Cassava, also known as yuca or manioc (Manihot esculenta Crantz), is a staple food in tropical and subtropical regions since it is an important source of carbohydrates. Nevertheless, it contains cyanogenic compounds including lotaustralin and linamarin, which have been shown by experimental models to affect brain structures such as the thalamus, the piriform cortex, the hippocampus, and others. These findings may explain the presence of such neurological diseases as konzo and tropical ataxic neuropathy. However, hippocampal involvement in the neurological alterations associated with the chemical compounds in cassava has yet to be explored.
MethodMale Wistar rats (3 months old), were assigned to 4 groups (n=8 per group) as follows: a vehicle-control group (receiving injectable solution 1μl) and three groups receiving linamarin (10, 15, and 20mM). The substances were microinjected intrahippocampally (CA1) every 24hours for 7 consecutive days, and their effects on locomotor activity, rotarod, and swim tests were assessed daily.
ResultsLinamarin microinjected into the dorsal hippocampus produced hyperactivity and loss of motor coordination which became more evident as treatment time increased. In the swim test, rats treated with linamarin displayed lateral rotation beginning on the fourth day of microinjection.
ConclusionsMicroinjection of linamarin into the dorsal hippocampus of the rat is associated with impaired motor coordination, suggesting that the dorsal hippocampus, among other brain structures, may be affected by the neurological changes associated with inappropriate consumption of cassava in humans.
La yuca, cassava o mandioca (Manihot esculenta Crantz) constituye uno de los alimentos básicos en regiones tropicales y subtropicales, por ser fuente importante de hidratos de carbono. No obstante, contiene compuestos cianogénicos, como linamarina y lotaustralina, que a nivel experimental se ha encontrado que afectan a estructuras cerebrales como el tálamo, la corteza piriforme y el hipocampo, entre otras, lo cual podría explicar algunas enfermedades neurológicas, como el konzo y la neuropatía atáxica tropical. Sin embargo, la participación del hipocampo en las alteraciones neurológicas asociadas a los componentes químicos de la yuca aún no ha sido identificada.
MétodoSe incluyeron ratas macho de 3 meses de edad (cepa Wistar), distribuidas en 4 grupos (n=8 cada grupo): un grupo vehículo (1μl de solución inyectable) y 3 grupos con linamarina (10, 15 y 20mM). Las sustancias fueron microinyectadas intrahipocampalmente (CA1) durante siete días consecutivos (cada 24h) y los efectos fueron evaluados diariamente en las pruebas de actividad locomotora, rota-rod y nado.
ResultadosLa microinyección de linamarina en el hipocampo dorsal produjo hiperactividad e incoordinación motora que fue acentuándose con los días de tratamiento. En la prueba de nado desplegaron la conducta de giro sobre su propio eje, a partir del cuarto día de microinyección.
ConclusiónLa microinyección de linamarina en el hipocampo dorsal de la rata se asocia a alteraciones en la coordinación motora, lo cual indica la participación del hipocampo dorsal, entre otras estructuras cerebrales, en las alteraciones neurológicas asociadas al consumo inapropiado de la yuca en el ser humano.
Manioc, also known as yuca or cassava (Manihot esculenta Crantz), is a vegetable staple food in many tropical and subtropical regions around the world. This crop is easy to grow and its roots are an important source of carbohydrates and some micronutrients essential for human nutrition.1 Unfortunately, it also contains such cyanogenic glycosides as linamarin (90%) and lotaustralin (10%)2; when consumed in large quantities, these substances cause a number of neurological symptoms which mainly manifest as motor and cognitive impairment.3
Excessive or inappropriate cassava consumption has been associated with 2 neurological diseases: tropical ataxic neuropathy (TAN) and epidemic spastic paraparesis (konzo). TAN is a syndrome characterised by sensory polyneuropathy, sensory ataxia, bilateral optic atrophy, and bilateral deafness which has been described in Tanzania,4 Sierra Leone,5 Nigeria,6,7 and India.8 Konzo is a neurological entity characterised by upper motor neuron damage. It initially causes irreversible, nonprogressive, and symmetrical spastic paraparesis or tetraparesis9 characterised by progressive weakness and spasticity of the lower limbs, which results in poor motor coordination. These neurological alterations have also been linked to continued and improper cassava consumption.10 The toxicity of the cyanogenic compounds of cassava mainly affects the brain structures involved in memory processing and integration, emotions, control of autonomic functions, smell, and motor function such as the thalamus, the piriform cortex, the hypothalamus, and the hippocampus.11
The hippocampus is involved in most neurodegenerative diseases; it seems to play a major role in the integration of motor responses associated with emotionally arousing events since it forms part of the emotional memory system.12,13 Multiple studies in rats have shown that consumption of the cycad Dioon spinulosum,14,15 or the intrahippocampal microinjection of one of its neurotoxic metabolites (methylazoxymethanol), induces motor alterations characterised by immobility and spinning in the forced swimming test.15,16 Similarly, rats receiving treatment with cassava root juice (with a linamarin concentration of 0.30mg/2mL) also developed such motor alterations as poor motor coordination and lateral swimming,17 which seemed to be associated, al least in part, with a decrease in the number of neurons in hippocampal area CA1.18 However, the effects of microinjections of linaramin into the dorsal hippocampus on motor activity and coordination are yet to be explored. This study aimed to determine the effects of intrahippocampal microinjection of linamarin on spontaneous motor activity (locomotor activity test) and motor coordination (rotarod and forced swimming tests) in Wistar rats.
