Some patients with acute ischaemic stroke occasionally present seizures at stroke onset.1 Non-contrast brain computed tomography (CT) has some limitations in this context, and early signs of ischaemia are frequently subtle.2 An epileptic seizure at stroke onset is considered a relative contraindication for intravenous recombinant tissue plasminogen activator (rt-PA) administration. The 2011 SEN stroke management guidelines3 acknowledge that this should not justify ruling out thrombolytic treatment when cerebral infarction is confirmed by neuroimaging techniques. The 2013 American Heart Association/American Stroke Association guidelines deem intravenous thrombolysis appropriate given evidence that the residual deficits are secondary to ischaemia and not to a postictal event.4
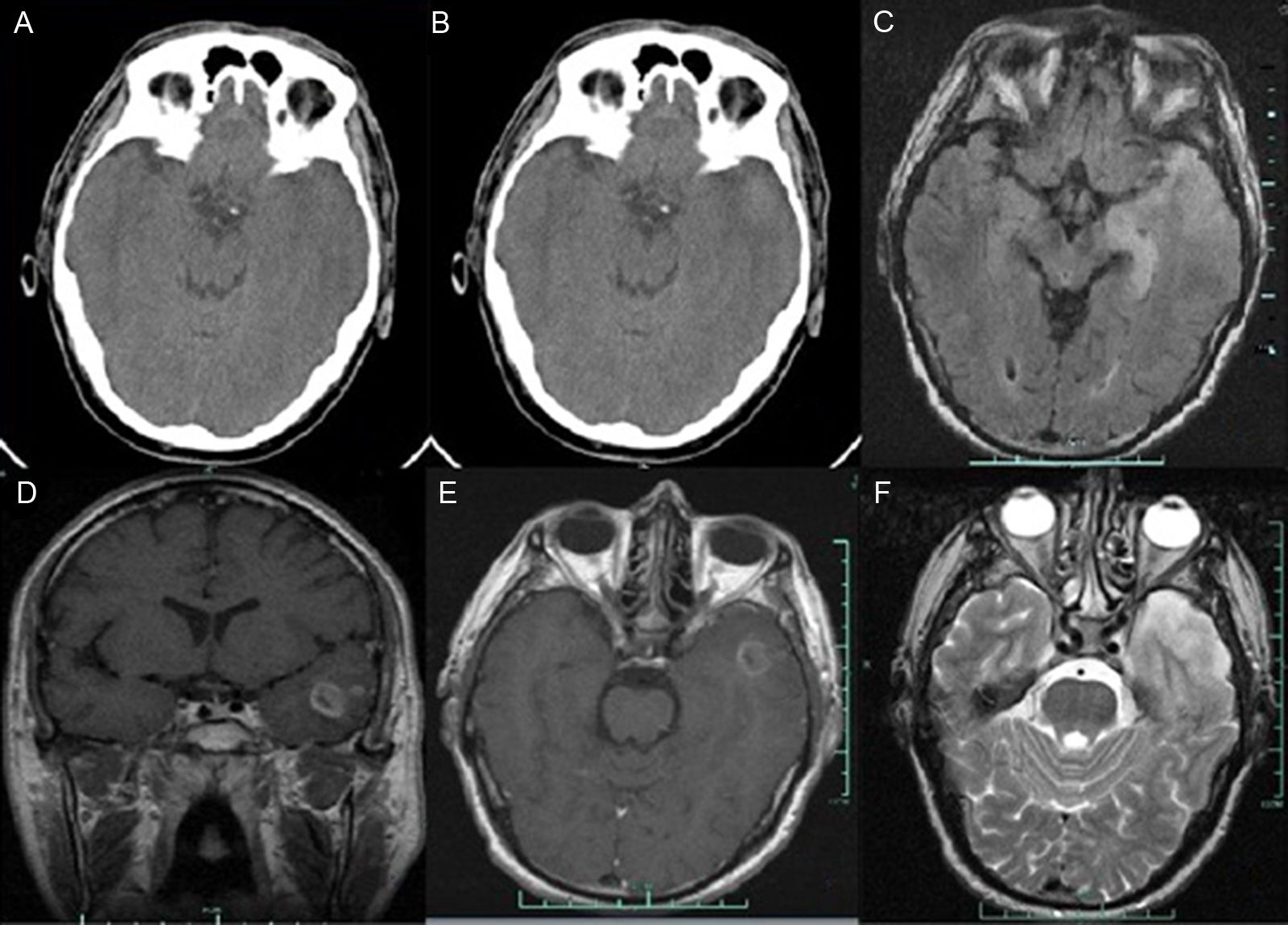
We present the case of a 46-year old man with a history of hypercholesterolaemia and moderate alcohol consumption, who attended our emergency department due to speech disturbances and right-sided motor deficit of sudden onset. He arrived 45minutes after symptom onset, presenting an arterial pressure of 168/92mm Hg and a heart rate of 109bpm. Auscultation was normal and the echocardiogram showed sinus rhythm. Neurological examination revealed global aphasia, right-sided hemiparesis of faciobrachial predominance, and right hemihypaesthesia. The National Institute of Health Stroke Scale (NIHSS) score was 15:2 in level of consciousness (LOC) questions, 2 in LOC commands, 1 in facial palsy, 4 in right motor arm, 2 in right motor leg, 1 in sensory, and 3 in language. One hour and 30minutes after symptom onset, the patient experienced a generalised tonic-clonic seizure which resolved in 20seconds after the intravenous administration of 10mg diazepam. A non-contrast brain CT scan performed with a Siemens Somatom® Emotion eco (16-slice configuration) 15minutes after the seizure yielded normal results (Fig. 1A). As we did not consider the patient's symptoms to be due to postictal changes, no change to the neurological deficits after the seizure was observed, and an advanced imaging scan could not be scheduled (time- and space-limited accessibility), we discussed the case and started treatment with intravenous alteplase, 2hours and 15minutes after symptom onset. Neurological improvement was observed (NIHSS 9:1 in LOC questions, 1 in LOC commands, 1 in facial palsy, 2 in right motor arm, 1 in right motor leg, 1 in sensory, and 2 in language). At 24hours, we observed mild aphasia with no motor or sensory deficit (NIHSS 2); the contrast brain CT scan (indicated after the epileptic seizure at onset) performed at 24hours showed a hyperdense area in the left temporal lobe (Fig. 1B). The echocardiogram and the carotid and vertebral echo-Doppler study yielded normal results. The brain MRI study (T1-, contrast T1-, T2-, T2*-, and diffusion-weighted, FLAIR sequences and apparent diffusion coefficient [ADC] maps) performed at 3 days revealed a glial tumour with cyst-like appearance in the left temporal lobe, extending to the uncus and hippocampus. It also revealed central necrosis with an infiltrative/expansive growth pattern (Fig. 1C-F). No haemorrhagic transformation or intratumoural bleeding was observed (Fig. 1C-F). Diffusion-weighted sequences and ADC maps did not show changes suggestive of cerebral ischaemia. At day 5 (with no previous corticosteroids or surgery), deficits resolved completely (NIHSS 0).
