Longitudinally extensive transverse myelitis (LETM) consists of an inflammatory lesion affecting 3 or more consecutive spinal cord segments. Onset may be acute or subacute. Aetiology varies, and includes tumours; infections; inflammation; demyelination; dysimmune, vascular, or metabolic disorders; and nutritional deficiencies.1,2 Despite its relatively low incidence (1–8 cases per million person-years), LETM is associated with considerable neurological morbidity. We present a case of LETM secondary to copper deficiency, which was initially thought to be due to concomitant vitamin B12 deficiency. Copper deficiency is an infrequent aetiology; very few cases have been reported, mainly in patients undergoing gastrectomy.3–5
Our patient was an 86-year-old woman of medium-high socioeconomic level and no relevant medical history. She began to experience subacute symptoms of paraesthesia in the lower limbs, mild sensory gait ataxia, and alterations in vibration sense at C7-T1 and joint mobility in the left lower limb. A complete blood count and radiological study revealed vitamin B12 deficiency. The patient was diagnosed with subacute combined spinal cord degeneration and started vitamin B12 replacement therapy (intramuscular administration; one vial per day for one week, followed by one vial per week for 4 weeks, and finally one vial per month), which achieved significant clinical improvements.
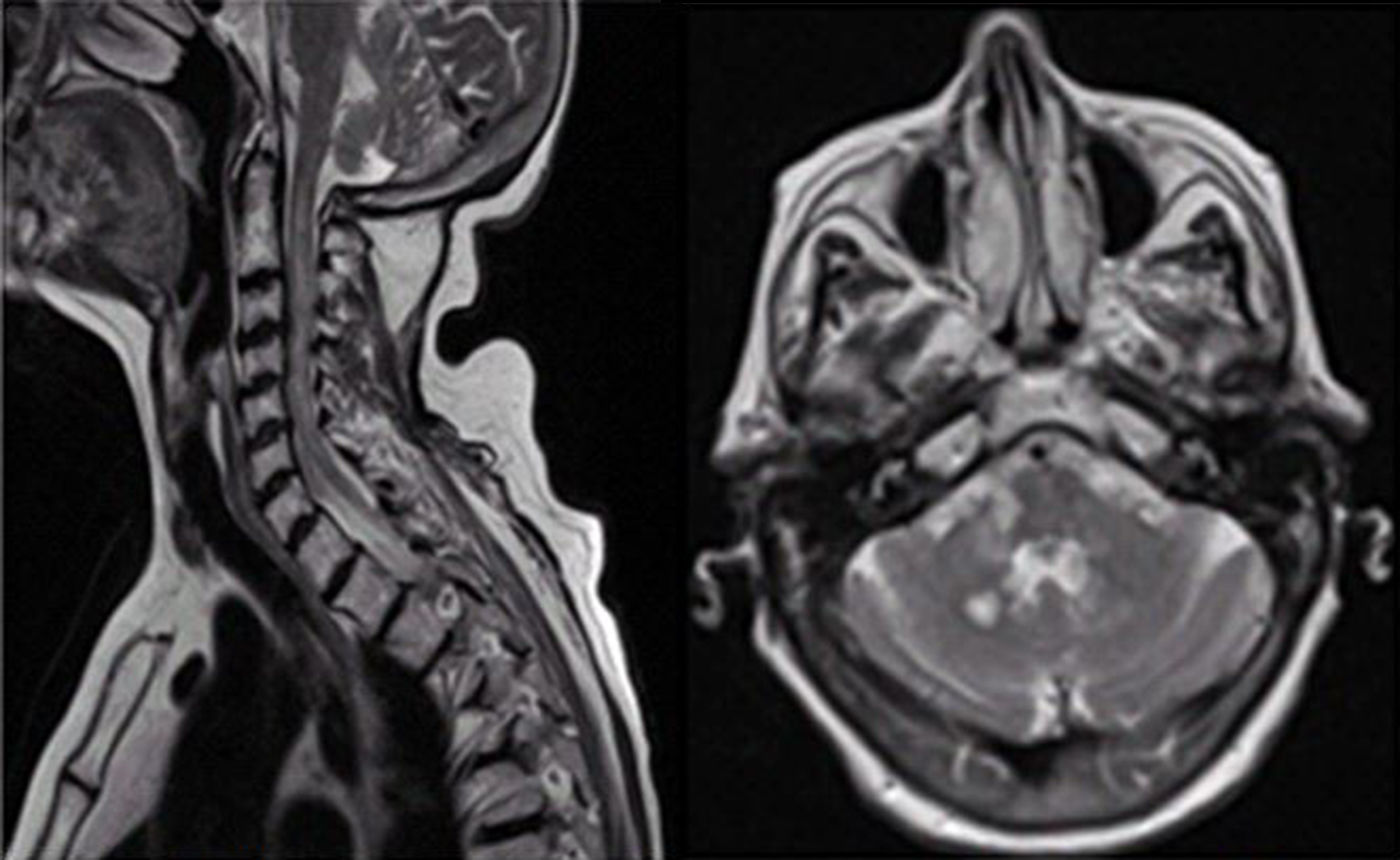
Six months later (while vitamin B12 replacement therapy was still being administered), symptoms worsened and the patient developed predominantly proximal right-sided brachial monoparesis accompanied by allodynia. The neurological examination revealed distal limb hypoalgesia, abolished arthrokinetic reflexes (except in the left arm), generalised hyporeflexia, and extensor plantar reflex in the right foot. The patient showed normal cognitive function. A complete blood count revealed normocytic normochromic anaemia (haemoglobin: 8.1g/dL) and normal vitamin B12 levels. A brain MRI scan (Fig. 1) showed multiple round lesions of varying