MethodsSubjectsWe used 32 three-month-old male Wistar rats weighing 250 to 300g at the beginning of the study. Rats were housed at room temperature (25±2°C) in transparent acrylic cages in a vivarium, with a 12:12 light–dark cycle (lights on at 7:00am). Rats had ad libitum access to water and food. Rats were managed following the international ethical standards put forward in the Guide for care and use of laboratory animals19 and official Mexican guidelines for the care and use of laboratory animals.20
Stereotactic surgeryAs described in previous studies, animals were deeply anaesthetised during the unilateral implantation of a guide cannula.21 Researchers used a stereotactic apparatus (Stoelting, Wood Dale, IL, USA) to immobilise the rats’ heads before performing a longitudinal incision on the skin to expose the skull. Taking the bregma as a reference, and following the stereotactic coordinates of the rat brain atlas created by Paxinos and Watson,22 researchers used a dental lab drill (Saeshin Dental Lab 35000 RPM, South Korea) to drill a hole to implant a cannula in the dorsal hippocampus (AP=−3.8mm; L=−2mm; H=−2mm) in CA1. A stainless steel guide cannula (8mm long, 0.7mm diameter) was subsequently implanted and secured to the skull with dental acrylic (Arias Distribuidora Dental; Tlalnepantla, Mexico). Four days after cannula implantation, rats underwent intrahippocampal microinjections of either linamarin or a vehicle to assess the effects of linamarin on behaviour.
Experimental groups and treatmentRats were randomly assigned to 4 groups (n=8 rats per group): one group received the vehicle (injectable solution) and the remaining 3 groups received linamarin dosed at different concentrations (10, 15, and 20mM), calculated based on studies by Soler-Martín et al.2 Microinjections of either the vehicle or linamarin were delivered every 24hours for 7 consecutive days using a guide cannula (10mm long, 0.7mm in diameter) consisting of a stainless steel needle measuring 0.7×32mm attached to a 10μL Hamilton syringe by means of a polyethylene tube. An automatic infusion pump (KD Scientific, Holliston, MA, USA) was used to microinject 1μL at a constant speed of 0.1μL/min for 10minutes. During this procedure rats were able to move freely. After microinjection, the injection cannula was left in place for 5 additional minutes to allow diffusion of the injected substance and prevent it from returning by capillarity. Immediately afterwards, rats underwent locomotor activity, rotarod, or forced swimming tests, as appropriate.
Locomotor activity testEach rat was placed inside an opaque acrylic cage (44×33×20cm) whose base was divided into squares measuring 11×11cm. We assessed the following: (a) number of squares crossed during the 5minutes that the test lasted (the rat was considered to have crossed a square when at least three-fourths of its body passed from one square to another); and (b) number of vertical behaviours (number of times when the rat exhibited vertical behaviour, that is, it was on its back legs). Crossed squares were an indicator of spontaneous motor activity, whereas vertical behaviour was also used to detect any potential alterations in motor coordination.
Rotarod testRats were trained on a rotarod (LE 8300, LSI Letica, Panlab Scientific Instruments, Barcelona, Spain) for 5 days before microinjections at a speed of 18rpm. After receiving the microinjections, rats were placed on the rotarod for assessment of latency to fall, that is, the time it takes the rat to fall off the rod. This variable is used to identify any alterations in motor coordination and balance.
