Behçet disease (BD) is a recurrent multisystemic inflammatory disease of unknown aetiology, with greater prevalence in Asian countries. The condition has been associated with specific histocompatibility antigens (HLAs) such as B5, B27, B56, and A26, while HLA-B*49 and HLA-B*03 are considered to be protective alleles.1 The autoimmune basis of the disease is currently subject to debate: although the disease has been associated with specific antibodies, their role in pathogenesis is controversial, with some authors considering it to be an autoinflammatory disease.2 Other features of BD are its peculiar geographic distribution along the former Silk Road between the Mediterranean and East Asia, and the varying prevalence of different clinical manifestations according to patients’ ethnicity.3 Neurological manifestations of BD, or neuro-Behçet disease (NBD), are present in only 5%-10% of patients.4,5 NBD can feature parenchymal (80% of cases) or non-parenchymal involvement.6 Non-parenchymal NBD affects vascular structures, with cerebral venous thrombosis (CVT) being the most frequent complication. Arteriovenous fistulae (AVF) are rare and have previously been described in the retina.7 We present the case of a 37-year-old patient with dural AVFs (DAVF) secondary to CVT as the initial neurological manifestation of BD.
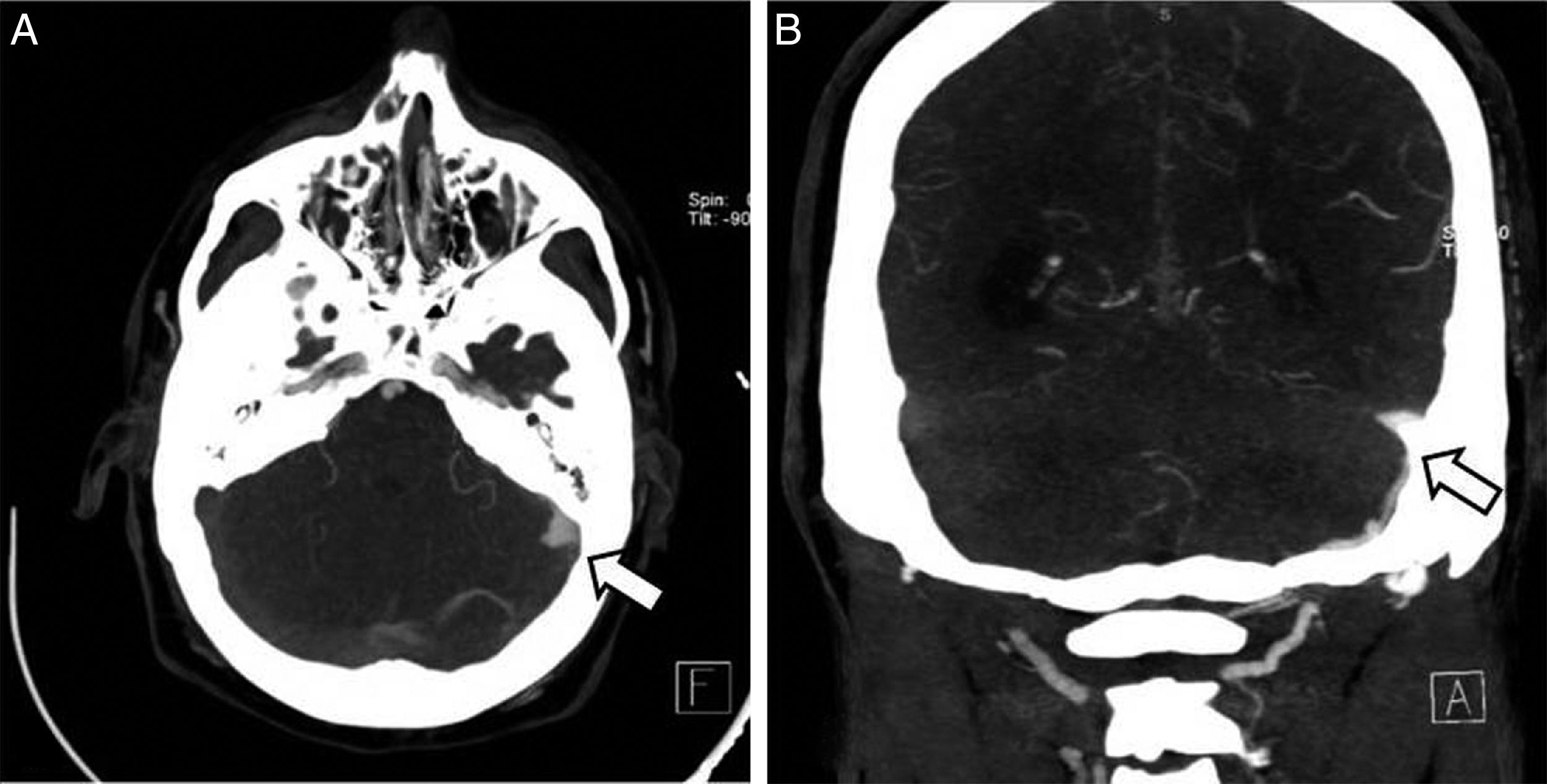
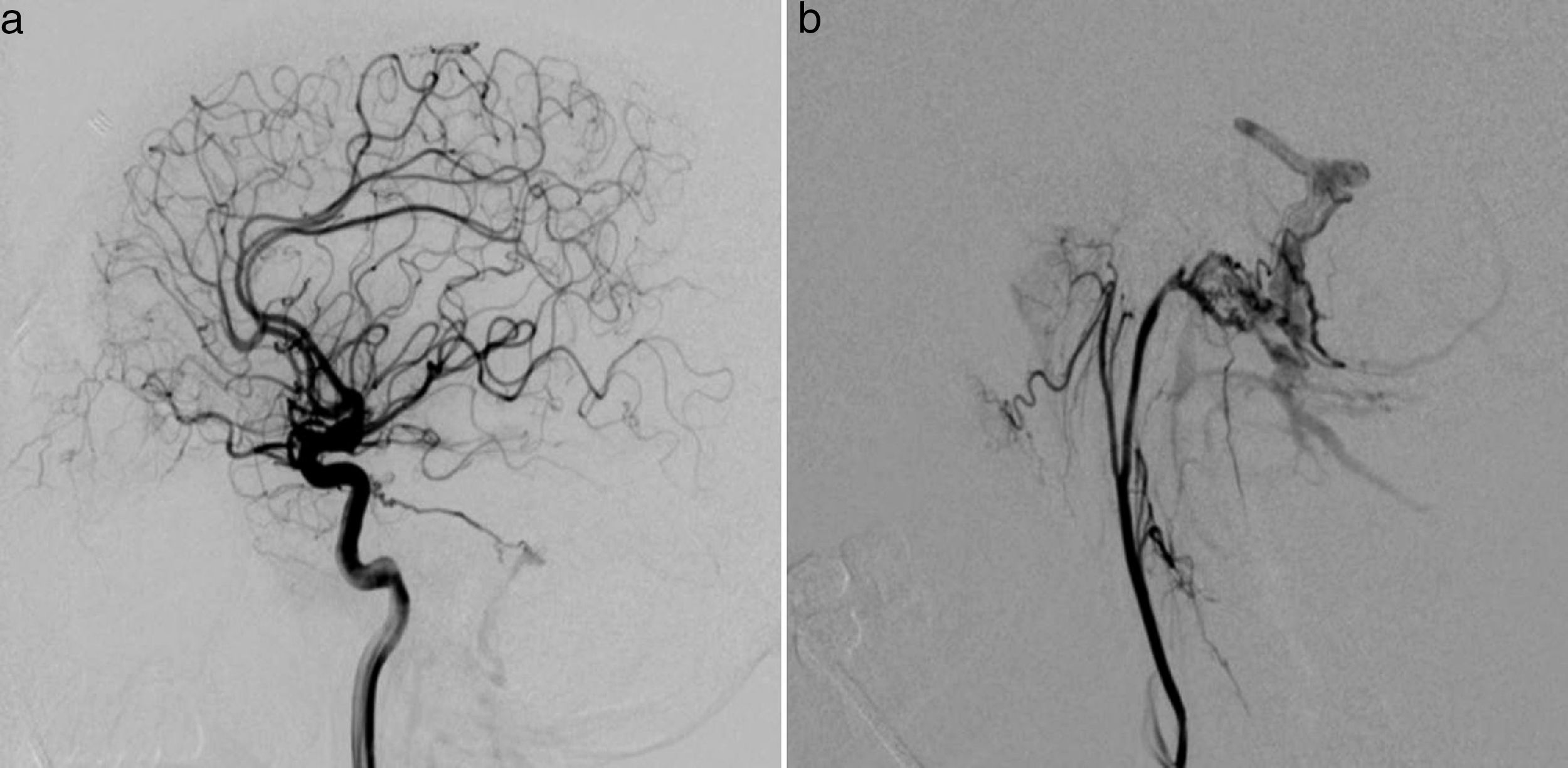
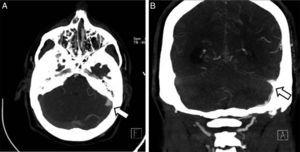
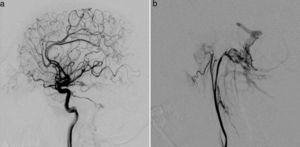
Clinical caseThe patient was a 37-year-old man who came to our hospital with subacute-onset headache, transient hypoaesthesia of the left arm, and sudden loss of consciousness. A brain CT scan revealed a right frontal focal subarachnoid haemorrhage (Fig. 1); however, a CT-angiography and arteriographic study found no abnormalities. Six months later, the patient returned to our centre after experiencing an episode of headache and dizziness. Another CT-angiography was performed, revealing anomalous vascular structures in the posterior fossa and an irregularity in the sigmoid sinus, accompanied by engorged branches of the external carotid artery (Fig. 1). A new cerebral arteriography showed the presence of 2 DAVFs: one in the posterior fossa, fed by the internal and external carotid arteries and with venous drainage through the sigmoid and transverse sinuses and cortical veins (type II a+b in the Cognard classification)8; and the other in the superior sagittal sinus, fed by the left meningeal artery, with cortical venous reflux (Cognard type III) (Fig. 2). A brain MRI with venography displayed thrombosis of the left transverse sinus.