size and morphology (some were ring-shaped with thick walls, others were ill-defined) in both cerebellar hemispheres and in the right bulbar region and middle cerebellar peduncle, with no diffusion restriction and some paramagnetic contrast uptake; no associated vasogenic oedema was observed. We also observed white matter involvement in the periauricular area and the globus pallidus bilaterally, with no contrast uptake. A full spine MRI scan revealed diffuse involvement of the posterior column, with patchy contrast uptake predominantly at C1-C5. A CT scan and a tumour marker test ruled out the presence of a tumour. Serological tests for HIV, syphilis, Lyme disease, and hepatitis C virus, and the autoimmunity study for thyroperoxidase (TPO) antibodies, complement, ANA, ENA, ANCA, and NMO-IgG yielded negative results (Table 1). A CSF analysis showed non-specific alterations, with moderate lymphocytosis and presence of oligoclonal bands. Regarding metabolic and nutritional parameters, plasma levels of vitamin B12, folic acid, zinc, and vitamins A and E were within normal ranges. Homocysteine levels were slightly elevated (13.9μmol/L). The latter finding was suggestive of vitamin B12 deficiency, despite normal plasma vitamin B12 levels. Total copper and serum ceruloplasmin levels were low (46.9μg/dL and 15.10mg/dL, respectively), with 24-h urine copper within the normal range. As the patient had not undergone gastrointestinal surgery, was not receiving iron supplementation, and had normal zinc levels, we performed a gastroscopy to determine whether malabsorption was responsible for the deficiency; we observed only mild, chronic antral gastritis. The patient was diagnosed with copper deficiency of unknown origin and started treatment with copper sulfate (2mg/24hours), oral and intramuscular vitamin B complex, and folic acid (5mg/24hours). The patient also received botulinum toxin injections in the right forearm, which improved spasticity and relieved pain.
Differential diagnosis of acquired, non-compressive myelopathies and the diagnostic process followed.