Forced swimming testWe placed rats in a glass tank (base: 26×29cm; height: 50cm) filled with water at a temperature of 25°C (±1°C). The water level was such that rats could touch the bottom of the tank with their back feet and tails. This test was used to evaluate the number of spins, that is, periods during which the rat was spinning rather than moving forward.14,16
We recorded video feed from all sessions of the locomotor activity and swimming tests. Two independent observers quantified open field test variables until reaching a concordance of at least 95%. In the forced swimming test, the variable ‘number of spins’ was quantified analysing the video recordings with a software tool (ANY-maze 4.73, Stoelting, Wood Dale, IL, USA).
Verification of the microinjection siteAfter completing the behavioural tests, rats were euthanised with pentobarbital (PiSA Agropecuaria, Guadalajara, Mexico) and transcardially perfused with 100mL of physiological saline (NaCl 0.9%) followed by 100mL of formaldehyde 30% (J.T. Baker, Ecatepec, Mexico). The microinjection site was marked with Evans blue. Brains were removed and cut into thick slices to analyse the site of microinjection under a light microscope and Paxinos and Watson's22 rat brain atlas was used as a reference. The statistical analysis included data from only those rats in which cannulas were shown to have been implanted correctly in hippocampal CA1.
Statistical analysisData were analysed with the 2-way repeated measures ANOVA; the 2 factors were treatment and days of treatment. For P-values≤.05, we used the post hoc Student–Newman–Keuls test. Results were expressed as means±SD.
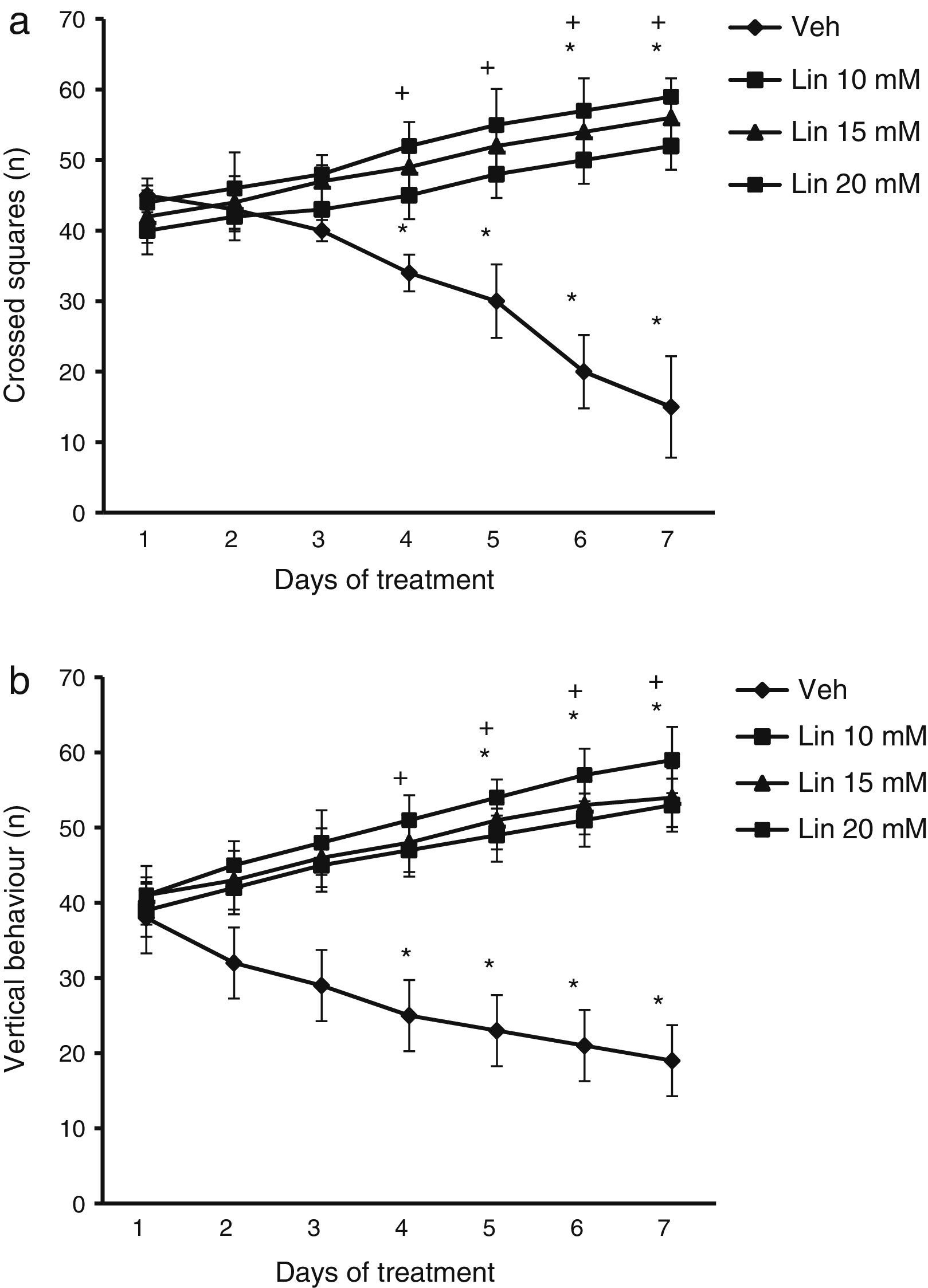
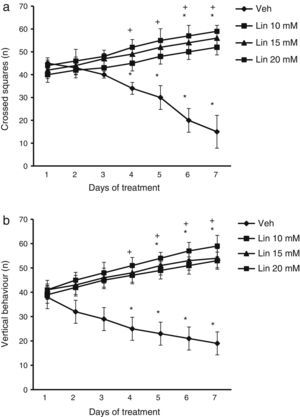
ResultsLocomotor activity testWe found significant differences in the number of crossed squares for treatment type (F [3.168]=2.735, P<.010), days of treatment (F [6.168]=1.687, P<.050), and the interaction between factors (F [18.168]=4.835, P<.010). According to the post hoc test, the number of crossed squares was significantly higher by day 4 of treatment with linamarin at different concentrations compared to day 1 of linamarin and compared to day 4 in the vehicle group (P<.05). This variable decreased significantly in the vehicle group from day 4 until the end of the study (Fig. 1).
Locomotor activity test. Regardless of linamarin concentration (Lin), the number of crossed squares (a) and vertical behaviours (b) increased beginning on day 4. The opposite effect was observed in the vehicle group (Veh). *P<.05 vs day 1 of treatment in the same group. **P<.05 vs the same day in the vehicle group. 2-way repeated measures ANOVA, post hoc Student–Newman–Keuls test.