The patient had a history of mouth ulcers and folliculitis. He reported no history of ocular pathology or genital ulcers; a pathergy test and coagulation study returned negative results. Positive results were found for histocompatibility antigen HLA-B51. BD was diagnosed according to the criteria established by the International Team for the Revision of the International Criteria for Behçet's Disease (ITR-ICBD).4
The Cognard classification distinguishes 5 types of DAVF according to drainage pattern, assisting physicians in assessing the risk of complication and in making treatment decisions. According to this classification, type II a+b and type III DAVFs, the types observed in our patient, present a high risk of bleeding.8 We therefore decided to occlude the DAVFs using Onyx®, a liquid embolic agent, combined with immunotherapy consisting of colchicine and low doses of prednisone and azathioprine. It was initially decided not to begin thrombolytic treatment due to the high risk of cerebral haemorrhagic complication. The patient's only systemic symptom in the following months was occasional mouth ulcers. A year later, he experienced recurring intense headache and tinnitus in the right ear. An MRI with venography and an arteriographic study were performed. These showed another large DAVF with 2 components: one in the torcula, with associated cortical venous drainage (Cognard type II a+b); and another affecting the right sigmoid and transverse sinuses, fed by branches of the right internal carotid artery (Cognard type I). These were successfully treated. We suspected that the recurring DAVFs were secondary to a chronic, non-recanalised CVT and decided to begin oral anticoagulant treatment in order to achieve an international normalised ratio score between 2 and 3. Azathioprine dose was doubled.
The patient did not present deep vein thrombosis or other vascular symptoms and remained radiologically stable during clinical follow-up of the anticoagulant treatment (8 months).
DiscussionCVT is the most common vascular complication in BD, accounting for 18% of NBD cases9; it occurs in approximately 8% of all patients with BD.10 Some authors have described specific characteristics of CVT in these patients, with a higher frequency of chronic or subacute course,11 greater involvement of the sagittal and transverse sinuses,12,13 and more favourable prognosis.11 The pathogenesis of thrombus formation in BD remains unknown; its high incidence in this condition is not explained by classic thrombotic factors.14
Regarding coagulation, many studies have attempted to identify specific disorders in some process or reaction in the coagulation cascade, such as protein C, protein S, high thrombomodulin concentrations, and alterations affecting antithrombin function and various levels of plasmatic fibrinolysis (both the activator [tissue plasminogen activator; t-PA] and the inhibitor [type I t-PA inhibitor]).14 Findings have been contradictory, however; this is probably due to a selection bias (patients in the active and inactive phase of the disease), methodological differences in laboratory testing, or due to the differing ethnicities of patients, as is the case with the roles that factor V Leiden and prothrombin gene mutation G20210A may play in thrombosis.15 Other, less frequent, forms of neurovascular involvement in BD include arterial dissections, arterial thrombosis, intracranial haemorrhage, and cerebral aneurysm.
DAVFs are a series of heterogeneous conditions, all of which involve arteriovenous shunts, or connections, between dural vessels. They represent 10%-15% of all vascular malformations with shunts.16 According to our literature search, DAVFs associated with BD have been described only once.17 In that case, the first neurological manifestation of the disease was focal subarachnoid haemorrhage, probably related to a cortical venous thrombosis. We interpreted our patient's DAVF as being the consequence of high vascular resistance caused by a chronic, non-recanalised CVT.
Regarding treatment of thrombosis in BD, no recommendations are available with respect to long-term anticoagulant treatment.18 On the one hand, it is essential to consider the potential for co-presence of arterial aneurysms and the associated haemorrhagic risk.19 On the other hand, the presence of an underlying inflammatory process causing the thrombus to adhere to the vascular wall20 has led many experts to consider only immunosuppressive treatment and not anticoagulation. A study including 64 patients with BD and CVT, 62 (96%) of whom were treated with anticoagulants, found that anticoagulants are safe and effective for these patients, and that immunosuppression had no additional benefit.13 However, a recent international expert consensus was unable to make a definitive recommendation on long-term anticoagulation/immunosuppression in patients with CVT and BD.6 In cases where the condition is recurrent or refractory to conventional treatment, physicians should consider biological treatment, particularly anti-TNF agents and interferon alpha. Despite having been shown to be very effective in patients with ocular or mucocutaneous manifestations of BD, the effectiveness of these drugs in patients with vascular involvement is currently only anecdotal; decisions should therefore be made case-by-case.21
Ours is the second published case of DAVF manifesting in the context of BD, presumably associated with chronic CVT. This complication may be the only manifestation of a silent CVT; treatment should be modified if it is detected.
Conflicts of interestNone.
Please cite this article as: Falgàs N, Borrego S, Llull L, Espinosa G, López-Rueda A, Blasco J, et al. Fístulas arteriovenosas durales recurrentes en paciente con enfermedad de Behçet. Neurología. 2017;32:623–626.