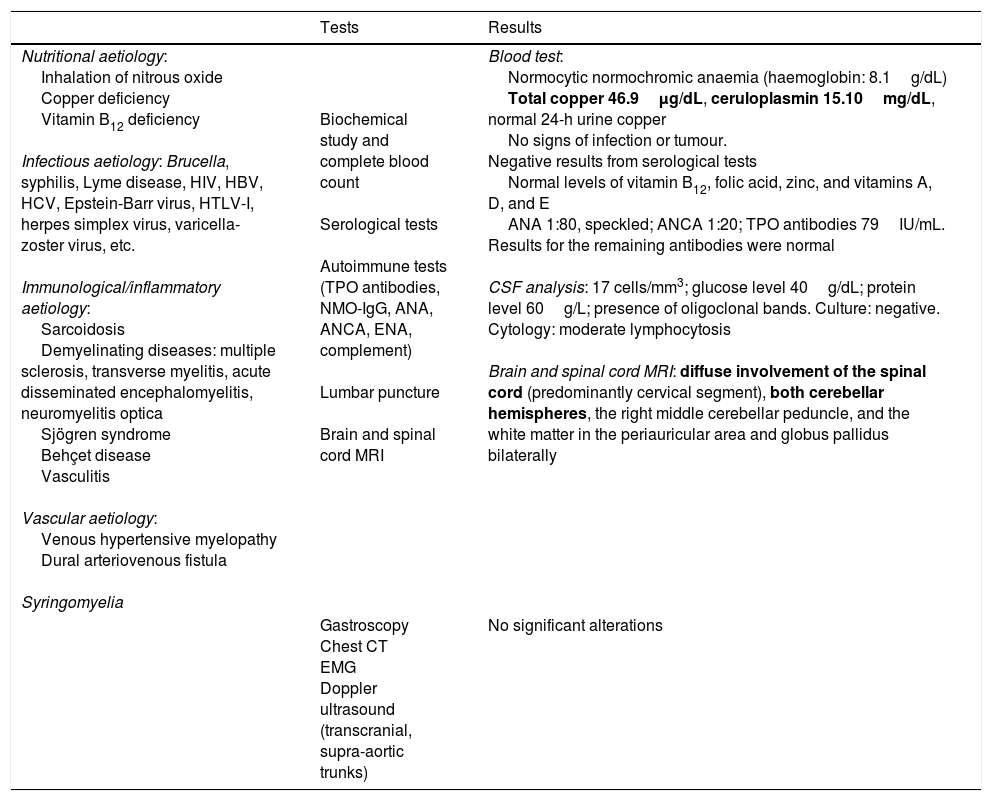
| Tests | Results | |
|---|---|---|
| Nutritional aetiology: Inhalation of nitrous oxide Copper deficiency Vitamin B12 deficiency Infectious aetiology: Brucella, syphilis, Lyme disease, HIV, HBV, HCV, Epstein-Barr virus, HTLV-I, herpes simplex virus, varicella-zoster virus, etc. Immunological/inflammatory aetiology: Sarcoidosis Demyelinating diseases: multiple sclerosis, transverse myelitis, acute disseminated encephalomyelitis, neuromyelitis optica Sjögren syndrome Behçet disease Vasculitis Vascular aetiology: Venous hypertensive myelopathy Dural arteriovenous fistula Syringomyelia | Biochemical study and complete blood count Serological tests Autoimmune tests (TPO antibodies, NMO-IgG, ANA, ANCA, ENA, complement) Lumbar puncture Brain and spinal cord MRI | Blood test: Normocytic normochromic anaemia (haemoglobin: 8.1g/dL) Total copper 46.9μg/dL, ceruloplasmin 15.10mg/dL, normal 24-h urine copper No signs of infection or tumour. Negative results from serological tests Normal levels of vitamin B12, folic acid, zinc, and vitamins A, D, and E ANA 1:80, speckled; ANCA 1:20; TPO antibodies 79IU/mL. Results for the remaining antibodies were normal CSF analysis: 17 cells/mm3; glucose level 40g/dL; protein level 60g/L; presence of oligoclonal bands. Culture: negative. Cytology: moderate lymphocytosis Brain and spinal cord MRI: diffuse involvement of the spinal cord (predominantly cervical segment), both cerebellar hemispheres, the right middle cerebellar peduncle, and the white matter in the periauricular area and globus pallidus bilaterally |
| Gastroscopy Chest CT EMG Doppler ultrasound (transcranial, supra-aortic trunks) | No significant alterations |
ANA: antinuclear antibodies; ANCA: anti-neutrophil cytoplasmic antibodies; CSF: cerebrospinal fluid; CT: computed tomography; EMG: electromyography; ENA: extractable nuclear antigens; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; HTLV-1: human T-lymphotropic virus type 1; MRI: magnetic resonance imaging; NMO-IgG: aquaporin-4 antibodies; TPO: thyroperoxidase antibodies.