Analysis of the number of vertical behaviours revealed significant differences for treatment type (F [3.168]=3.618, P<.015), days of treatment (F [6.168]=2.367, P<.050), and the interaction between factors (F [18.168]=15.689, P<.001). The post hoc test showed that regardless of linamarin concentration, the number of vertical behaviours increased significantly (P<.05) from day 4 of treatment compared to the vehicle group for the same day. The same was true for days 5 to 7 in the treatment group compared to day 1 in the same group. This variable decreased significantly in the vehicle group between days 4 and 7 (Fig. 1).
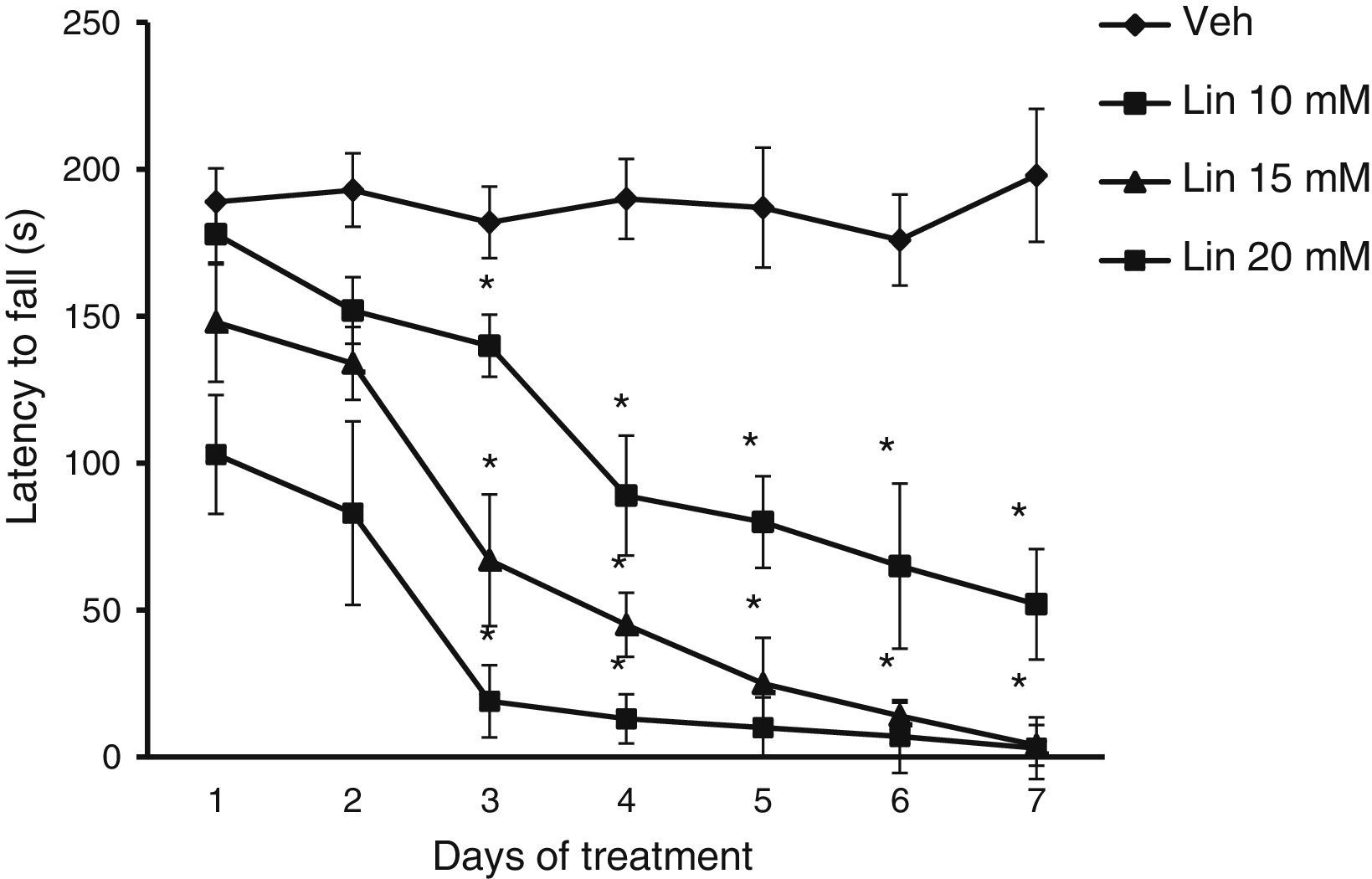
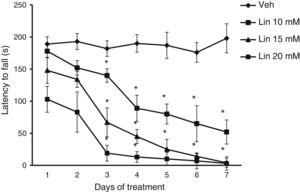
Rotarod testLatency to fall showed significant differences for treatment type (F [3.168]=28.585, P<.001), days of treatment (F [6.168]=3.162, P<.050), and the interaction between factors (F [18.168]=2735, P<.011). The post hoc test showed that regardless of linamarin concentration in treated rats, latency to fall decreased significantly (P<.05) from day 3 compared to the same day in the vehicle group and also compared to day 1 of linamarin treatment. This trend became more marked as the study progressed. Latency to fall did not change significantly in the vehicle group during the study period (Fig. 2).
Rotarod test. Regardless of linamarin concentration (Lin), latency to fall decreased gradually over the study period, a trend that was not seen in the vehicle group (Veh). *P<.05 vs the same day in vehicle group and day 1 in each experimental group. 2-way repeated measures ANOVA, post hoc Student–Newman–Keuls test.
