Lymphomatoid granulomatosis is a B-cell lymphoproliferative disorder associated with Epstein-Barr virus (EBV) infection, and characterised by a reactive T-cell infiltrate with an angiocentric, angiodestructive growth pattern.1–3 In exceptional cases, the condition may affect the central nervous system (CNS) exclusively.3–5
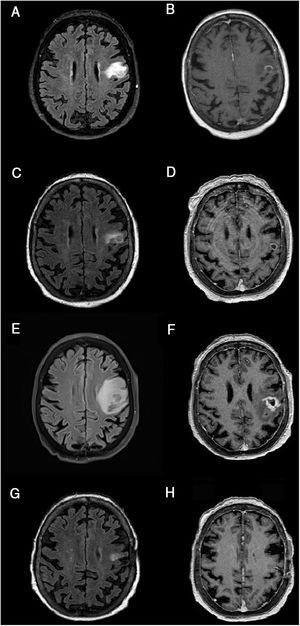
We present the case of a 56-year-old woman who consulted due to repeated episodes of transient dysarthria. She had history of arterial hypertension, dyslipidaemia, and chronic kidney disease. She had undergone kidney transplantation and had been receiving immunosuppressive therapy since 1981; at the time of consultation, she was receiving prednisone (5 mg/24 h), tacrolimus (2 mg/24 h), and mycophenolate mofetil (360 mg/12 h). An emergency CT scan revealed a hypodense lesion in the left frontal lobe. During hospitalisation, she presented episodes of anarthria and clonic movements affecting the perioral region, suggestive of opercular epilepsy, and started treatment with levetiracetam dosed at 500 mg/12 hours. Graft failure was also observed, and haemodialysis was restarted. A brain MRI scan (Fig. 1) revealed a corticosubcortical lesion in the left frontal lobe, vasogenic oedema, and ring contrast enhancement; differential diagnosis between infectious and neoplastic processes was considered. Empirical antibiotic therapy was started and immunosuppressive therapy suspended. Whole-body CT and PET/CT scans revealed no abnormalities. CSF biochemical analysis yielded normal results, with negative results for immunophenotyping, cytology, and microbiology studies (cryptococcal antigen screening; venereal disease research laboratory test; polymerase chain reaction assay for Toxoplasma gondii, Listeria monocytogenes, and John Cunningham virus; fungal and mycobacterial cultures). The patient remained asymptomatic after starting antiepileptic treatment. Radiological improvements were observed after 21 days of antibiotic therapy (Fig. 1). Two months later, she presented progressive speech impairment with naming difficulties, right facial paresis, and right faciobrachial clonic movements, which were controlled with levetiracetam (1000 mg/12 h) and lacosamide (50 mg/12 h). An MRI scan revealed increased lesion size and oedema extension. Results from a brain biopsy (Fig. 2) revealed grade 3 lymphomatoid granulomatosis. Additional whole-body CT and PET/CT scans were performed to detect any metastasis, yielding normal results. After a multidisciplinary assessment, radiation therapy and chemotherapy (R-CHOP, rituximab) were ruled out due to the patient’s poor health status and history of chronic kidney disease. Treatment was started with ibrutinib (a Bruton’s tyrosine kinase inhibitor) at 560 mg/day for compassionate use; tolerance was good. After 8 months of treatment, the patient presented a partial clinical improvement (mild anomia and facial paresis) and significant radiological improvement (Fig. 1).
Brain MRI scan showing lesion progression. A-B) At admission. C-D) After 21 days of treatment. E-F) After 2 months of treatment. G-H) After 3 months of treatment. FLAIR (A, C, E, G) and gadolinium-enhanced T1-weighted sequences (B, D, F, H), axial plane. Images show the progression of a corticosubcortical lesion located in the left precentral gyrus/frontal operculum; the lesion is rounded and well defined, and appears iso- and hyperintense on FLAIR sequences (A, C, E, G) and hypointense on T1-weighted sequences (B, D, F, H), surrounded by vasogenic oedema and with contrast ring enhancement (B, D, F, H). Lesion size at admission was 15 × 12 mm (anteroposterior × lateromedial) (A). After a slight decrease in lesion size (12 × 7.5 mm) and significant improvements in the perilesional oedema (C), with contrast ring enhancement remaining nearly unchanged (D), remarkable increases were observed in lesion size (16 × 16 mm) and vasogenic oedema (E), as compared to the previous study (D). The lesion also changed in terms of morphology (more heterogeneous) (D), and displayed more extensive and irregular contrast uptake (F). Significant improvements were observed 3 months after onset of treatment with ibrutinib, with a decrease in lesion size and improvement in oedema (G); contrast uptake was barely perceptible (H). Changes were observed after craniotomy (biopsy) (G, H).