Brain CT: (A) Findings from the initial non-contrast cranial CT scan were normal. (B) Contrast brain CT image at 24hours revealing a hyperdense area in the left temporal lobe, infiltrating the ipsilateral Sylvian fissure with no contrast uptake. Brain MRI: (C) Axial T1-weighted sequence. A hyperintense lesion was observed in the left temporal lobe. (D) Coronal contrast T1-weighted sequence. An irregular lesion with a hypointense centre (ring enhancement) was identified. (E) Axial contrast T1-weighted sequence. The scan revealed an irregular area with low contrast with a cyst-like component and central necrosis exerting a mass effect with left temporal horn effacement. F) Axial T2-weighted sequence. A left temporal hyperintense lesion (oedema) was observed. MRI results: glial tumour in the left temporal lobe expanding into the uncus and hippocampus with a cyst-like appearance and central necrosis with an infiltrative/expansive growth pattern.
The patient was transferred to the neurosurgery department for tumour resection. Histological findings were compatible with grade III anaplastic astrocytoma.
There is limited scientific information on the use of alteplase in patients with astrocytomas mimicking stroke.5 To our knowledge, there are only 2 published cases of patients treated with rt-PA due to suspected acute ischaemic stroke; both were finally diagnosed with glioblastoma multiforme.6,7
Our patient was symptomatic for more than 24hours; however, no signs of lesions caused by cerebrovascular disease were observed. We believe that the stroke-like symptoms were caused by infiltration of the tumour into the Sylvian fissure, surrounding the left middle cerebral artery as in the case reported by García et al.6 However, we cannot definitively rule out an ischaemia associated with the brain tumour and resolved by thrombolysis.6
Few cases of patients with epileptic seizures manifesting at stroke onset and safe use of rt-PA have been published,6,8 and cases of patients with stroke-like conditions treated with thrombolysis and finally diagnosed as brain tumour are rare.9 The Copenhagen Stroke Study suggests that an epileptic seizure at stroke onset may involve a large area of hypoperfused but potentially salvageable brain tissue.10
We would like to highlight that: (1) thrombolysis in a patient with astrocytoma was safe, (2) a patient with an epileptic seizure at onset of a stroke-like event may present a tumour; (3) non-contrast brain CT is not sufficient to detect neoplasms, particularly in the middle cranial fossa (artefacts are frequent in this location), potentially causing an astrocytoma to go unnoticed; and (4) brain MRI is useful since it displays the middle cranial fossa better than brain CT, helping to rule out tumour vs ischaemic lesion. Likewise, an astrocytoma may go unnoticed in non-contrast brain CT images.
As far as we are aware, this is the first case of safe use of alteplase in a patient with grade III anaplastic astrocytoma mimicking stroke with no haemorrhagic transformation.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Prof. Pablo Vivanco for his help in drafting this manuscript.
Please cite this article as: Ros Forteza FJ, Pantazi I, Cardoso A. Trombólisis segura en astrocitoma de fosa craneal media. Neurología. 2018;33:199–201.