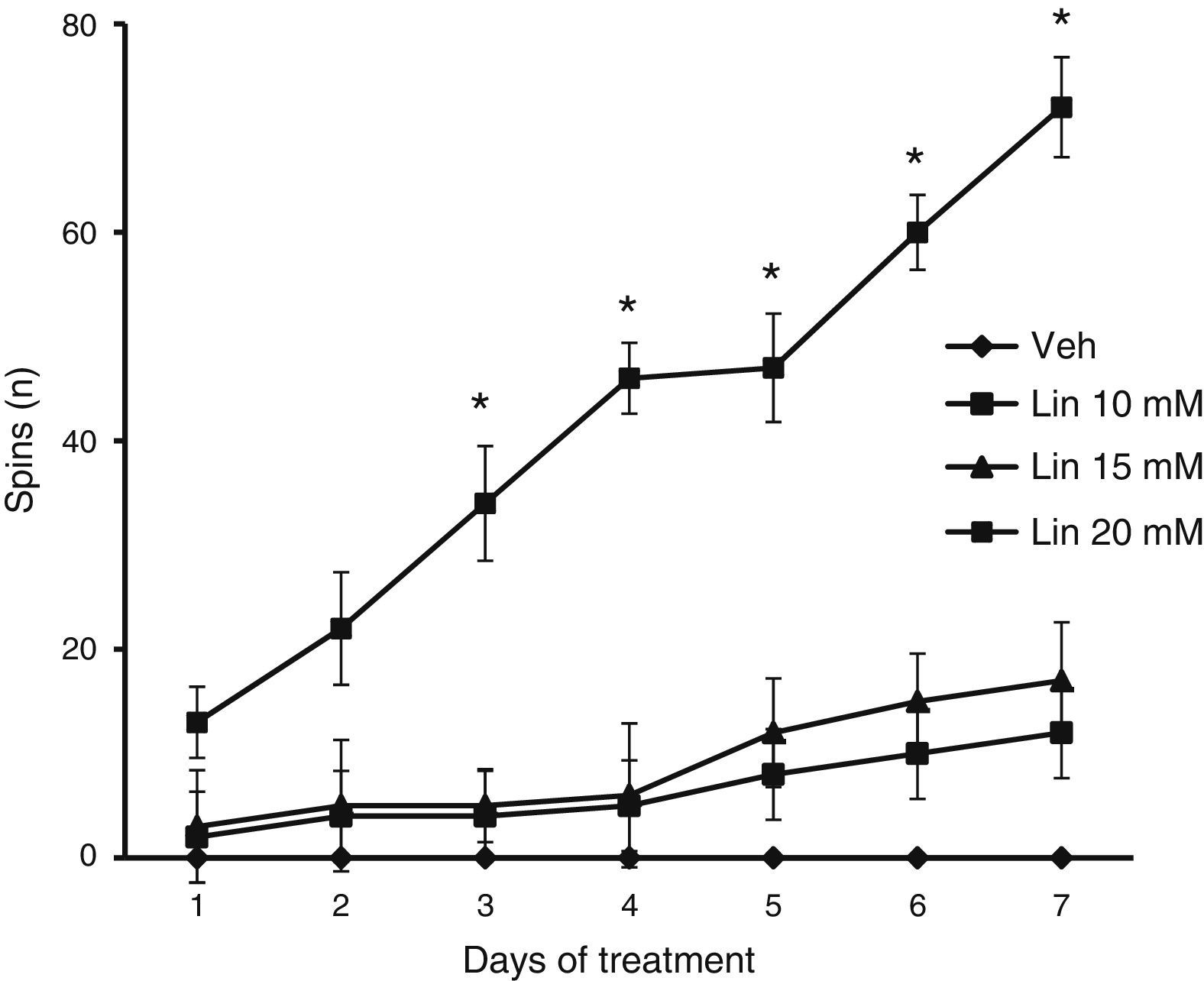
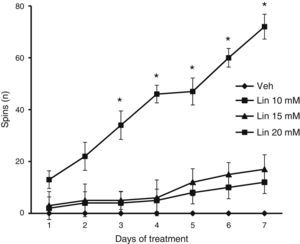
We found significant differences in the spin count for treatment type (F [3.168]=2.793, P<.040), days of treatment (F [6.168]=12.566, P<.050), and the interaction between factors (F [18.168]=2.370, P<.025). According to the post hoc test, only the group taking linamarin at a concentration of 20mM showed a gradual and significant increase in spin count from day 3 onward compared to all other groups on the same days, and compared to the same group on day 1 (P<.05). The vehicle group showed no spinning behaviour at any time throughout the study period (Fig. 3).
Forced swimming test. Only linamarin concentrations (Lin) of 20mM resulted in a gradual and significant increase in spinning; changes were significant from day 3. The vehicle group (Veh) showed no spinning behaviour during the study period. *P<.05 vs day 1 in the same group and the same day in the vehicle group. 2-way repeated measures ANOVA, post hoc Student–Newman–Keuls test.
