these diseases that do not express typical morphological alterations, but which are able to modify the normal patterns of neuronal discharge and dissemination.29
Comparative analysis of the study variables showed statistically significant differences only for age at seizure onset. This variable was lower in patients with favourable results, which is consistent with prior studies.30–32 It is an interesting fact that men tended to experience better postoperative outcomes, with a difference approaching the pre-established threshold for statistical significance (P=.06). Studies of TLE patients by Burneo et al.,33 Varoglu et al.,34 and Aull-Watschinger et al.35 have shown better postoperative outcomes in men. However, other authors did not confirm this association.1,36 At present, there are no conclusive explanations for these observations; however, researchers hypothesise that changes in antiepileptic drug levels related to increased cytochrome P450 3A4 activity, pregnancy, menopause, or menstruation could explain why women might have higher seizure recurrence rates than men, who present more stable levels and better treatment adherence after surgery.34,37
In addition, we observed that patients with good postoperative results presented a shorter mean time from epilepsy diagnosis to surgery, with differences that tended to be statistically significant; however, this could not be conclusively demonstrated due to the sample size. This observation suggests that TLE patients could also benefit from early surgical treatment and supports having them assessed by a multidisciplinary epilepsy surgery group as soon as treatment with 2 antiepileptic drugs fails.38–43 This measure has been associated with better seizure control, better quality of life, and fewer changes in cognitive function.38,40–44
Bias controlIn our study, patients were assessed using 1 and 1.5T MR scans. There is only one high-resolution imaging scanner in Colombia (3T) and it is quite far from where patients were studied (some 1060km). This could explain why rates of negative results were higher than in other series.7 With the aim of eliminating this potential bias, this study included only those patients whose specimens had no histological alterations, according to studies carried out by at least one pathologist with formal training (a fellowship) in neuropathology and experience studying tissue samples obtained from epilepsy surgery.
FundingThe current study received funding from the Fundación Centro Colombiano de Epilepsia y Enfermedades Neurológicas (a private and non-profit organisation) and Centro Latinoamericano de Investigación en Epilepsia, Cartagena de Indias, Colombia.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the multidisciplinary epilepsy surgery group at Fundación Centro Colombiano de Epilepsia y Enfermedades Neurológicas for their hard work and dedication to patients in providing treatment and rehabilitation. We also thank Dr Lucía Niño-Hernández for helping us draft our manuscript, and Cristina Lozano for her assistance with the English translation of the abstract.
Please cite this article as: Benedetti-Isaac JC, Torres-Zambrano M, Fandiño-Franky J, Dussán-Ordóñez J, Herrera-Trujillo A, Guerra-Olivares R, et al. Resultados quirúrgicos a largo plazo en pacientes con epilepsia fármaco-resistente del lóbulo temporal sin anormalidades histológicas. Neurología. 2013;28:543–549.