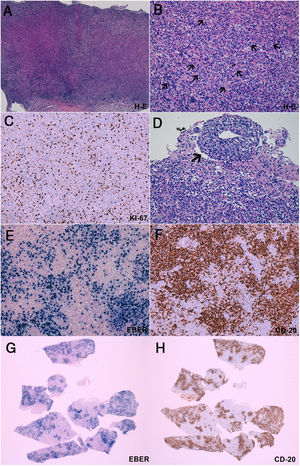
Brain biopsy. A-B) Haematoxylin-eosin staining showed intense polymorphous inflammatory infiltrate containing lymphocytes, plasma cells, and histiocytes, intermingled with large, atypical cells (B, arrows), some of which were close to blood vessels (B, arrowheads). C) The Ki67 proliferation index was > 40%. These cells tend to form aggregates of >50 cells (B) and present an angiocentric and angiodestructive growth pattern (D). Immunohistochemical studies (E, F, G, H) revealed that these atypical cells express CD20 (present in B-cells) and EBER (Epstein-Barr virus–encoded RNA, which indicates Epstein-Barr virus infection). A comparison of histology sections revealed a nearly complete overlap between EBER-expressing and CD20-expressing cells (G, H). All these findings are diagnostic of an EBV-associated B-cell lymphoproliferative disorder, compatible with lymphomatoid granulomatosis grade 3.
Lymphomatoid granulomatosis is an extremely rare type of mature B-cell neoplasm.1,2,5–7 It is most common in men aged 40-60 years.1–5,7 The condition frequently affects immunosuppressed patients,1,2,7 although cases have also been reported in immunocompetent individuals.2,4,5 It is an atypical EBV-associated B-cell lymphoproliferative disorder1,2,7,8 involved in the development of different types of lymphoproliferative neoplasms.9 Although the exact pathophysiological relationship between EBV infection and lymphomatoid granulomatosis is not understood, immunosuppression-dependent EBV reactivation is believed to promote the expression of oncogenes, which in turn promote lymphoproliferation.1,4 Lymphocytic aggregates appear around a necrotic central region, due to the angiocentric and angiodestructive behaviour of the disorder.1,7,8 Lymphomatoid granulomatosis is classified into 3 grades, depending on the extension of necrosis (absent, patchy, or extensive) and the number of EBV-positive B cells (< 5, 5-20, or > 20 per high-power field).1,3,7,8 Lymphomatoid granulomatosis grade 1 or 2, considered indolent processes, may improve with corticosteroid therapy.2 The prognosis of lymphomatoid granulomatosis is poor; the condition is equivalent to diffuse large B-cell lymphoma, and requires aggressive treatment with chemotherapy and/or radiation therapy.1–3 The lung is the most frequently affected organ (90%). Secondary dissemination to the CNS is not infrequent (25%-30%),1,3 but primary, isolated CNS involvement is exceptional, with only 49 cases reported to date.2–5 Cases not involving the lungs are atypical, and are therefore associated with greater diagnostic delays; this may have a negative impact on prognosis, since low-grade lymphomatoid granulomatosis can progress to more aggressive forms.1,10 Neurologists should therefore be familiar with this entity. In patients with history of transplantation, lymphomatoid granulomatosis should be differentiated from post-transplant lymphoproliferative disorder; this B-cell proliferative disorder is also associated with EBV infection but does not present reactive T-cell infiltration or necrosis.2,4,8,11,12 In immunocompetent individuals, lymphomatoid granulomatosis may be misdiagnosed as glioblastoma multiforme due to the presence of necrosis. Lymphomatoid granulomatosis associated with brainstem involvement may be indistinguishable from CLIPPERS syndrome.13 These similarities between lymphomatoid granulomatosis and other diseases make diagnosis even more challenging. Furthermore, in our case, chronic kidney disease and limitations with drug transportation across the blood-brain barrier constituted a therapeutic challenge. Ibrutinib, a drug currently under study for the treatment of primary CNS lymphoma,14 was administered for compassionate use due to its ability to cross the blood-brain barrier and its limited renal clearance.15 This is the first reported case of primary lymphomatoid granulomatosis of the CNS treated with ibrutinib; the drug may constitute a treatment option for patients ineligible for R-CHOP.
Our case is interesting in that lymphomatoid granulomatosis exclusively involved the CNS and was treated with ibrutinib. Neurologists should be familiar with this entity in order to prevent diagnostic delays.
DisclosuresAll authors made intellectual contributions to this study and approved the final version of the manuscript.
Confidentiality of patient data was preserved.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to sincerely thank Dr Zaldumbide Dueñas (anatomical pathology department, Hospital Universitario de Cruces) for her constant collaboration with the neurology department, and particularly in this case for her insightful explanation of anatomical pathology images.
Please cite this article as: Moreno-Estébanez A, González-Pinto T, Agirre-Beitia G, González LM. Granulomatosis linfomatoide primaria del sistema nervioso central: un reto diagnóstico. Neurología. 2021;36:625–628.