Ischaemic stroke due to a calcified embolus is an extremely rare complication of cardiac surgery. Treatment for these cases has not been clearly defined. We present our experience with a patient whose middle cerebral artery (MCA) was occluded by a calcified embolus after aortic valve surgery; the occlusion was successfully managed using neurointerventional techniques.
Our patient was a 77-year-old woman with aortic valve stenosis who had been admitted at our hospital to undergo aortic valve replacement. Four months before admission she began to experience exercise-induced dyspnoea. She had a history of hypertension and diabetes. The echocardiogram showed a normal left ventricular ejection fraction, mild left ventricular hypertrophy, mild bilateral atrial enlargement, and severe aortic stenosis. The patient was treated with acetylsalicylic acid, simvastatin, torasemide, and metformin. During the preoperative assessment, she was alert and oriented with no sensory or motor deficits and displaying normal gait. Laboratory test results were within normal limits. The patient underwent a thoracotomy and aortic valve replacement (porcine valve). An intraoperative transoesophageal echocardiogram revealed no thrombi in the left atrium.
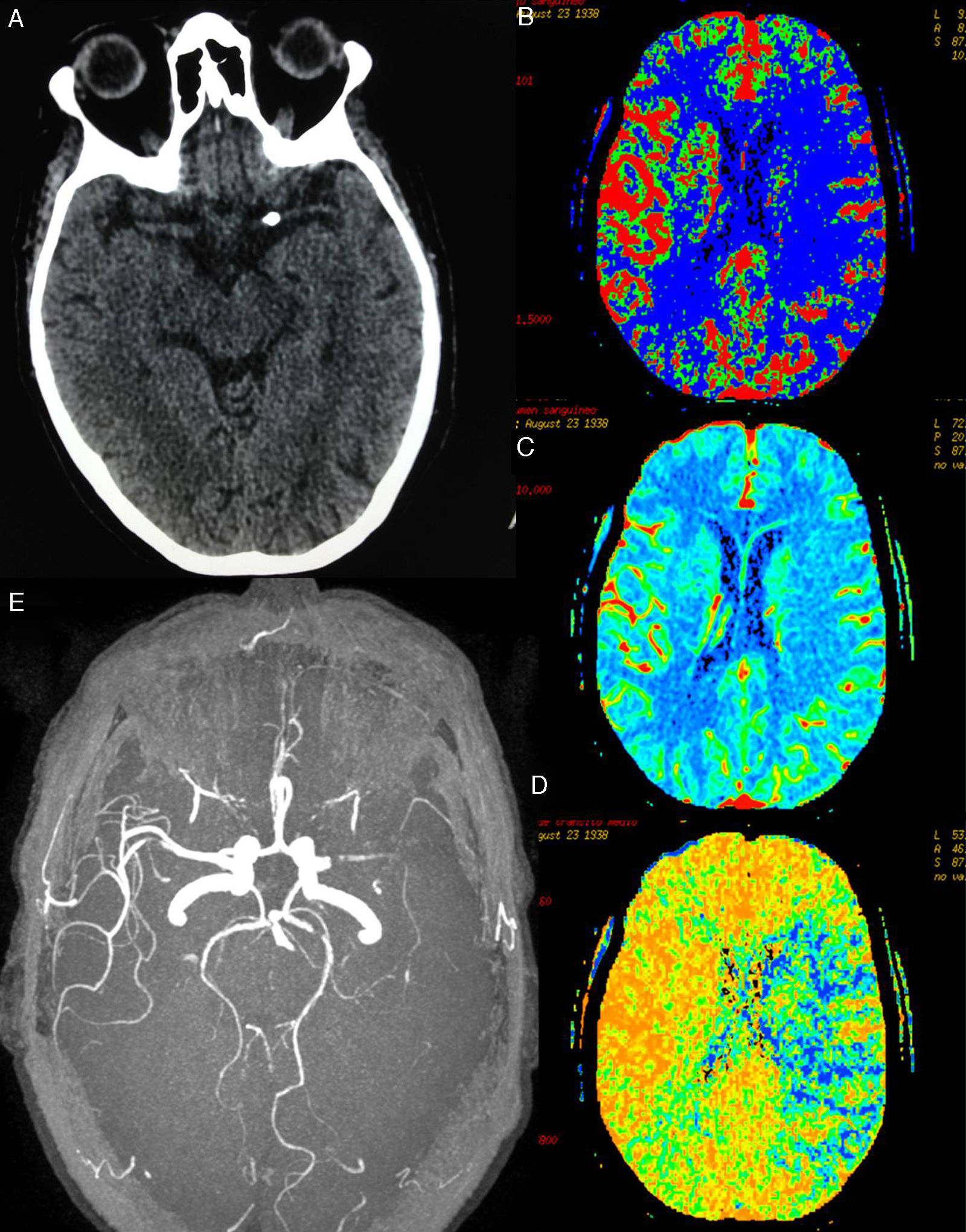
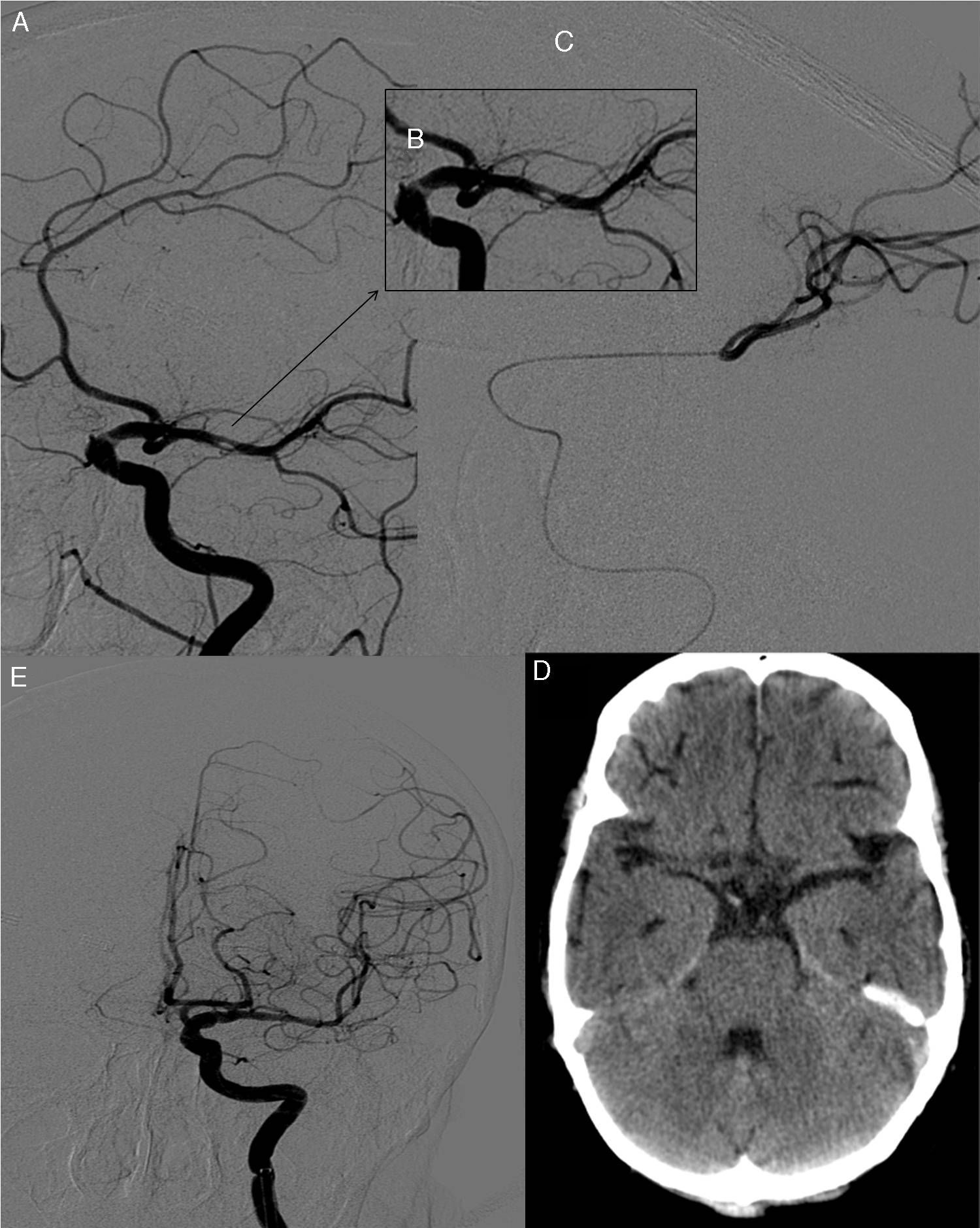
At day 1 after surgery, after extubation and upon waking up at the cardiac postoperative care unit, our patient displayed drowsiness, right hemiparesis, and global aphasia; these clinical symptoms were compatible with ischaemic stroke of the left MCA. In-hospital code stroke was activated. Our patient's NIHSS score at that moment was 18. An emergency brain CT scan revealed a high-density rounded mass in the area corresponding to the proximal segment of the left MCA. A CT perfusion map showed a large ischaemic penumbra in the territory of the left MCA. MR angiography revealed interrupted blood flow distal to the origin of the left MCA (Fig. 1). Thrombolytic therapy was ruled out since our patient had recently undergone surgery. She received endovascular treatment under general anaesthesia: stentriever-based thrombectomy successfully restored circulation with a single pass (Fig. 2). The results of the histology study of the extracted fragment were compatible with a calcium embolus. The patient was discharged after 7 days and the outcome was very favourable: she scored 1 on the mRS at 3 months.