Abnormal findings are shown in bold.
Gait progressively improved in the months following onset of copper replacement (the patient needed a crutch to walk long distances); the mild paraesthesia and pain in the left arm persisted, with slight paresis in the right arm and difficulty performing manipulation tasks with the right hand. At a sensory level, arthrokinetic reflexes were completely restored; the patient was left with decreased tactile sensitivity in the right arm (up to the elbow) and decreased vibration sense in the legs and right hand. Follow-up blood tests revealed that anaemia had resolved and copper levels were within the normal range. Neuroimaging revealed small, nearly imperceptible, foci of myelopathy in the cervical spinal cord, with slight contrast uptake and focal atrophy, in addition to small, non-specific hyperintensities in the cerebellum on T2-weighted sequences, with no associated contrast uptake or oedema.6
Determining the aetiology of LETM requires a combination of several tests. Although the presence and characteristics of certain radiological findings may guide diagnosis (multiple small spinal cord lesions are indicative of systemic lupus erythematosus or multiple sclerosis, whereas extensive lesions at multiple levels suggest vasculitis), no specific characteristics for each condition have been defined. Likewise, the aetiology cannot be determined based on the symptoms. Differential diagnosis therefore requires a thorough analysis including a biochemical study, a complete blood count, serological tests, autoimmunity tests (ANA, ANCA, TPO antibodies, complement, ENA, NMO-IgG), and a nutritional study (copper, vitamin B12, folic acid). Tumour screening tests and an FDG-PET scan should be performed when there is suspicion of a paraneoplastic origin.4,7–9
Although the exact role of copper deficiency in acute myelopathy is yet to be determined, copper is known to be essential to the proper functioning of the nervous system. The mineral is present throughout the brain, particularly in the basal ganglia, hippocampus, and cerebellum. The central nervous system contains several copper-dependent enzymes: tyrosinase, peptidylglycine alpha-amidating monooxygenase, copper/zinc superoxide dismutase, ceruloplasmine, hephaestin, dopamine beta-hydroxylase, and cytochrome c oxidase.5,10 Copper deficiency has an estimated prevalence of 23%11 and is associated with haematological alterations in 78% of cases (68% present anaemia and 50% leukopaenia), brain and spinal cord MRI alterations in 47% of cases, and neurological alterations in 48%12; these neurological alterations include myelopathy associated with symptoms of subacute combined degeneration. Although the literature includes some cases of patients with similar CSF results to our own (pleocytosis and presence of oligoclonal bands), most patients show normal CSF results (copper deficiency of paraneoplastic origin may be associated with elevated protein levels and mild pleocytosis). The inflammation secondary to neurodegeneration (with demyelination probably secondary to copper deficiency) that occurs during this condition may be responsible for these alterations. Up to 47% of the patients have a history of gastrointestinal surgery, whereas the cause is unknown in 20% of cases.13 Other more prevalent conditions, such as myelopathy secondary to vitamin B12 deficiency or to varicella-zoster virus infection, may present with similar symptoms, which adds to the complexity of diagnosing this condition (Table 1).
We may conclude that LETM due to copper deficiency is a rare entity whose clinical and radiological features make it indistinguishable from other more prevalent conditions, such as combined spinal cord degeneration secondary to vitamin B12 deficiency. The co-occurrence of these 2 conditions, especially in patients with no history of gastrectomy and in whom malabsorption syndrome is not suspected, delays the onset of copper replacement therapy, resulting in potentially irreversible neurological sequelae.
Please cite this article as: Urtiaga S, Terrero R, Malumbres M, Pinel A. Mielopatía por déficit de cobre: la gran simuladora. Neurología. 2018;33:278–281