In the present study, intrahippocampal administration of linamarin resulted in impaired motor coordination and activity. This may serve as an experimental model of the motor alterations observed in humans with neurological disorders secondary to excessive cassava consumption. Locomotor activity is frequently used to quantify displacement, exploration, and anxiety under specific experimental circumstances.23,24 In the present study, the locomotor activity test allowed us to identify hyperactivity, characterised by an increase in the number of crossed squares and vertical behaviours. These behavioural changes were caused by linamarin microinjection into the dorsal hippocampus rather than by the procedure itself (surgery and microinjection) since the vehicle group did not show any of those alterations. Our findings are in line with those reported by previous studies.16,21 The increase in these 2 types of behaviour suggests that linamarin microinjections into the dorsal hippocampus induced neuronal damage, thereby preventing the consolidation of visuospatial memory in a process that may be linked to the apparent state of ‘locomotor hyperactivity’. This hypothesis is supported by the fact that intact experimental animals or those receiving the vehicle display a gradual decrease in locomotor activity and vertical behaviour after performing the locomotor activity test several times,25 as occurred in our vehicle group. One possible explanation is that rats learn about and adapt to the conditions of the cage, which becomes familiar, resulting in a decreased need for exploration and spontaneous motor activity.26 However, rats treated with linamarin show hippocampal damage, which is likely to impair learning and memory consolidation. If this is the case, rats would not recognise the conditions of the cage and would therefore explore as with any new or unknown setting. We should highlight that increased locomotor activity over repeated sessions of the locomotor activity test has also been observed in rats undergoing dorsal hippocampus microinjection of neurotoxic cycad derivatives or receiving cassava root juice orally.15–17,27 These findings in rats may coincide with clinical reports of patients who after excessive or inappropriate cassava consumption developed a neurological disorder consisting of motor alterations and learning and memory impairment.3,28
As previously mentioned, patients with neuropathies secondary to cassava consumption also develop poor motor coordination among other symptoms.29,30 In the present study, the rats receiving linamarin at different concentrations showed decreased latency to fall regardless of the number of days of treatment. The rotarod test assesses motor coordination and balance. It is assumed that intact (healthy) animals are able to remain on the rod during longer periods since they have intact limb coordination and balance.31 For this reason, the rotarod test is used to assess the degree of CNS damage and the effects of substances on motor coordination and balance.32 Animals with CNS damage, or those treated with neurotoxic or sedative agents that affect motor activity, will show a reduced latency to fall on this test,33,34 as we observed in our study. Rats also displayed spinning behaviour in the forced swimming tests after receiving linamarine microinjections into the dorsal hippocampus. This behaviour has been proposed as an indicator of poor motor coordination: affected rats cannot control their limbs to swim correctly, but control rats can.14 Spinning has also been observed after dorsal hippocampus microinjections of neurotoxic cycad derivatives, such as methylazoxymethanol.15,16 In our study, spinning during the swimming test was not linked to damage to the vestibular system: rats with vestibular damage display this type of behaviour not only in the forced swimming test but also in the locomotor activity test (they walk in circles),12,35 but this behaviour was not observed in our sample. This indicates that behavioural alterations in the swimming test were linked to damage at a motor level rather than at the vestibular level. Spinning has been associated with poor motor coordination in the back legs of rats35; the alterations observed in our sample may therefore be similar to those in patients with induced TAN and konzo, apparently due to inappropriate cassava consumption.3,10,30
Although identifying the mechanisms underlying motor impairment after linamarin microinjection into the dorsal hippocampus was not among the purposes of the present study, we are able to offer a plausible explanation. Linamarin and other cyanogenic compounds have the ability to cause neuronal hyperexcitation in the hippocampus: these neurotoxic compounds hyperstimulate the hippocampus by overactivating ionotropic NMDA receptors, which leads to excitotoxicity and neuronal death. This may partially explain the alterations seen in humans with TAN or konzo.36–38 Our results offer a new perspective for future studies aiming to identify the neuronal mechanisms underlying neurological changes caused by the neurotoxic compounds in cassava and cassava derivatives.
Conflict of interestThe authors have no conflict of interest to declare.
This study was partially supported by the study group for the biology, chemistry, and molecular functionality of vegetable metabolites (UV-GC-235) at Universidad Veracruzana.
Please cite this article as: Rivadeneyra-Domínguez E, Rodríguez-Landa JF. Alteraciones motoras inducidas por la microinyección de linamarina en el hipocampo dorsal de la rata Wistar. Neurología. 2016;31:516–522.