(A) Brain CT showing calcium deposition in the lumen of the proximal segment of the left MCA. (B)–(D) CT-perfusion maps of cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) showing a large area of the left MCA territory with increased MTT (in blue, D) and decreased CBF (in blue, B). The CBV map (C) shows no significant decreases in volume; the affected area corresponds to the large penumbra. (E) Preocclusive stenosis of the left M1 segment in MRI angiography 3D sequences.
(A) Selective angiography of the left internal carotid artery showing a calcific embolus in the left MCA. (B) Angiography close-up image showing nearly complete occlusion of the vessel. (C) Embolectomy using TREVO; the procedure was successful after a single pass, as shown by the follow-up angiography (D). (E) Follow-up brain CT scan showing no calcific material in the area of the left MCA.
Aortic valve stenosis due to calcification is the most common acquired heart valve disorder in developed countries. Surgery is frequently recommended to treat symptoms and reduce associated mortality in these cases.1 Aortic valve calcification may be a source of emboli travelling to the brain. Mechanical manipulation of cardiac valves during diagnosis or treatment may predispose patients to calcified cerebral emboli, although these blockages may also appear spontaneously or secondary to endocarditis on rare occasions.2,3
Around 20% of patients with prosthetic heart valves will experience a cardioembolic stroke within 15 years after valve replacement.4 On the other hand, ischaemic stroke due to perioperative thromboembolism or hypoperfusion is a well-known complication of cardiovascular surgery. This type of surgery is associated with a high risk of perioperative stroke, which may be attributed to an increase in particles and the likelihood of air embolisation; calcific embolisation is extremely rare.5 In a prospective series of more than 16000 patients undergoing cardiac surgery with a mean of 12 months of follow-up after the intervention, short-term risk of stroke was 4.8% after aortic valve replacement, 8.8% after mitral valve replacement, and 9.7% after multiple valve replacement.6
Perioperative stroke can be classified as either early-onset, occurring during extubation (intraoperative), or late-onset, occurring after extubation (postoperative). Tarakji et al.7 studied more than 45000 patients and reported different risk factors for early- and late-onset stroke, which may be linked to different pathogenic mechanisms.
Calcified cerebral emboli were first demonstrated on CT by Yock in 1981.8 CT is normally less sensitive than MRI for detecting acute ischaemic stroke but more sensitive for locating calcified cerebral emboli.9
Diagnosis of ischaemic stroke due to a calcified cerebral embolus is usually fast since this type of stroke occurs in hospitalised patients who are subject to thorough haemodynamic and neurological monitoring in intensive care or cardiac surgery reanimation units; however, treatment is difficult and controversial. The stroke guidelines available to date recommend avoiding systemic thrombolysis within the first 14 days after major surgery,10 which means that most patients experiencing perioperative stroke are not eligible for this treatment; also, when it can be used, it may have little effect due to the type of embolic material involved.11 A promising alternative to major surgery is endovascular treatment using thrombus removal devices. Five recently published randomised clinical trials (MR CLEAN, EXTEND-IA, ESCAPE, SWIFT PRIME, and REVASCAT) have shown that endovascular treatment is highly beneficial for patients with intracranial internal carotid artery or MCA occlusions when performed up to 6hours after symptom onset. In these studies, the number of patients needed to treat with thrombus removal devices for one additional patient to be functionally independent at 90 days ranged between 3 and 7 patients.12–16 Extrapolating these data to the perioperative period is not possible since very few cases of calcific cerebral embolism have been successfully treated with thrombus removal devices.17,18 This procedure may cause migration of calcific material, leading to vessel occlusion. One team successfully wedged the calcific embolus against the vessel wall using balloon-mounted coronary stents; this procedure, however, does carry a risk of vessel rupture.19 In our case, the fragment was successfully removed with a stentriever after a single pass and the patient experienced no complications.
Our case demonstrates, on the one hand, the usefulness of CT for detecting a calcified cerebral embolus after aortic valve replacement and, on the other, the need to activate code stroke when hospitalised patients experience a stroke, since early diagnosis combined with endovascular treatment may improve outcomes in these patients.
This study was presented in part at the 67th Annual Meeting of the Spanish Society of Neurology.
☆☆ Please cite this article as: Ramírez-Moreno JM, Trinidad-Ruiz M, Ceberino D, Fernández de Alarcón L. Trombectomía mecánica en un ictus isquémico debido a embolia cerebral cálcica. Neurología. 2017;32:270–273.